Abstract
Background
Pulmonary drug delivery is an efficient way to deliver drugs directly to the site of action i.e., lungs or to the blood circulation with minimum systemic effects. Recent emergence of coronavirus disease-2019 (COVID-19) and expeditious development of nanoparticle-based vaccines have recently reignited considerable interest in designing inhalable nanoparticle-based drug delivery systems as next-generation respiratory therapeutics.
Area covered
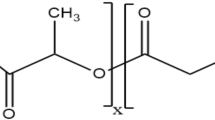
In this review, dry powder inhaler (DPI) formulations based on polymeric nanoparticles have been reviewed. It comprehensively describes various biodegradable and biocompatible synthetic (e.g. polylactic-glycolic acid, polyethylene glycol, polyethylenimine, and polycaprolactone) and natural (e.g. alginate, chitosan, dextran, hyaluronic acid, and gelatin) polymers used in formulating nanoparticle-based dry powder inhalers. This review covers the most recent drugs encapsulated in synthetic-based and natural-based biocompatible nanocarriers used in DPIs, providing latest approaches for treating various respiratory and pulmonary disorders.
Expert Opinion
DPIs comprised of biocompatible biodegradable polymeric nanocarriers exhibited favorable particle properties and aerodynamic properties. In addition, these polymeric nanocarriers are chemically versatile in being either synthetic or natural. This chemical versatility enables versatility in the various types of drugs that can be incorporated into DPIs provided that the needed particle properties and aerodynamic properties are maintained.




Similar content being viewed by others
References
Abdelaziz HM, Gaber M, Abd-Elwakil MM, Mabrouk MT, Elgohary MM et al (2018) Inhalable particulate drug delivery systems for lung cancer therapy: nanoparticles, microparticles, nanocomposites and nanoaggregates. J Controlled Release 269:374–392
Abdelrady H, Hathout RM, Osman R, Saleem I, Mortada ND (2019) Exploiting gelatin nanocarriers in the pulmonary delivery of methotrexate for lung cancer therapy. Eur J Pharm Sci 133:115–126
Abourehab MS, Rajendran RR, Singh A, Pramanik S, Shrivastav P et al (2022) Alginate as a Promising Biopolymer in Drug Delivery and Wound Healing: a review of the state-of-the-art. Int J Mol Sci 23:9035–9035
Al-Obaidi H, Granger A, Hibbard T, Opesanwo S (2021) Pulmonary Drug Delivery of antimicrobials and Anticancer drugs using solid dispersions. Pharmaceutics 13:1056–1056
Al-Qadi S, Grenha A, Carrión-Recio D, Seijo B, Remuñán-López C (2012) Microencapsulated chitosan nanoparticles for pulmonary protein delivery: in vivo evaluation of insulin-loaded formulations. J Controlled Release 157:383–390
Archer E, Torretti M, Madbouly S (2021) Biopolymers and composites: Processing and characterization. CRC Press, pp 255–278
Avramović N, Mandić B, Savić-Radojević A, Simić T (2020) Polymeric nanocarriers of drug Delivery systems in Cancer Therapy. Pharmaceutics 2020. Page 298 12 12:298–298
Baleeiro RB, Rietscher R, Diedrich A, Czaplewska JA, Lehr C-M et al (2021) Preparation of Poly-Lactic-Co-Glycolic Acid Nanoparticles in a Dry Powder Formulation for Pulmonary Antigen Delivery. Pharmaceutics 2021, Vol. 13, Page 1196 13:1196–1196
Cazzola M, Cavalli F, Usmani OS, Rogliani P (2020) Advances in pulmonary drug delivery devices for the treatment of chronic obstructive pulmonary disease. Expert Opin Drug Deliv 17:635–646
Ceschan NE, Rosas MD, Olivera ME, Dugour AV, Figueroa JM et al (2020) Development of a carrier-free dry powder ofloxacin formulation with enhanced aerosolization Properties. J Pharm Sci 109:2787–2797
Chan HW, Chow S, Zhang X, Zhao Y, Tong HHY et al (2023) Inhalable Nanoparticle-based Dry Powder Formulations for Respiratory Diseases: Challenges and Strategies for Translational Research. AAPS PharmSciTech 2023 24:4 24:1–28
Chandel A, Goyal AK, Ghosh G, Rath G (2019) Recent advances in aerosolised drug delivery. Biomed Pharmacother 112:108601–108601
Chenthamara D, Subramaniam S, Ramakrishnan SG, Krishnaswamy S, Essa MM et al (2019) Therapeutic efficacy of nanoparticles and routes of administration. Biomaterials Res 23:20–20
Choi HS, Ashitate Y, Lee JH, Kim SH, Matsui A et al (2010) Rapid translocation of nanoparticles from the lung airspaces to the body. Nature Biotechnology 2010 28:12 28:1300–1303
Craparo EF, Drago SE, Quaglia F, Ungaro F, Cavallaro G (2022) Development of a novel rapamycin loaded nano- into micro-formulation for treatment of lung inflammation. Drug Delivery and Translational Research 12:1859–1872
D’souza AA, Shegokar R (2016) Polyethylene glycol (PEG): a versatile polymer for pharmaceutical applications. Expert Opin Drug Deliv 13:1257–1275
Dailey L, Jekel N, Fink L, Gessler T, Schmehl T et al (2006) Investigation of the proinflammatory potential of biodegradable nanoparticle drug delivery systems in the lung. Toxicol Appl Pharmacol 215:100–108
Debnath SK, Saisivam S, Omri A (2017) PLGA Ethionamide nanoparticles for Pulmonary Delivery: Development and in vivo evaluation of dry powder inhaler. J Pharm Biomed Anal 145:854–859
Dehghan S, Kheiri MT, Abnous K, Eskandari M, Tafaghodi M (2018) Preparation, characterization and immunological evaluation of alginate nanoparticles loaded with whole inactivated influenza virus: dry powder formulation for nasal immunization in rabbits. Microb Pathog 115:74–85
Díaz-Montes E (2021) Dextran: sources, structures, and Properties. Polysaccharides 2:554–565
Eedara BB, Alabsi W, Encinas-Basurto D, Polt R, Mansour HM (2021) Spray-dried inhalable powder formulations of therapeutic proteins and peptides. AAPS PharmSciTech 22:1–12
El Baihary D, Osman R, Abdel-Bar HM, Sammour OA (2019) Pharmacokinetic/pulmokinetic analysis of optimized lung targeted spray dried ketotifen-dextran core shell nanocomplexes–in-microparticles. Int J Biol Macromol 139:678–687
Elmowafy E, Soliman ME (2019) Losartan-chitosan/dextran sulfate microplex as a carrier to lung therapeutics: dry powder inhalation, aerodynamic profile and pulmonary tolerability. Int J Biol Macromol 136:220–229
Elzoghby AO (2013) Gelatin-based nanoparticles as drug and gene delivery systems: reviewing three decades of research. J Controlled Release 172:1075–1091
Foox M, Zilberman M (2015) Drug delivery from gelatin-based systems. Expert Opin Drug Deliv 12:1547–1563
Gaikwad SS, Pathare SR, More MA, Waykhinde NA, Laddha UD et al (2023) Dry powder inhaler with the technical and practical obstacles, and forthcoming platform strategies. J Controlled Release 355:292–311
Guo Y, Bera H, Shi C, Zhang L, Cun D et al (2021) Pharmaceutical strategies to extend pulmonary exposure of inhaled medicines. Acta Pharm Sinica B 11:2565–2584
Han W, Wang L, Li Q, Ma B, He C et al (2022) A review: current status and emerging developments on natural polymer-based Electrospun fibers. Macromol Rapid Commun 43
Ihara D, Hattori N, Horimasu Y, Masuda T, Nakashima T et al (2015) Histological quantification of Gene Silencing by Intratracheal Administration of Dry Powdered Small-interfering RNA/Chitosan Complexes in the murine lung. Pharm Res 32:3877–3885
Islam N, Richard D (2019) Inhaled micro/nanoparticulate anticancer drug formulations: an emerging targeted drug delivery strategy for lung cancers. Curr Cancer Drug Targets 19:162–178
Jin Z, Gao Q, Wu K, Ouyang J, Guo W et al (2023) Harnessing inhaled nanoparticles to overcome the pulmonary barrier for respiratory disease therapy. Adv Drug Del Rev:115111
Kalombo L, Lemmer Y, Semete-Makokotlela B, Ramalapa B, Nkuna P et al (2019) Spray-Dried, Nanoencapsulated, multi-drug anti-tuberculosis therapy aimed at once Weekly Administration for the duration of treatment. Nanomaterials 9.
Keil TWM, Feldmann DP, Costabile G, Zhong Q, Da Rocha S et al (2019) Characterization of spray dried powders with nucleic acid-containing PEI nanoparticles. Eur J Pharm Biopharm 143:61–69
Kho K, Hadinoto K (2011) Optimizing aerosolization efficiency of dry-powder aggregates of thermally-sensitive polymeric nanoparticles produced by spray-freeze-drying. Powder Technol 214:169–176
Kulvanich P, Sinsuebpol C, Chatchawalsaisin J (2013) Preparation and in vivo absorption evaluation of spray dried powders containing salmon calcitonin loaded chitosan nanoparticles for pulmonary delivery. Drug Des Devel Ther:861–861
Kumar A, Vimal A, Kumar A (2016) Why Chitosan? From properties to perspective of mucosal drug delivery. Int J Biol Macromol 91:615–622
Kumar R, Thakur AK, Chaudhari P, Banerjee N (2021) Particle size reduction techniques of pharmaceutical compounds for the enhancement of their dissolution rate and bioavailability. J Pharm Innov:1–20
Lee KY, Mooney DJ (2012) Alginate: properties and biomedical applications. Prog Polym Sci 37:106–126
Lim YH, Tiemann KM, Hunstad DA, Elsabahy M, Wooley KL (2016) Polymeric nanoparticles in development for treatment of Pulmonary Infectious diseases. Wiley interdisciplinary reviews. Nanomed Nanobiotechnol 8:842–842
Lin X, Hu Y, Liu L, Su L, Li N et al (2018) Physical Stability of Amorphous Solid dispersions: a physicochemical perspective with thermodynamic, kinetic and environmental aspects. Pharm Res 35
Liu Q, Guan J, Sun Z, Shen X, Li L et al (2019) Influence of stabilizer type and concentration on the lung deposition and retention of resveratrol nanosuspension-in-microparticles. Int J Pharm 569:118562–118562
Ma Z, Garrido-Maestu A, Jeong KC (2017) Application, mode of action, and in vivo activity of chitosan and its micro- and nanoparticles as antimicrobial agents: a review. Carbohydr Polym 176:257–265
Malamatari M, Charisi A, Malamataris S, Kachrimanis K, Nikolakakis I (2020) Spray drying for the Preparation of nanoparticle-based drug formulations as dry powders for inhalation. Processes 8:788–788
Mansour HM, Rhee Y-S, Wu X (2009) Nanomedicine in pulmonary delivery. Int J Nanomed 4:299–319
Marianecci C, Marzio LD, Rinaldi F, Carafa M, Alhaique F (2011) Pulmonary delivery: innovative approaches and perspectives. J Biomater Nanobiotechnol 02:567–575
Meenach SA, Vogt FG, Anderson KW, Hilt JZ, Mcgarry RC et al (2013) Design, physicochemical characterization, and optimization of organic solution advanced spray-dried inhalable dipalmitoylphosphatidylcholine (DPPC) and dipalmitoylphosphatidylethanolamine poly(ethylene glycol) (DPPE-PEG) microparticles and nanoparticles for targeted respiratory nanomedicine delivery as dry powder inhalation aerosols. Int J Nanomed 8:275–293
Meenach SA, Anderson KW, Hilt JZ, Mcgarry RC, Mansour HM (2014) High-performing dry powder inhalers of paclitaxel DPPC/DPPG lung surfactant-mimic multifunctional particles in lung cancer: physicochemical characterization, in vitro aerosol dispersion, and cellular studies. AAPS PharmSciTech 15:1574–1587
Mukhtar M, Pallagi E, Csóka I, Benke E, Farkas Á et al (2020) Aerodynamic properties and in silico deposition of isoniazid loaded chitosan/thiolated chitosan and hyaluronic acid hybrid nanoplex DPIs as a potential TB treatment. Int J Biol Macromol 165:3007–3019
Muralidharan P, Mallory E, Malapit M, Hayes D, Mansour HM (2014) Inhalable PEGylated phospholipid nanocarriers and PEGylated therapeutics for respiratory delivery as aerosolized colloidal dispersions and dry powder inhalers. Pharmaceutics 6:333–353
Muralidharan P, Malapit M, Mallory E, Hayes D, Mansour HM (2015) Inhalable nanoparticulate powders for respiratory delivery. Nanomed Nanotechnol Biol Med 11:1189–1199
Muralidharan P, Mallory EK, Malapit M, Phan H, Ledford JG et al (2020) Advanced design and development of nanoparticle/microparticle dual-drug combination lactose carrier-free dry powder inhalation aerosols. RSC Adv 10:41846–41856
Okuda T, Morishita M, Mizutani K, Shibayama A, Okazaki M et al (2018) Development of spray-freeze-dried siRNA/PEI powder for inhalation with high aerosol performance and strong pulmonary gene silencing activity. J Controlled Release 279:99–113
Otto DP, Otto A, De Villiers MM (2015) Differences in physicochemical properties to consider in the design, evaluation and choice between microparticles and nanoparticles for drug delivery. Expert Opin Drug Deliv 12:763–777
Patel P, Raval M, Manvar A, Airao V, Bhatt V et al (2022) Lung cancer targeting efficiency of Silibinin loaded Poly Caprolactone/Pluronic F68 inhalable nanoparticles: in vitro and in vivo study. PLoS ONE 17:e0267257
Pilcer G, Amighi K (2010) Formulation strategy and use of excipients in pulmonary drug delivery. Int J Pharm 392:1–19
Popov A, Schopf L, Bourassa J, Chen H (2016) Enhanced pulmonary delivery of fluticasone propionate in rodents by mucus-penetrating nanoparticles. Int J Pharm 502:188–197
Qiu Y, Man RC, Liao Q, Kung KL, Chow MY et al (2019) Effective mRNA pulmonary delivery by dry powder formulation of PEGylated synthetic KL4 peptide. J Controlled Release 314:102–115
Quiñones JP, Peniche H, Peniche C (2018) Chitosan Based Self-assembled nanoparticles in drug delivery. Polymers 10:235–235
Rawal T, Parmar R, Tyagi RK, Butani S (2017) Rifampicin loaded chitosan nanoparticle dry powder presents an improved therapeutic approach for alveolar tuberculosis. Colloids Surf B Biointerfaces 154:321–330
Rosière R, Van Woensel M, Mathieu V, Langer I, Mathivet T et al (2016) Development and evaluation of well-tolerated and tumor-penetrating polymeric micelle-based dry powders for inhaled anti-cancer chemotherapy. Int J Pharm 501:148–159
Ruge CA, Kirch J, Lehr C-M (2013) Pulmonary drug delivery: from generating aerosols to overcoming biological barriers—therapeutic possibilities and technological challenges. The Lancet Respiratory Medicine 1:402–413
Ruzycki CA, Tavernini S, Martin AR, Finlay WH (2022) Characterization of dry powder inhaler performance through experimental methods.114518
Rytting E, Nguyen J, Wang X, Kissel TJEOODD (2008) Biodegradable polymeric nanocarriers for pulmonary drug delivery. 5:629–639
Sadikot RT, Kolanjiyil AV, Kleinstreuer C, Rubinstein I (2017) Nanomedicine for treatment of Acute Lung Injury and Acute Respiratory Distress Syndrome. Biomed Hub 2:1–12
Scherließ R, Janke J (2021) Preparation of poly-lactic-co-glycolic acid nanoparticles in a dry powder formulation for pulmonary antigen delivery. Pharmaceutics 13:1196
Scherließ R, Bock S, Bungert N, Neustock A, Valentin L (2022) Particle engineering in dry powders for inhalation. Eur J Pharm Sci 172:106158–106158
Scolari IR, Páez PL, Sánchez-Borzone ME, Granero GE (2019) Promising Chitosan-Coated Alginate-Tween 80 nanoparticles as Rifampicin Coadministered ascorbic acid delivery carrier against Mycobacterium tuberculosis. AAPS PharmSciTech 20:1–21
Sharma P, Mehta M, Dhanjal DS, Kaur S, Gupta G et al (2019) Emerging trends in the novel drug delivery approaches for the treatment of lung cancer. Chem -Biol Interact 309:108720–108720
Shi M, Mchugh KJ (2023) Strategies for overcoming protein and peptide instability in biodegradable drug delivery systems. Adv Drug Del Rev 199:114904–114904
Smyth HDC, Hickey AJ (2005) Carriers in drug powder delivery. Am J Drug Delivery 3:117–132
Szekalska M, Puciłowska A, Szymańska E, Ciosek P, Winnicka K (2016) Alginate: current use and future perspectives in Pharmaceutical and Biomedical Applications. Int J Polym Sci 2016:1–17
Topal GR, Devrim B, Eryilmaz M, Bozkir A (2018) Design of ciprofloxacin-loaded nano-and microcomposite particles for dry powder inhaler formulations: preparation, in vitro characterisation, and antimicrobial efficacy. J Microencaps 35:533–547
Torrico Guzmán EA, Sun Q, Meenach SA (2019) Development and evaluation of Paclitaxel-Loaded Aerosol Nanocomposite microparticles and their efficacy against air-grown Lung Cancer Tumor spheroids. ACS Biomaterials Science and Engineering 5:6570–6580
Tran TT, Vidaillac C, Yu H, Yong VFL, Roizman D et al (2018) A new therapeutic avenue for bronchiectasis: dry powder inhaler of ciprofloxacin nanoplex exhibits superior ex vivo mucus permeability and antibacterial efficacy to its native ciprofloxacin counterpart. Int J Pharm 547:368–376
Tran TT, Amalina N, Cheow WS, Hadinoto K (2020) Effects of storage on the stability and aerosolization efficiency of dry powder inhaler formulation of plasmid DNA-Chitosan nanoparticles. J Drug Deliv Sci Technol 59:101866–101866
Ullah F, Shah KU, Shah SU, Nawaz A, Nawaz T et al (2022) Synthesis, characterization and in Vitro evaluation of Chitosan nanoparticles physically admixed with Lactose microspheres for Pulmonary Delivery of Montelukast. Polym 2022 14:3564–3564
Ungaro F, D’angelo I, Coletta C, D’emmanuele Di Villa Bianca R, Sorrentino R et al (2012) Dry powders based on PLGA nanoparticles for pulmonary delivery of antibiotics: modulation of encapsulation efficiency, release rate and lung deposition pattern by hydrophilic polymers. J Controlled Release 157:149–159
Valente SA, Silva LM, Lopes GR, Sarmento B, Coimbra MA et al (2022) Polysaccharide-based formulations as potential carriers for pulmonary delivery– a review of their properties and fates. Carbohydr Polym 277:118784–118784
Vanza JD, Lalani JR, Patel RB, Patel MR (2023) DOE supported optimization of biodegradable polymeric nanoparticles based dry powder inhaler for targeted delivery of afatinib in non-small cell lung cancer. J Drug Deliv Sci Technol 84:104554–104554
Xu Y, Harinck L, Lokras AG, Gerde P, Selg E et al (2022) Leucine improves the aerosol performance of dry powder inhaler formulations of siRNA-loaded nanoparticles. Int J Pharm 621:121758–121758
Yang MY, Chan JGY, Chan HK (2014) Pulmonary drug delivery by powder aerosols. J Controlled Release 193:228–240
Yang M-S, Kang J-H, Kim D-W, Park C-W (2023) Recent developments in dry powder inhalation (DPI) formulations for lung-targeted drug delivery. J Pharm Invest:1–18
Yıldız-Peköz A, Ehrhardt C (2020) Advances in Pulmonary Drug Delivery. Pharmaceutics 2020, Vol. 12, Page 911 12:911–911
Yoo NY, Youn YS, Oh NM, Oh KT, Lee DK et al (2011) Antioxidant encapsulated porous poly(lactide-co-glycolide) microparticles for developing long acting inhalation system. Colloids Surf B Biointerfaces 88:419–424
Yu H, Teo J, Chew JW, Hadinoto K (2016) Dry powder inhaler formulation of high-payload antibiotic nanoparticle complex intended for bronchiectasis therapy: spray drying versus spray freeze drying preparation. Int J Pharm 499:38–46
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors (D. Encinas-Basurto, B.B. Eedara, and H.M. Mansour) declare that they have no conflict of interest.
Statement of human and animal rights
This article does not contain any studies with human nor animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Encinas-Basurto, D., Eedara, B.B. & Mansour, H.M. Biocompatible biodegradable polymeric nanocarriers in dry powder inhalers (DPIs) for pulmonary inhalation delivery. J. Pharm. Investig. 54, 145–160 (2024). https://doi.org/10.1007/s40005-024-00671-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-024-00671-0