Abstract
Background
Cardiac magnetic resonance imaging (CMR) is the modality of choice for quantification of myocardial iron overload in β-thalassemia major patients using the T2* sequence. CMR feature tracking (FT) is a recent magnetic resonance imaging tool that gives an idea about myocardial fibers deformation; thus, it can detect early impairment in myocardial function even before the reduction in ejection fraction.
Methods
This study aims to assess the ability of left ventricular CMR-FT in the early detection of systolic dysfunction in β thalassemia major patients and to correlate it with the degree of myocardial iron overload measured by CMR T2*. This prospective study enrolled 57 β thalassemia major patients who received long-term blood transfusion and 20 healthy controls. CMR was used to evaluate left ventricular volumes, ejection fraction, and the amount of myocardial T2*. A two-dimensional left ventricular FT analysis was performed. Both global and segmental left ventricular strain values were obtained.
Results
The mean global circumferential strain (GCS) and global radial strain (GRS) values were significantly lower in patients compared to control (P = 0.002 and P = 0.006, respectively). No correlation was found between T2* values and ejection fraction; however, there was a significant correlation between T2* values and GCS and GRS (P = 0.012 and P = 0.025, respectively) in thalassemia patients. Regional strain revealed significantly lower values of GCS and GRS in basal regions compared to apical ones (P = 0.000).
Conclusions
Our study revealed that CMR-FT can play a role in the early detection of systolic impairment in thalassemia patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Β Thalassemia major is a group of hereditary disorders causing chronic hemolytic anemia with the affected children requiring blood transfusions during their whole life. Lifelong blood transfusions cause increased iron absorption subsequently leading to myocardial iron overload (MIO) which is not completely reversible and represents the major cause of cardiac functional impairment and is the main etiology for morbidity and mortality [1, 2].
Early adequate treatment with iron chelation therapy before the development of heart failure can reverse MIO-induced cardiomyopathy, hence the need for its early detection [1]. β Thalassemia major patients receive iron chelation therapy after about 20 to 25 units of red blood cells transfusion. The cutoff value for the start of iron chelation therapy is a serum ferritin level of more than 1,000 ng/mL and hepatic iron concentration of more than 3 mg/gm measured either by liver biopsy or noninvasive cardiac magnetic resonance imaging (CMR) [3].
CMR is the modality of choice for quantification of MIO in thalassemia patients using noninvasive T2* sequence [4, 5]. CMR is the gold standard modality for the evaluation of ventricular function and early prediction of cardiac complications in thalassemia patients [2].
Many studies evaluated the role of left ventricular myocardial strain derived from echocardiography for early detection of impaired myocardial function in thalassemia patients [6,7,8]. CMR feature tracking (FT) is a recent magnetic resonance imaging tool that represents myocardial fibers deformation and also reflects early ventricular dysfunction [9, 10]. CMR-FT myocardial strain analysis has been studied in different heart diseases and was reported to have better prognostic information than ejection fraction [11]. Very few studies evaluated the regional analysis of left myocardial strain in different myocardial regions. They reported that the myocardial regions are not uniformly affected in β thalassemia patients with MIO [6]. Myocardial strain analysis can be used as a tool to identify high-risk patients who may need intensification of their chelation therapy and prioritized to get T2* CMR studies [1,2,3,4,5,6,7].
This study aims to assess the ability of CMR-FT left ventricular strain analysis for the early detection of systolic dysfunction in β thalassemia patients and to correlate it with the degree of MIO measured by CMR T2*.
Methods
Study population
This study is a prospective study conducted from January 2022 to December 2023. A total of 57 consecutive β thalassemia major patients received long-term blood transfusions (ranging from 1 to 4 packs/mo) were enrolled in the study. All study participants underwent CMR and CMR-FT. Among these 11 patients were diagnosed with MIO based on CMR T2* values. Patients with septal T2* less than 20 ms were considered to have MIO while others with septal T2* more than 20 ms were considered to be without MIO [12]. There were 46 patients (80.8%) without MIO and 11 patients (19.2%) with MIO. From the 11 patients with MIO, one had mild MIO (T2*, 15–20 ms), seven had moderate MIO (T2*, 10–15 ms) and three had severe MIO (T2*, < 10 ms). Twenty healthy subjects without any cardiac symptoms or previous diagnosis of cardiac condition were included as a control group. Exclusion criteria included patients with contraindications to magnetic resonance imaging, or patients suffering from another cardiac disease.
CMR protocol
CMR was performed using a 1.5-T magnet (Philips Ingenia). Multiple slices during different phases of the cardiac cycle were obtained in the short-axis, axial, three and four-chamber planes by using free breathing retrospective electrocardiogram-gated steady-state free precession sequence. The following parameters were used: time of repetition, 3.20–3.65 ms; time to echo, 1.60–1.83 ms; field of view, 270 mm2; slice thickness, 5 mm; 30 cardiac phases; and no slice gap. T2* images were performed using black blood gradient echo sequence using time of repetition of 120 ms, different time to echo with 15 numbers of echoes, and flip angle of 20°. Signal intensity was measured in T2* images at different time to echo by a region of interest placed in the interventricular septum. Patients were categorized into two groups according to T2* values, patients with MIO when T2* < 20 ms and patients without MIO when T2* > 20 ms.
CMR-FT interpretation
Two-dimensional (2D) FT analysis was performed offline using dedicated cardiac imaging software (CVI42, Circle Cardiovascular Imaging). Two radiologists with 10- and 7-years of experience in CMR performed the analysis in two separate reading sessions. They were blinded to the patient’s clinical data. Endocardial and epicardial contours of left ventricle (LV) were traced at the end-diastolic phase in short-axis and two-, three- and four-chamber planes. Automated tracking was visually reviewed, and the epicardial and endocardial contours were manually adjusted when required. The mean global longitudinal strain (GLS) analysis was performed using long-axis planes (two-, three- and four-chamber planes). The mean global radial strain (GRS) and global circumferential strain (GCS) analyses were performed using short-axis planes, as well as the regional strain analysis in the 16 myocardial segments. Global strain values, strain curves, strain rate curves, polar maps and cooler-coded images were obtained. Regional strain values were recorded for apical, mid, and basal regions (Fig. 1).
Feature traking analysis in a patient with β-thalassaemia major (A, B) Analysis of cardiac magnetic resonance imaging feature tracking strain of short-axis and four-chamber cine images. Color map and curve of (C, D) global longitudinal strain (GLS), (E, F) global circumferential strain (GCS), and (G, H) global radial strain (GRS) in a patient with thalassaemia major with preserved left ventricular ejection fraction
Statistical analysis
Data were analyzed using IBM SPSS ver. 22.0 (IBM Corp). The Kolmogorov–Smirnov test was performed to determine the normality of the distribution of quantitative data. Quantitative data were described as mean standard deviation or median and range according to the normality test. The Mann–Whitney U-test and Student t-test were used to compare two groups for non-normally and normally distributed data, respectively. Interobserver agreement for strain analysis was evaluated in 40 β thalassemia major patients using intraclass correlation coefficients (ICCs) at 95% confidence intervals (CIs). The Pearson correlation test was used to correlate T2* with LV ejection fraction (EF) and global LV myocardial strain values. The one-way analysis of variance (ANOVA) test was performed to compare basal, mid, and apical strain values. Receiver operating characteristic (ROC) curve was performed to detect the cutoff value of GCS and GRS for differentiating β-thalassemia major patients from controls with the calculation of area under the curve (AUC), 95% CIs, sensitivity, and specificity. The chosen cutoff point was based on best detected cutoff point retrieved from the curve that yields highest sensitivity and specificity and for combined parameters; multivariate analysis was firstly done and then a new variable was detected by saved probability and from which we retrieve the best detected cutoff point. P-values were considered significant when they were less than 0.05.
Results
Patient demographics and clinical data
This study included 57 patients with β-thalassemia major (28 men [49.1%] and 29 women [50.9%]), with median age of 27 years (range, 18–50 years); and 20 healthy subjects (11 men [55.0%] and nine women [45.0%]) with median age of 29 years (range, 18–49 years) as the control group. Patients with β-thalassemia major were then divided into two groups according to T2* CMR results, 46 patients (80.8%) without MIO and 11 patients (19.2%) with MIO (one had mild MIO, seven had moderate MIO, and three had severe MIO).
The median serum ferritin in patients with MIO had trends to be higher than in patients without MIO (2,500 ng/mL vs. 1,766.5 ng/mL) but this difference did not reach statistical significance (P = 0.190). The median white blood cells, hemoglobin, platelets, liver enzymes (alanine transaminase and aspartate transferase), and albumin are displayed in Table 1, with no significant difference between both studied groups.
CMR parameters
The mean LV EF was significantly lower in thalassemia patients compared to the control group (P = 0.003). Also, patients had higher mean LV end-diastolic volume index compared to the control group (101. mL/m2 vs. 75.6 mL/m2, P = 0.042). Comparison between different CMR parameters between thalassemia patients and control was demonstrated in Table 2.
CMR-FT global strain parameters
On comparing CMR-FT LV myocardial strain values between patients and control, the mean GCS and GRS values were significantly impaired in patients compared to the control group (P = 0.002 and P = 0.006, respectively). The mean GLS tends to be lower in patients compared to the control group, but did not reach statistical significance (P = 0.980) (Table 2, Fig. 2). There was no significant difference between patients with and without MIO as regards to GLS (P = 0.678), GCS (P = 0.572), and GRS (P = 0.771) (Table 3). An example of T2* and LV strain analysis in a patient with β-thalassemia major and mild myocardial iron overload is shown at Fig. 3.
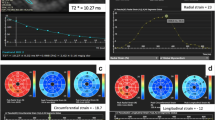
T2* and left ventricular strain analysis in a patient with β-thalassamia major and mild myocardial iron overload, (A) detected by T2* short-axis, (B) showing T2* of 19.3 ms and myocardial iron concentration of 1.21 mg/g. C Segmental global radial strain (GRS) and global circumferential strain (GCS). Strain curves for (D) GCS, (E) GRS, and (F) global longitudinal strain (GLS)
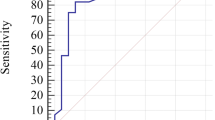
ROC analysis revealed an AUC of 0.818 (95% CI, 0.709–0.928) with a GCS cutoff value of –16.95%, sensitivity of 72%, and specificity of 88% for GCS (P = 0.003). While for GRS, the cutoff value of 27.2% showed an AUC of 0.792 (95% CI, 0.664–0.920), sensitivity of 63%, and specificity of 78% (P = 0.006) (Fig. 4 A, B).
Receiver operating characteristic (ROC) curve analysis for differentiating β thalassaemia major patients from the control group. A ROC curve analysis of the left ventricle global circumferential strain (GCS) showing an area under the curve of 0.818 (P = 0.003). B ROC curve analysis of the left ventricle global radial strain (GRS) showing an area under the curve of 0.792 (P = 0.006)
The mean GCS and GRS values were lower in β-thalassemia patients without MIO compared to the control group (P = 0.012 and P = 0.007, respectively). However, the mean GLS values did not show a significant difference between the two groups (P = 0.420) (Table 4).
CMR-FT regional strain parameters
LV regional myocardial strain values revealed statistically significant differences between the basal, mid and apical segments regarding GRS, GRS rate, GCS and GCS rate (P < 0.001 for all). Post hoc analysis in the one-way ANOVA test between regional myocardial strain values in the basal, mid, and apical regions revealed statistically significant differences between basal and apical regions concerning GRS, GRS rate, and GCS (P = 0.000 for all). There was a statistically significant difference between mid and apical regions concerning GRS (P = 0.014), GRS rate (P = 0.003), GCS (P = 0.050), and GCS rate (P = 0.001). GCS rate was significantly different between basal and mid regions (P = 0.001). However, GRS (P = 0.246), GRS rate (P = 0.621), and GCS (P = 0.090) did not show statistically significant differences between basal and mid regions. The mean base to apex ratio was 0.82% ± 0.27% for GRS and 0.86% ± 0.2% for GCS (Table 5).
Interobserver reliability of CMR-FT parameters
Interobserver agreement for LV strain analysis showed moderate agreement for GLS (ICC, 0.7; 95% CI, 0.4–0.8) and good agreement for both GCS (ICC, 0.86; 95% CI, 0.7–0.9) and GRS (ICC, 0.85; 95% CI, 0.7–0.9).
Correlations between T2* values with LV EF, global myocardial strain values, and base to apex ratio
In this study we found no correlation between T2* values and LV EF in thalassemia patients, (r = 0.23, P = 0.875). However, there was a significant positive correlation between T2* values and GCS values (r = 0.361, P = 0.012). We also found a significant negative correlation between T2* values and GRS values (r = –0.323, P = 0.025). GLS values did not significantly correlate with T2* values. There was no significant correlation between base to apex ratio and T2* values (P = 0.250 for GRS ratio and P = 0.225 for GCS ratio) (Fig. 5).
Correlation between T2* and (A) left ventricle (LV) ejection fraction (EF), (B) LV global longitudinal strain (GLS), (C) LV global circumferential strain (GCS), (D) LV global radial strain (GRS), (E) basal to apical LV GCS ratio, (F) basal to apical LV GRS ratio. Significant correlation was revealed between T2* and LV GCS (r = 0.361, P = 0.012) and LV GRS (r = –0.323, P = 0.025). However, no significant correlation was found between T2* and other parameters
Discussion
The main findings of this study are as follows. First, LV strain parameters (GCS and GRS) were significantly lower in thalassemia patients compared to control group. Second, thalassemia patients without MIO showed significantly lower LV GCS and LV GRS compared to control group. Third, there was significant correlation between LV GCS and LV GRS and T2* values even in absence of significant correlation with LV EF. Fourth, LV GCS and LV GRS values were lower in the basal and mid myocardial segments compared to the apical segments.
The intension from this study was not to replace the gold standard T2* used for detection of MIO but to detect the early impairment in LV function even in absence of MIO, as absence of MIO doesn’t mean that the myocardium is healthy, so the intension was to provide additional information from routine CMR sequences to detect early myocardial impairment.
Iron-induced cardiomyopathy is the main cause of death in thalassemia patients with chronic blood transfusion. Initially, excess iron saturates the myocardial iron storage in its bounded form. During this period, myocardial T2* value decreases, yet cardiac functions generally remain within normal limits. If myocardial iron is not cleared at this stage, iron exceeds the storage capacity of the myocardium, and free iron appears, which is more toxic to the myocardial cells and results in impaired myocardial function [12, 13]. Although, it appears that the main pathophysiologic event is related to iron overload, other physiologic, biomolecular, and structural factors play a role in affecting the myocardium [14].
Conventional echo cardiography has failed to identify the early myocardial dysfunction among β-thalassemia major patients, thus speckle tracking echocardiography (STE) has been lately used to detect early myocardial deformation [15]. We noted heterogeneity in the methods that were used in previous studies for evaluation of STE-derived LV strain parameters in β-thalassemia major patients, as some studies used 2D STE [15,16,17,18,19,20,21,22,23,24,25,26,27,28],while others used 3D STE [23, 29,30,31]. Regarding 2D-STE for LV GLS estimation, some studies acquired it from single apical four-chamber view [25, 28, 29], others acquired it from the combination of apical four- and two-chamber views [27, 32, 33], while in the remaining studies it was acquired from the combined apical four-, three-, and two-chamber views [16, 18,19,20,21,22, 24, 34, 35]. All previously mentioned studies agreed on using parasternal short-axis view for acquiring 2D-STE–derived GCS and GRS indices [16, 21, 26,27,28,29, 31, 32]. This technical difference can explain the different results among the literature. As, when comparing patient with controls, some studies reported significant difference between the two groups regarding GLS [18, 19, 29, 30, 34], GCS [16, 29, 31] or GRS [27]. While others reported no significant difference between the two groups regarding GLS [16, 24, 25], GCS [26], or GRS [28]. In addition, some of the previously mentioned studies reported significant difference between cases with MIO and cases without MIO regarding GLS or GCS [35], However others found no significant difference between the two groups regarding GLS [36], GCS [16, 20, 29], or GRS [16, 29].
Recently, CMR-FT has been used for detection of myocardial deformation. CMR has the privilege of high contrast and spatial resolution, multiplanar imaging and being nonoperator dependent unlike echo. Regarding CMR-FT parameters, previous studies agreed on the method by which they acquire these parameters. As they obtained GLS using four-chamber with or without two- and three-chamber views while GCS and GRS were acquired using short-axis view [1, 2, 14, 15]. Many previous studies agreed that LV strain is impaired in thalassemia patients, however, there were controversies on the best strain parameter that can detect the early impairment in cardiac function [1, 2, 14, 15]. Previous studies on CMR and autopsy findings revealed that in thalassemia patients, the subepicardial layer of the myocardium is the main site of iron deposition [15]. The subepicardial layer is the most important marker of cardiac rotation [37]. It was reported that the deformation of the subepicardial layer is reflected as impaired LV GRS [37] and LV GCS [1]. This can explain our findings that the mean GCS and GRS values were significantly lower in thalassemia patients compared to the control group.
LV GLS reflects the longitudinal shortening of the subendocardial layer [1, 11]. Since MIO begins in the subepicardial layer, it is expected that GLS may be spared till late stages of the disease [1]. This fact can explain our results that there was no significant difference between patients and control group regarding GLS values. However, some previous studies reported that GLS was the most valuable strain parameter in detecting early LV dysfunction in thalassemia patients. However, these studies did not reach an explanation for this finding, and they did not correlate the GLS values with the T2* values [8, 38].
Unlike previously mentioned studies, there has been a great debate regarding correlation between LV myocardial strain, either obtained by STE or by CMR, and T2* values and so the degree of MIO. Most of the previous studies that used STE to evaluate the LV strain in thalassemia patients reported a significant correlation between GLS and T2* [8, 20, 35, 38,39,40], only two studies reported significant correlation between GCS and T2* [20, 35], yet none of the previously mentioned studies found significant correlation between GRS and T2* (Table 6 [8, 20, 35, 38,39,40]). Regarding strain parameters derived from CMR, three previous studies reported significant correlation between GRS and T2* [1, 3, 37], one study reported a significant correlation between GCS and T2* [13], and one study significant correlation between GLS and T2* (Table 6 [37]). This variability among previously reported results can be explained by the hypothesis that not only MIO is the cause of myocardial damage in thalassemia patients. Those patients suffer from chronic anemia which can cause chronic hypoxia explaining the affection of LV subendocardial layer and so reduction of GLS regardless of the degree of iron overload as mentioned in some of previous studies [1]. Thalassemia patients also receive repeated blood transfusion among their life increasing the possibility of having viral infection such as hepatitis C. Also, various degrees of genetic factors, and chronic state of immunodeficiency in these patients can contribute in myocardial damage [14]. This explains why the relation between strain values (reflecting LV function) and T2* (reflecting degree of MIO) is not linear and not constant among studies. So, although early detection of MIO is important, absence of MIO does not exclude myocardial affection and hence those patients require surveillance of their cardiac function for early detection of myocardial impairment allowing them to receive adequate treatment as early as possible. In agreement with some of the previous studies, we found a significant correlation between both LV myocardial strain parameters (GRS and GCS) and T2* values in thalassemia patients despite the absence of a significant correlation between EF and T2* values. Accordingly, this supports the potential of using CMR strain analysis for the early detection of subtle systolic dysfunction in thalassemia patients before it can be detected by conventional functional CMR even in absence of significant MIO.
The results regarding the LV CMR-FT parameters between β-thalassemia major patients with and without MIO are controversial. While some studies reported that only GRS values were significantly different between the two groups [1], others found a significant difference in GCS [2, 14, 15] and GLS [2]. Odoardo et al. [32], used STE strain analysis to assess myocardial strains in 55 thalassemia patients and they reported significant difference regarding GCS and GRS between patients and control; however, they found no significant difference regarding the three strain parameters between thalassemia patients with and without MIO. In agreement with the previously mentioned study, we found no statistically significant difference in GLS and GCS values between patients with MIO and patients without MIO. However, GRS was slightly lower in patients with MIO compared to those without MIO, but this difference did not reach statistical significance. This finding supports the claim that the relationship between MIO and myocardial dysfunction is not linear as there are other factors contributing factors to the myocardial damage other than MIO. This also might be explained by the smaller number of patients with MIO enrolled in our study.
It was reported that some thalassemia patients with MIO, especially those with higher levels of serum ferritin, develop restrictive LV filling in response to MIO [41]. This may be the explanation of our findings as we noted that end-diastolic volume index and end-systolic volume index were significantly lower in thalassemia patients with MIO compared to those without MIO, although there was no significant difference regarding the end-diastolic wall mass index among the two groups. Also, the serum ferritin was higher in cases with MIO compared to those without MIO although this difference did not reach a statistical significance.
Alis et al. [13] found in their study that thalassemia patients without MIO (with T2* > 20 ms) showed significantly lower GCS and GRS values compared to the control group, but they found no significant difference between the two groups regarding GLS. On the other hand, Asadian et al. [37] found that GLS was the only strain parameter that was different between the two groups. In our study, both GCS and GRS were lower in thalassemia patients without MIO compared to the control group. This finding can be explained by the claim that the affection of LV myocardium in those patients might be multifactorial including inflammatory, molecular, and immunologic factors and not only secondary to MIO [14].
Segmental strain analysis in this study revealed significantly lower LV strain values (GRS and GCS) in the basal and mid myocardial segments compared to the apical segments, suggesting the possibility of sparing the apical segments until late in the disease. These results were in line with Rao et al. [7] who reported a statistically significant difference between the apical and basal segments for both longitudinal and circumferential strains. This might be explained by the hypothesis that iron deposition early in the disease is not uniform throughout the myocardium and tends to spar apical segments [7, 16].
This study has several limitations. First, the small number of patients especially the MIO group (11 cases), further research on a larger number of patients is needed for a better understanding of the role of CMR strain analysis in thalassemia patients. Second, this cross-sectional study showed impaired LV strain values in thalassemia cases, we did not perform future follow-up for cardiac events. Longitudinal studies are recommended to evaluate the prognostic role of CMR strain for assessment of systolic function. Third, the study was executed in a single-center arrangement with a single-vendor application, further multicenter studies are needed to establish the role of strain analysis in thalassemia patients.
Conclusions
β-Thalassemia major patients can have myocardial dysfunction even in absence of MIO. CMR-FT strain parameters can play an important role as a marker for the early detection of systolic impairment in those patients. The correlation of myocardial strain parameters with T2* values may help in the identification of high-risk groups who may need modification of the dose of their iron chelation therapy.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- ANOVA:
-
Analysis of variance
- AUC:
-
Area under the curve
- CI:
-
Confidence interval
- CMR:
-
Cardiac magnetic resonance imaging
- D:
-
Dimensional
- EF:
-
Ejection fraction
- FT:
-
Feature tracking
- GCS:
-
Global circumferential strain
- GLS:
-
Global longitudinal strain
- GRS:
-
Global radial strain
- ICC:
-
Intraclass correlation coefficient
- LV:
-
Left ventricle
- MIO:
-
Myocardial iron overload
- ROC:
-
Receiver operating characteristic
- STE:
-
Speckle tracking echocardiography
References
Ojha V, Ganga KP, Seth T, Roy A, Naik N, Jagia P, et al. Role of CMR feature-tracking derived left ventricular strain in predicting myocardial iron overload and assessing myocardial contractile dysfunction in patients with thalassemia major. Eur Radiol. 2021;31:6184–92.
Rezaeian N, Asadian S, Parsaee M, Toloueitabar Y, Hemmati Komasi MM, Shayan L, et al. The predictive role of cardiac magnetic resonance imaging in determining thalassemia patients with intermediately to highly probable pulmonary hypertension. Echocardiography. 2021;38:1769–77.
Das KM, Baskaki UM, Pulinchani A, Ali HM, Almanssori TM, Gorkom KV, et al. Significance of cardiac magnetic resonance feature tracking of the right ventricle in predicting subclinical dysfunction in patients with thalassemia major. Diagnostics (Basel). 2022;12:1920.
Batouty NM, Sobh DM, Tawfik AM. Native myocardial T1 mapping in β-thalassemia major patients with and without iron overload. Acta Haematol Pol. 2023;54:48–9.
Ramazzotti A, Pepe A, Positano V, Rossi G, De Marchi D, Brizi MG, et al. Multicenter validation of the magnetic resonance T2* technique for segmental and global quantification of myocardial iron. J Magn Reson Imaging. 2009;30:62–8.
Hamdy AM. Use of strain and tissue velocity imaging for early detection of regional myocardial dysfunction in patients with beta thalassemia. Eur J Echocardiogr. 2007;8:102–9.
Rao S, Hasan BS, Hoodbhoy Z, Habib I, Mohsin S, Tomredle R, et al. Role of myocardial deformation imaging in transfusion-dependent thalassemia: correlation with severity of myocardial siderosis. Prog Pediatr Cardiol. 2023;68: 101607.
Pizzino F, Meloni A, Terrizzi A, Casini T, Spasiano A, Cosmi C, et al. Detection of myocardial iron overload by two-dimensional speckle tracking in patients with beta-thalassaemia major: a combined echocardiographic and T2* segmental CMR study. Int J Cardiovasc Imaging. 2018;34:263–71.
Chitiboi T, Axel L. Magnetic resonance imaging of myocardial strain: a review of current approaches. J Magn Reson Imaging. 2017;46:1263–80.
Tawfik AM, Sobh DM, Gadelhak B, Zedan MM, Sobh HM, Eid R, et al. Right ventricular strain analysis by tissue tracking cardiac magnetic resonance imaging in pediatric patients with end-stage renal disease. J Thorac Imaging. 2024;39:49–56.
Sobh DM, Batouty NM, Tawfik AM, Gadelhak B, Elmokadem AH, Hammad A, et al. Left Ventricular strain analysis by tissue tracking-cardiac magnetic resonance for early detection of cardiac dysfunction in children with end-stage renal disease. J Magn Reson Imaging. 2021;54:1476–85.
Anderson LJ, Holden S, Davis B, Prescott E, Charrier CC, Bunce NH, et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171–9.
Alis D, Asmakutlu O, Topel C, Sahin AA, Karaarslan E. Association between left ventricular strain and cardiac iron load in beta-thalassaemia major: a cardiac magnetic resonance study. Acta Cardiol. 2022;77:71–80.
Gujja P, Rosing DR, Tripodi DJ, Shizukuda Y. Iron overload cardiomyopathy: better understanding of an increasing disorder. J Am Coll Cardiol. 2010;56:1001–12.
Patsourakos D, Aggeli C, Dimitroglou Y, Delicou S, Xydaki K, Koukos M, et al. Speckle tracking echocardiography and β-thalassemia major: a systematic review. Ann Hematol. 2023 Aug 1 [Epub]. https://doi.org/10.1007/s00277-023-05380-6
El-Shanshory M, Tolba O, El-Shafiey R, Elgamasy M, Hablas N, Mawlana W. Cardiac iron overload by MRI in children with B-thalassemia major and its correlation with cardiac function by echocardiography. J Pediatr Hematol Oncol. 2020;42:398–402.
AbdelMassih AF, Salama KM, Ghobrial C, Haroun B, Rahman MA. Discrepancy in patterns of myocardial involvement in beta-thalassaemia vs. sickle cell anaemia. Acta Cardiol. 2020;75:442–9.
Nashat M, Khedr LA, Khairat E, Elsheikh E. Evaluation of right and left ventricular function using speckle-tracking echocardiography in thalassemic patients. Ann Pediatr Cardiol. 2021;14:476–84.
Sayed NM, Mashahit MA, El-Husseiny NM, Ali RA, Ahmed GA, Ibrahim MK. Cardiac structural and functional changes evaluated by echocardiography and two-dimensional strain in patients with beta thalassemia. Nat Sci. 2021;19:61–6.
Fattahi H, Parsaee M, Rezaeian N, Azarkeivan A, Meimand SE, Mohammadi K, et al. Comparison between two and three-dimensional speckle-tracking echocardiography and cardiac T2* magnetic resonance imaging in ß-thalassemia. Res Cardiovasc Med. 2021;10:7–13.
Cheung YF, Liang XC, Chan GC, Wong SJ, Ha SY. Myocardial deformation in patients with Beta-thalassemia major: a speckle tracking echocardiographic study. Echocardiography. 2010;27:253–9.
Barbero U, Fornari F, Gagliardi M, Fava A, Giorgi M, Alunni G, et al. Myocardial longitudinal strain as the first herald of cardiac impairment in very early iron overload state: an echocardiography and biosusceptometry study on beta-thalassemia patients. Am J Cardiovasc Dis. 2021;11:555–63.
Hanneman K, Nguyen ET, Thavendiranathan P, Ward R, Greiser A, Jolly MP, et al. Quantification of myocardial extracellular volume fraction with cardiac MR imaging in thalassemia major. Radiology. 2016;279:720–30.
Okay M, Coteli C, Unal S, Hazırolan T, Karabulut E, Ozer N, et al. Strain imaging by speckle tracking for the assessment of diastolic dysfunction in beta-thalassemia major patients. Acta Medica. 2021;52:57–61.
Cheung YF, So EK, Hwang GY, Chan GC, Ha SY. Left and right atrial function and remodeling in beta-thalassaemia major. Pediatr Cardiol. 2019;40:1001–8.
Parsaee M, Saedi S, Joghataei P, Azarkeivan A, Alizadeh SZ. Value of speckle tracking echocardiography for detection of clinically silent left ventricular dysfunction in patients with β-thalassemia. Hematology. 2017;22:554–8.
Chen MR, Ko HS, Chao TF, Liu HC, Kuo JY, Bulwer BE, et al. Relation of myocardial systolic mechanics to serum ferritin level as a prognosticator in thalassemia patients undergoing repeated transfusion. Echocardiography. 2015;32:79–88.
Karamanou AG, Hamodraka ES, Vrakas SC, Paraskevaidis I, Lekakis I, Kremastinos DT. Assessment of left ventricular and atrial diastolic function using two-dimensional (2D) strain imaging in patients with β-thalassemia major. Eur J Haematol. 2014;92:59–65.
Eroğlu AG, Uluğ N, Karakaş H, Yüksel EK, Akyel NG, Çığ G, et al. Evaluation of left ventricular function and myocardial deformation in children with beta-thalassemia major by real-time three-dimensional (four-dimensional) and speckle tracking echocardiography. Echocardiography. 2022;39:1307–15.
Patsourakos D, Aggeli C, Gatzoulis KA, Delicou S, Dimitroglou Y, Xydaki K, et al. Left atrial deformation indices in β-thalassemia major patients. Ann Hematol. 2022;101:1473–83.
El Razaky OA, El-Shanshory MR, El-Shehaby WA, Hables NM, Elshamia AM, Fayed AM, et al. Left ventricular regional function in children with beta thalassemia with no cardiac manifestations (four-dimensional echocardiographic study). Indian J Hematol Blood Transfus. 2019;35:750–7.
Di Odoardo LA, Giuditta M, Cassinerio E, Roghi A, Pedrotti P, Vicenzi M, et al. Myocardial deformation in iron overload cardiomyopathy: speckle tracking imaging in a beta-thalassemia major population. Intern Emerg Med. 2017;12:799–809.
Ari ME, Ekici F, Çetin İİ, Tavil EB, Yaralı N, Işık P, et al. Assessment of left ventricular functions and myocardial iron load with tissue Doppler and speckle tracking echocardiography and T2* MRI in patients with β-thalassemia major. Echocardiography. 2017;34:383–9.
El-Shanshory M, Tolba O, El-Shafiey R, Mawlana W, Ibrahim M, El-Gamasy M. Cardioprotective effects of spirulina therapy in children with beta-thalassemia major. J Pediatr Hematol Oncol. 2019;41:202–6.
Garceau P, Nguyen ET, Carasso S, Ross H, Pendergrast J, Moravsky G, et al. Quantification of myocardial iron deposition by two-dimensional speckle tracking in patients with β-thalassaemia major and Blackfan-Diamond anaemia. Heart. 2011;97:388–93.
Wood JC. Estimating tissue iron burden: current status and future prospects. Br J Haematol. 2015;170:15–28.
Asadian S, Rezaeian N, Hosseini L, Toloueitabar Y, Komasi MM, Shayan L. How does iron deposition modify the myocardium?: a feature-tracking cardiac magnetic resonance study. Int J Cardiovasc Imaging. 2021;37:3269–77.
Cusmà Piccione M, Piraino B, Zito C, Khandheria BK, Di Bella G, De Gregorio C, et al. Early identification of cardiovascular involvement in patients with β-thalassemia major. Am J Cardiol. 2013;112:1246–51.
Li SJ, Hwang YY, Ha SY, Chan GC, Mok AS, Wong SJ, et al. Role of three-dimensional speckle tracking echocardiography in the quantification of myocardial iron overload in patients with beta-thalassemia major. Echocardiography. 2016;33:1361–7.
Abtahi F, Abdi A, Jamshidi S, Karimi M, Babaei-Beigi MA, Attar A. Global longitudinal strain as an Indicator of cardiac Iron overload in thalassemia patients. Cardiovasc Ultrasound. 2019;17:24.
Kremastinos DT, Farmakis D. Iron overload cardiomyopathy in clinical practice. Circulation. 2011;124:2253–63.
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
Study concept and design were proposed by AMT and DMS. Database search done by BNG and SEA. Pathological report analysis done by MAES and SEA. Technical or material support was provided by SEA, AAAEA. Analysis and interpretation of data and the drafting of the manuscript were done by NMB, RK, and MAES. Revision of the manuscript was done by MG and MAH. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Institutional Research Board of Faculty of Medicine, Mansoura University (No. R.23.06.2236.R1). Written informed consents were obtained from all patients included in the study.
Consent for publication
The participants in the study were informed and consented to the possibility of research publication. Authors hereby transfer, assign or otherwise convey all copyright ownership to the Journal of Cardiovascular Imaging if such work is published in that journal.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Batouty, N.M., Tawfik, A.M., Sobh, D.M. et al. Global and regional cardiac magnetic resonance feature tracking left ventricular strain analysis in assessing early myocardial disease in β thalassemia major patients. J Cardiovasc Imaging 32, 18 (2024). https://doi.org/10.1186/s44348-024-00026-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s44348-024-00026-1