Abstract
Canine health is consistently affected by the circulation of the H3N2 strain of canine influenza virus (CIV). Prior research has indicated that the isolation rate of H3N2 CIVs in dogs has gradually increased in China, and these viruses have progressively adapted to humans over the course of their evolution within canine hosts, posing a significant public health threat. However, the key factors influencing the spread of CIVs remain elusive. From January 2020 to December 2022, during the COVID-19 pandemic, strict epidemic prevention policies were implemented in China. Thus, this measure provides an ideal model for studying factors influencing the prevalence of CIVs. In this study, we continuously monitored the prevalence of CIVs in China before and after the COVID-19 pandemic. We found that the pathogen detection rate and seropositivity rate of domestic CIVs significantly declined after the implementation of epidemic control measures. However, after restrictions on human movement were lifted in 2023, the circulation of CIVs gradually increased. Our results demonstrate that restricting human activity directly impacts the epidemic caused by CIVs and provide a theoretical basis for the implementation of control measures during outbreaks of zoonotic diseases in human companion animals.
Similar content being viewed by others
Introduction
The predominant subtypes of influenza viruses circulating in canine populations are the equine-origin H3N8 and avian-origin H3N2 subtypes of canine influenza viruses (CIVs) [1]. From 2005 to 2006, avian-origin H3N2 CIV was first reported in dogs in Asia (including Korea and China), and effective transmission between dogs was demonstrated [2, 3]. In April 2015, H3N2 CIV, which is prevalent in Asia, spread among canine populations in the United States, causing respiratory diseases in thousands of dogs [4]. Dogs, as significant companion animals to humans, can serve as vectors for the transmission of zoonotic diseases to humans [5]. Canine influenza can be caused by a variety of influenza A viruses, including equine-origin H3N8 and avian-origin H3N2 viruses, which are both established lineages in dogs worldwide. CIV usually causes mild respiratory symptoms, and CIV-infected dogs often recover without treatment. As a consequence, animal owners and veterinarians often neglect treating CIV infections, creating an opportunity for CIVs to circulate and further adapt in dogs. Mutations leading to better growth in dogs could enhance infectiousness in other mammals (e.g., humans). In a previous study, we conducted a systematic and comparative identification of the biological characteristics of H3N2 CIVs globally isolated over a span of 10 years, showing that the isolation rate of H3N2 CIVs in dogs has gradually increased and that their adaptability to mammals has further increased [6]. Therefore, H3N2 CIVs pose a serious threat to human health. However, the factors influencing the incidence of CIVs are still unclear.
The activity ranges of dogs, which are significant companion animals to humans, are affected by changes in human social distancing. During the COVID-19 pandemic, China promptly enforced emergency health measures, which entailed imposing restrictions on public activities and shutting down public spaces [7]. Strict containment and feasible suppression strategies during the emergency stage of the COVID-19 pandemic with core measures including lockdown in endemic areas, travel restrictions and physical distancing successfully stopped the spread of COVID-19 in China and had positive impacts on the incidence and mortality of zoonotic diseases, especially those with a relatively high number of annual cases [8]. These limitations affected both humans and their companion animals, providing an ideal model for studying factors influencing the prevalence of canine influenza, a human companion animal disease.
In this study, we used the Veterinary Teaching Hospital of China Agricultural University (CAU), which has the most medical cases, as the monitoring site and continuously monitored the prevalence of CIVs in China before and after the COVID-19 pandemic. Our findings indicated that following the implementation of epidemic prevention and control measures in China, the pathogen detection rate and seropositivity rate of CIVs significantly decreased. However, after the lifting of personnel movement restrictions, the circulation of CIVs gradually increased. This study demonstrated that during the COVID-19 epidemic, prevention and control policies effectively suppressed the spread of CIVs. Our research suggests that human activities are a significant factor influencing the prevalence of CIVs and provides a theoretical basis for the implementation of preventive and control measures for other zoonotic diseases in companion animals.
Results
Isolation of viruses and collection of clinical data
We previously found that H3N2 CIVs, the Haemagglutinin (HA) gene of which belongs to clade 5, became dominant in China, and these CIVs pose a great threat to public health. The Veterinary Teaching Hospital of CAU has the greatest number of medical cases (90,000 cases/year) in China. Thus, we established the Veterinary Teaching Hospital of CAU as a monitoring site and conducted a continuous epidemic investigation of CIVs. From 2022 to 2023, throat swabs were collected from dogs with respiratory symptoms. Real-time RT‒PCR was utilized to amplify the matrix gene and determine the positivity of CIV. A total of 790 samples were collected, 14 of which (1.70%) were positive for CIV. The specific clinical symptoms of these positive patients from 2022 to 2023 are detailed in Table 1. According to the previous CIV surveillance data [6, 9], the positive rates for each year increased from 6.17% in 2016 to 10.13% in 2019 in the Veterinary Teaching Hospital of CAU (Fig 1). However, in December 2019, the COVID-19 epidemic occurred in Wuhan, and on January 23, 2020, intervention measures were gradually implemented nationwide. The Animal Hospital of CAU was closed from January to April 2020, and thorough disinfection measures were implemented. Notably, from April 2020 to December 2022, we observed a significant decrease in the percentage of CIV-positive individuals (Fig. 1), which was only 0.79% (7/1010). In contrast, on January 8, 2023, nationwide intervention measures were lifted, and we observed a gradual increase in the positivity rate of CIV, although no virus was detected from April to August 2023, possibly because this period was not the peak season for CIV circulation. From September to December 2023, the CIV positivity rate was 4.97% (9/181). These results indicate that COVID-19 epidemic intervention measures significantly reduced the incidence of CIV.
The percentage of canine influenza virus-positive dogs with respiratory symptoms between 2016 and 2023. From 2016 to 2023, throat swabs were collected from dogs with respiratory symptoms, and real-time RT‒PCR was used to determine CIV positivity. The bar chart represents the positivity rate of CIV for each month, with the values above indicating the number of positive samples for each year
Phylogenetic analysis
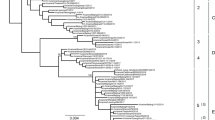
To characterize the evolution of CIVs, we sequenced the HA and Neuraminidase (NA) genes of the CIV-positive samples detected from 2022 to 2023 and conducted a phylogenetic analysis of the HA gene of these viruses. As shown in Fig. 2 and Fig. 3, all sequenced samples were identified as H3N2 CIV, with their HA gene segments falling within Clade 5.1, a clade that formed after 2019 in China.
Antigenic analysis
Our previous study showed that global H3N2 CIV has seven antigenic groups (A–G) [6]. To determine whether there is antigenic variation in the H3N2 CIV isolates from 2023, we investigated their antigenicity using antisera against different antigenic groups of H3N2 CIV. As shown in Table 2, the H3N2 CIVs isolated in 2023 exhibited the same antigenic spectrum as strains from antigenic group G, characterized by the highest reactivity with antisera from antigenic group G strains, which was 4–16 times greater than that with antisera from other antigenic groups.
Serological analysis
To further determine the effect of COVID-19 epidemic intervention measures on the incidence of CIV, we collected 460 canine serum samples from November 2022 to December 2023 (with an average of 33 ± 7 samples per month). According to the WHO vaccine evaluation guidelines, an HI antibody titer ≥40 indicates a 50% protection rate against influenza A viruses. Therefore, we defined an HI antibody titer ≥40 as the threshold for estimating infection. Among the tested serum samples, 19 (4.13%) were positive for canine H3N2 influenza virus-specific antibodies (Fig. 4). We found that the trend of the serum positivity rate during the tested period was consistent with the trend of the virus isolation rate. In 2022, during the epidemic control period, all 65 tested serum samples were negative. Importantly, from September 2023 onward, the rate of serum positivity for canine H3N2 influenza virus-specific antibodies significantly increased (6.25% – 9.68%) (Fig. 5). These results further demonstrate that COVID-19 epidemic intervention measures can significantly reduce the incidence of CIV.
HI titers in serum samples from dogs positive for canine H3N2 virus. In total, 460 serum specimens were tested with an HI assay in 2023, and HI antibody titers ≥40 were considered positive. The number of positive serum samples for each virus is shown. GMTs and 95% confidence intervals are indicated by long and short horizontal lines, respectively
The seroprevalence of influenza viruses in domestic dogs in 2023. Serum samples were collected from domestic dogs at the Veterinary Teaching Hospital of China Agricultural University and analyzed for hemagglutination inhibition (HI). The bar chart represents the monthly positivity rate of H3N2 antibodies in serum
Discussion
Due to the serious threat that H3N2 CIVs pose to human health, in this study, we continuously monitored the prevalence of CIVs in China before and after the COVID-19 pandemic. We found that epidemic prevention and control measures for the COVID-19 pandemic restricting personnel movement significantly suppressed the prevalence of CIVs, indicating that human activity is an important factor influencing the spread of CIVs.
Previous research has shown that the implementation of strict prevention and control measures during the COVID-19 pandemic has significantly inhibited the occurrence of human diseases. Measures restricting personnel movement implemented in Southeast Asia and Latin America in April 2020, along with school closures and reduced travel, led to a significant decrease in dengue fever cases among the population [10]. Xu et al. found that strict epidemic prevention and control policies in China reduced the incidence rates of various diseases, including H7N9, hemorrhagic fever, anthrax, brucellosis, leptospirosis, echinococcosis, and schistosomiasis, among the population in 2020 compared with the previous 5 years. Compared with the same period in 2015–2019, zoonotic diseases showed significant declines of 25.79% in the emergency stages of the COVID-19 pandemic [8]. Restrictions on human activities also reduce contact between companion animals, but their impact on companion animal diseases has not yet been reported. Therefore, epidemic prevention and control measures against the COVID-19 pandemic provide an ideal model for studying factors affecting the prevalence of companion animal diseases such as canine influenza. In this study, our results indicated that epidemic prevention and control measures during the COVID-19 pandemic significantly reduced the pathogen detection rate and seropositivity rate of CIVs, demonstrating the direct impact of restricting human activities on the prevalence of CIVs.
The prevalence of H3N2 CIVs in dog populations poses a serious public health threat. Additionally, both avian α2,3-linked sialic acid receptors and human α2,6-linked sialic acid receptors have been found in the respiratory tract of dogs, suggesting that dogs are vulnerable to natural influenza virus infections originating from avian (H3N2 and H5N1) or human (pdmH1N1 and H3N2) sources [1, 11]. Human influenza viruses (H1N1/2009 and H3N2) reportedly breach the species barrier to infect dogs [1, 12]. When multiple influenza virus strains infect dogs simultaneously, new reassorted viruses can be generated through the exchange of gene segments between influenza viruses. A novel H3N1 CIV, formed by reassortment between H3N2 CIV and pandemic H1N1 virus, was discovered in dogs in Korea from 2007 to 2010 [13]. Subsequently, recombination between H3N2 CIVs and other influenza viruses (including H1N1/2009 and H5N1 viruses) has been reported [14, 15]. Therefore, continuous surveillance of CIVs in dogs is necessary to assess their public health risk. Once an outbreak of canine influenza or other zoonotic diseases occurs in the canine population, measures can be taken to control the spread and development of the disease by restricting human or companion animal activities.
Materials and methods
Virus identification and isolation
In 2023, throat swabs were taken from dogs displaying respiratory symptoms at the Veterinary Teaching Hospital of CAU. Throat swabs were collected for virus isolation and placed in 1.0 mL of transmission medium, which consisted of 50% glycerol in PBS along with antibiotics, following previously described protocols [16]. We amplified the matrix gene by real-time reverse transcription (RT‒PCR) using the Influenza A Virus V8 Rapid Real-Time RT‒PCR Detection Kit ( Beijing Anheal Laboratories Co. Ltd., China, Beijing) [17]. For the swab-based RT‒qPCR assay, a sample is considered positive if either matrix gene shows Ct values <40. Positive PCR products were individually inoculated into 9-day-old embryonic eggs, which were allowed to incubate at 35 °C for 72 hours. Afterward, the allantoic fluid was collected and examined for hemagglutination activity using 1% chicken red blood cells (cRBCs). If the sample was positive for hemagglutination activity, the HA gene and NA gene segments were identified by direct sequencing of the PCR products. Viral gene amplification and sequencing were carried out as previously described [18]. The viruses were preserved in a freezer at −80 °C.
Phylogenetic and molecular analyses
This study utilized H3N2 CIV isolates obtained from the NCBI and GISAID databases and selected based on factors such as isolation time and location. Phylogenetic analyses were conducted on alignment regions with minimal sequence gaps. Maximum likelihood phylogenies for each segment were constructed using RAxML (version 8) via CIPRES Science Gateway, running 1000 bootstrap replicates, with the GTR + gamma + I nucleotide substitution model.
Antigenic analyses
Hemagglutination inhibition (HI) assays were utilized to conduct the antigenic characterization of H3N2 viruses isolated in this and previous studies. Our previous research revealed that global H3N2 CIV forms seven antigenic groups (A–G) [9]. In this study, we selected sera prepared in previous research: ferret antisera raised against Canine/GuangDong/1/2006 (Cn/GD/1/06, group A), Canine/Beijing/358/2009 (Cn/BJ/358/09, group B), Canine/Beijing/362/2009 (Cn/BJ/362/09, group B), Canine/Beijing/256/2015 (Cn/BJ/256/15, group C), Canine/Beijing/265/2015 (Cn/BJ/265/15, group C), Canine/Illinois/M17-05782-7-1/2017 (Cn/IL/M17-05782-7-17, group D), Canine/Beijing/137/2017 (Cn/BJ/137/17, group E), Canine/Beijing/147/2017 (Cn/BJ/147/17, group E), Canine/Fujian/1109/2018 (Cn/FJ/1109/18, group F), Canine/Guangzhou/011/2019 (Cn/GZ/011/19, group G), and Canine/Beijing/1115/2019 (Cn/2018). All HI assays were performed as previously described and following WHO guidelines [19].
Serological studies
Serum samples were collected from domestic pet dogs and presented to the Veterinary Teaching Hospital of CAU between January 2022 and June 2023 and analyzed for hemagglutination inhibition (HI). In this study, Cn/BJ/106/23 (group G) H3N2 CIV was used as the detection antigen. According to WHO guidelines for vaccine evaluation, HI antibody titers of 40 suggest a 50% protection rate against influenza A virus [20, 21]. Therefore, we established HI antibody titers of 32 as the cutoff for determining infection rates [22, 23].
Availability of data and materials
All data and materials are available for free for non-profit institutions.
References
Song D, Kang B, Lee C, Jung K, Ha G, Kang D, et al. Transmission of avian influenza virus (H3N2) to dogs. Emerg Infect Dis. 2008;14(5):741–6.
Li S, Shi Z, Jiao P, Zhang G, Zhong Z, Tian W, et al. Avian-origin H3N2 canine influenza A viruses in Southern China. Infect Genet Evol. 2010;10(8):1286–8.
Lee YN, Lee DH, Lee HJ, Park JK, Yuk SS, Sung HJ, et al. Evidence of H3N2 canine influenza virus infection before 2007. Vet Rec. 2012;171(19):477.
Voorhees IEH, Glaser AL, Toohey-Kurth K, Newbury S, Dalziel BD, Dubovi EJ, et al. Spread of canine influenza A(H3N2) virus United States. Emerg Infect Dis. 2017;23(12):1950–7.
Ou J, Zheng F, Cheng J, Ye SS, Ye C, Jia K, et al. Isolation and genetic characterization of emerging H3N2 canine influenza virus in Guangdong Province, Southern China, 2018–2021. Front Vet Sci. 2022;9:810855.
Chen M, Lyu Y, Wu F, Zhang Y, Li H, Wang R, et al. Increased public health threat of avian-origin H3N2 influenza virus caused by its evolution in dogs. eLife. 2023;12:e83470.
Zhang X, Luo W, Zhu J. Top-down and bottom-up lockdown: evidence from COVID-19 prevention and control in China. J Chin Polit Sci. 2021;26(1):189–211.
Ma C, Guo X, Wang L, Li W, Liu S, Lin F, et al. The impact of the COVID-19 pandemic on the incidence and mortality of zoonotic diseases in China. BMJ Glob Health. 2022;7(1):e007109.
Chen M, Wang R, Pei Y, Zhang T, Lyu Y, McLaughlin J, et al. Surveillance and characterization of avian-origin H3N2 canine influenza viruses in 2021 in China. One Health Adv. 2024;2(1):2.
Chen Y, Li N, Lourenço J, Wang L, Cazelles B, Dong L, et al. Measuring the effects of COVID-19-related disruption on dengue transmission in southeast Asia and Latin America: a statistical modelling study. Lancet Infect Dis. 2022;22(5):657–67.
Lin Y, Zhao YB, Zeng XJ, Lu CP, Liu YJ. Complete genome sequence of an H3N2 canine influenza virus from dogs in Jiangsu, China. J Virol. 2012;86(20):11402.
Sun Y, Shen Y, Zhang X, Wang Q, Liu L, Han X, et al. A serological survey of canine H3N2, pandemic H1N1/09 and human seasonal H3N2 influenza viruses in dogs in China. Vet Microbiol. 2014;168(1):193–6.
Song D, Moon HJ, An DJ, Jeoung HY, Kim H, Yeom MJ, et al. A novel reassortant canine H3N1 influenza virus between pandemic H1N1 and canine H3N2 influenza viruses in Korea. J Gen Virol. 2012;93(Pt 3):551–4.
Moon H, Hong M, Kim JK, Seon B, Na W, Park SJ, et al. H3N2 canine influenza virus with the matrix gene from the pandemic A/H1N1 virus: infection dynamics in dogs and ferrets. Epidemiol Infect. 2015;143(4):772–80.
Su S, Cao N, Chen J, Zhao F, Li H, Zhao M, et al. Complete genome sequence of an avian-origin H3N2 canine influenza A virus isolated in farmed dogs in southern China. J Virol. 2012;86(18):10238.
Zhang P, Tang Y, Liu X, Peng D, Liu W, Liu H, et al. Characterization of H9N2 influenza viruses isolated from vaccinated flocks in an integrated broiler chicken operation in eastern China during a 5 year period (1998–2002). J Gen Virol. 2008;89(Pt 12):3102–12.
Sun Y, Sun S, Ma J, Tan Y, Du L, Shen Y, et al. Identification and characterization of avian-origin H3N2 canine influenza viruses in northern China during 2009–2010. Virology. 2013;435(2):301–7.
Sun Y, Pu J, Jiang Z, Guan T, Xia Y, Xu Q, et al. Genotypic evolution and antigenic drift of H9N2 influenza viruses in China from 1994 to 2008. Vet Microbiol. 2010;146(3–4):215–25.
Wang Z, Li H, Li Y, Wu Z, Ai H, Zhang M, et al. Mixed selling of different poultry species facilitates emergence of public-health-threating avian influenza viruses. Emerg Microbes Infect. 2023;12(1):2214255.
de Jong JC, Palache AM, Beyer WE, Rimmelzwaan GF, Boon AC, Osterhaus AD. Haemagglutination-inhibiting antibody to influenza virus. Dev Biol (Basel). 2003;115:63–73.
Eichelberger M, Golding H, Hess M, Weir J, Subbarao K, Luke CJ, et al. FDA/NIH/WHO public workshop on immune correlates of protection against influenza A viruses in support of pandemic vaccine development, Bethesda, Maryland, US, December 10–11, 2007. Vaccine. 2008;26(34):4299–303.
Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361(20):1945–52.
Lerdsamran H, Pittayawonganon C, Pooruk P, Mungaomklang A, Iamsirithaworn S, Thongcharoen P, et al. Serological response to the 2009 pandemic influenza A (H1N1) virus for disease diagnosis and estimating the infection rate in Thai population. PLoS One. 2011;6(1):e16164.
Acknowledgments
We thank all the authors and laboratories for assisting with this project.
Funding
This work was supported by the Major Project of Agricultural Biological Breeding (No. 2023ZD0405304).
Author information
Authors and Affiliations
Contributions
M.C., R.W., Q.Z., Q.T., Y.L. and D.W. collected the data; Z.W., L.L., H.S., J.P., J.L. and Y.S. analyzed and interpreted the data; H.A., T.T. and Y.S. wrote the manuscript. All authors reviewed, revised, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was granted exemption by the China Agricultural University Laboratory Animal Welfare and Animal Experimental Ethical Review Committee because no experimental procedures were conducted that could endanger the animals' life or health.
Consent for publication
Not applicable.
Competing interests
No potential conflicts of interest were reported by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, T., Ai, H., Chen, M. et al. COVID-19 restrictions limit the circulation of H3N2 canine influenza virus in China. One Health Adv. 2, 19 (2024). https://doi.org/10.1186/s44280-024-00053-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s44280-024-00053-z