Abstract
Background
In 2022, fewer than half of persons with tuberculosis (TB) had access to molecular diagnostic tests for TB due to their high costs. Studies have found that the use of artificial intelligence (AI) software for chest X-ray (CXR) interpretation and sputum specimen pooling can each reduce the cost of testing. We modeled the combination of both strategies to estimate potential savings in consumables that could be used to expand access to molecular diagnostics.
Methods
We obtained Xpert testing and positivity data segmented into deciles by AI probability scores for TB from the community- and healthcare facility-based active case finding conducted in Bangladesh, Nigeria, Viet Nam, and Zambia. AI scores in the model were based on CAD4TB version 7 (Zambia) and qXR (all other countries). We modeled four ordinal screening and testing approaches involving AI-aided CXR interpretation to indicate individual and pooled testing. Setting a false negative rate of 5%, for each approach we calculated additional and cumulative savings over the baseline of universal Xpert testing, as well as the theoretical expansion in diagnostic coverage.
Results
In each country, the optimal screening and testing approach was to use AI to rule out testing in deciles with low AI scores and to guide pooled vs individual testing in persons with moderate and high AI scores, respectively. This approach yielded cumulative savings in Xpert tests over baseline ranging from 50.8% in Zambia to 57.5% in Nigeria and 61.5% in Bangladesh and Viet Nam. Using these savings, diagnostic coverage theoretically could be expanded by 34% to 160% across the different approaches and countries.
Conclusions
Using AI software data generated during CXR interpretation to inform a differentiated pooled testing strategy may optimize TB diagnostic test use, and could extend molecular tests to more people who need them. The optimal AI thresholds and pooled testing strategy varied across countries, which suggests that bespoke screening and testing approaches may be needed for differing populations and settings.
Similar content being viewed by others
Background
When the World Health Organization (WHO) recommended the Xpert MTB/RIF assay (Cepheid; Sunnyvale, CA, USA) for diagnosis of tuberculosis (TB) in 2010, it was heralded as a game-changer [1]. This novel technology of cartridge-based nucleic acid amplification test (NAAT) ushered in a new era of progress in TB diagnostics and was followed shortly thereafter by the second molecular WHO-recommended rapid diagnostic test (mWRD), the Molbio Truenat [2]. Today, the TB diagnostic pipeline is healthier than ever, with at least 35 other NAATs for diagnosing TB in development [3].
Despite the bright future, only 47% of people newly diagnosed with TB received a mWRD as their initial test in 2022 [4, 5]. Meanwhile, most persons with TB were still diagnosed by smear microscopy, the same method Robert Koch used to isolate M. tuberculosis as the etiologic agent in 1882 [6]. Among the many causes for this diagnostic coverage gap, a major reason is cost [7, 8]. Despite recent price reductions of consumables and reagents [9], ensuring universal mWRD coverage globally may cost over $1 billion per year [10, 11].
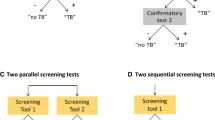
To mitigate high mWRD costs, a screening step can be used to rule out people with a low probability of TB disease [12]. Among various options, chest X-ray (CXR) has become the screening tool of choice in many settings due to the ability to identify the large cohort of asymptomatic people with TB [13, 14]. More recently, computer-aided detection (CAD) and artificial intelligence (AI) platforms to support CXR reading, such as qXR (Qure.ai; Mumbai, India), CAD4TB (Delft Imaging; Delft, the Netherlands), and other platforms, have gained popularity with value propositions ranging from capacity creation to overcome the lack of trained human readers to workload reduction through triaging of normal CXR images [15]. For individuals 15 years and above, CAD/AI may be used in place of human reading for screening and triage, and working alongside human readers to help automate and standardize interpretation [12]. Several evaluations of CAD/AI compared to human readers have shown that the technology performs as well or better than expert human readers [16,17,18]. CAD/AI offers a key advantage over human readers through its provision of a continuous abnormality score (ranging from 0–1 or 0–100) which confers a likelihood of TB among people screened, unlike humans who will produce a dichotomous outcome (TB suggestive or not), and grants greater flexibility to tailor follow-on testing to limited public health budgets [19].
Another recent process innovation to address the high costs of laboratory tests in resource-constrained settings that was effectively employed during the COVID-19 pandemic is specimen pooling. This method involves mixing specimens for a two-step hierarchical diagnostic algorithm that foregoes individual testing in the event of a negative pooled sample [20]. While initial TB pooling studies had found that dilution of the bacterial load can lead to lower levels of detection and potentially missed diagnoses [21], more recent evaluations have reported sensitivities of 98–100% with the Xpert MTB/RIF Ultra assay (Xpert Ultra) [22,23,24]. This has led to reported reductions in TB testing costs of 57–87% depending on the pool size [25]. A recent cost-effectiveness analysis reported a 34.9% decrease in costs when comparing pooled to individual Xpert testing [26].
The benefits of pooling are proportional to the prevalence of the disease in the target population. The lower the prevalence, the higher the theoretical savings. This raises the utility of pooling in high-throughput, low-yield approaches, such as active case finding (ACF) campaigns where large numbers of individuals need to be screened and tested to detect a person with TB. ACF is an important component of all high-burden countries’ national TB response and is critical to reach those people who are less likely or able to get care in public facilities. However, it is more expensive to conduct outreach, and ways to reduce costs are urgently needed to reach all persons with TB, especially those with subclinical TB [27, 28].
Here, we evaluated the theoretical impact of using AI outputs to inform different pooling strategies based on Xpert testing data collected during ACF campaigns in four high TB burden settings with the goal of modeling diagnostic savings and theoretical expansion of access to mWRDs.
Methods
Study design
This was a retrospective analysis of ACF campaigns using AI probability scores to model the incremental reduction in Xpert cartridge consumption.
Data sources
Data were obtained from four implementers located in high TB burden countries of Bangladesh, Nigeria, Viet Nam, and Zambia. These data consisted of aggregate AI abnormality scores and Xpert test results from community- and facility-based case-finding campaigns conducted between 2014 and 2017 in Bangladesh and 2022 and 2023 in all other countries. These campaigns targeted a heterogeneous mix of vulnerable populations particular to each ACF setting and country.
All countries used CXR with slightly different modalities to screen for TB but presumptive TB was identified by either symptom screening or an abnormal chest radiograph. Screening methods for each country are summarized here. In Bangladesh, standalone screening centers supported referrals of health-seeking symptomatic individuals in outpatient care departments of public and private sectors to conduct facility-based CXR screening and Xpert testing in Dhaka [29]. In Nigeria, mobile teams conducted active outreach events in remote rural areas using ultra-portable X-rays among people with limited access to healthcare services, such as pastoralists and nomadic populations using verbal symptom screening and CXR in parallel [30]. Viet Nam delivered community-based ACF campaigns in rural and urban areas focused on household contacts, older persons, urban poor, and people with a history of TB with verbal symptom screening and CXR in parallel [31, 32]. Zambia used a portable X-ray system in health facilities to screen clinic attendees and household contacts who were symptomatic [33]. Nigeria and Viet Nam used qXR v3 (Qure.ai, India), and Zambia used CAD4TB7 (Delft Imaging, The Netherlands) for the generation of the AI probability score. Bangladesh used qXR v3 and CAD4TB6 for the same dataset, but only qXR v3 results from the former were used due to concordance with the software used in Nigeria and Viet Nam. Xpert MTB/RIF (Bangladesh and Nigeria) or the newer Xpert MTB/RIF Ultra assay (Viet Nam and Zambia) was used for diagnostic testing of distinct individuals in all countries. Both CAD4TB and qXR interpret CXR images as DICOM files and produce a TB abnormality score that confers a likelihood that the individual has TB and therefore should be tested. CAD4TB provides a score ranging from 0 to 100, while qXR produces a score ranging from 0 to 1 [34].
Model structure
Each participating country provided Xpert testing data aggregated by deciles (Dm, where m = 1 to 10) of AI probability scores in the range of 0–100 for CAD4TB, i.e., D1: AI score = 0–9, D2: 10–19 …, and, similarly, 0–0.99 for qXR. We calculated the positivity rate (pm) for each Dm. We assigned testing thresholds for each country based on the decile (Dm) where ~95% of cases would be detected (C) with Xpert testing starting at that decile to have only ~ 5% missed cases (M) as a result of not employing Xpert testing in the previous deciles (Additional file 1: Table S1, S2, S3). The 5% rate of missed TB cases was chosen to acknowledge the real-life resource limitations of testing all people with Xpert, while still maintaining a high level of detection.
To model the theoretical savings, we calculated the theoretical number of diagnostic tests per person needed to achieve the same positivity through pooling for a two-step hierarchical testing strategy [35]. The difference between the total actual number of individual tests performed and the theoretical number of tests per person when employing the pooled testing strategy represented the number of tests saved. For the primary analysis, we assumed a pool size of four based on prior studies and that both individual and pooled testing were 100% sensitive and specific [36, 37]. We then modeled four ordinal screening and testing approaches with increasing complexity to estimate incremental savings compared to the previous approach (Fig. 1):
-
1.
Baseline approach: all people with presumptive TB receive individual Xpert tests as per the original datasets;
-
2.
CXR approach: individual Xpert tests in all deciles for which ΣM ~ 5%;
-
3.
Indiscriminate pooling approach: pooled Xpert tests in all deciles for which ΣM ~ 5%;
-
4.
AI-guided pooling approach: a combination of pooled and individual Xpert tests in all deciles for which ΣM ~ 5%, with individual Xpert testing in deciles with a pm ≥ 20%. The 20% cutoff was chosen based on the peak level of savings that pooling would generate at various levels of positivity based on the outputs of our model.
While we aimed to achieve a ΣC ~ 95% (≡ ΣM ~ 5%) for screening and testing approaches, the actual values were 95.7% (Bangladesh), 95.3% (Nigeria), 94.9% (Viet Nam), and 96.7% (Zambia).
Data analysis
We described the number of tests performed, positive results, and positivity rates in total and for each AI-score decile. We further calculated the theoretical number and proportion of tests saved incrementally between each screening and testing approach and cumulatively over the baseline. To characterize the optimal approach in each country, we identified the deciles below which testing could be foregone and the deciles at which individual testing instead of pooled testing would save tests. The number of tests saved was multiplied by the current price for an Xpert Ultra cartridge ($7.97) to calculate crude cost savings and subsequently divided by the number of positive test results for a unit-cost estimate per person diagnosed with TB [9]. To offer an alternative perspective to cost savings, we estimated the ratio of additional tests that could be performed with the savings over the theoretical number of tests needed for the current cohort as a measure of the extent to which access to mWRDs could have been expanded by employing the optimal screening and testing approach.
For the sensitivity analyses, we modeled using pool size of three [22] and relaxed the 100% sensitivity and specificity assumption of the pooling method to 95% and 98%, respectively, based on systematic review findings from pooling with Xpert Ultra as Xpert MTB/RIF will be discontinued in 2024 using the datasets from all countries [23]. We used binGroup2 package in R for the analysis and have publicly availed all data and analysis code [38, 39].
Results
Dataset characteristics
In the two facility-based ACF settings, Bangladesh and Zambia performed 24,079 and 2353 Xpert tests with a matching corresponding AI result, respectively. In the community-based ACF settings, Nigeria performed 1021 and Viet Nam performed 5074 tests. Positivity rates were 15.3% (3679/24,079) in Bangladesh, 11.6% (273/2353) in Zambia, 9.0% (455/5,074) in Viet Nam, and 8.3% (85/1021) in Nigeria (Fig. 2).
In terms of testing distribution by AI-score decile, most countries exhibited similar U-shaped patterns except Zambia (Fig. 3A). In Zambia, almost half (49.3%) of the tests were conducted in D5–D6. Meanwhile, 40.6% of Xpert tests in Bangladesh had an AI score in the lowest decile (D1), which was higher than Viet Nam (22.1%), Nigeria (17.4%), and Zambia (2.5%). Meanwhile, the testing rate in the AI-scores D4–D5, were higher in Nigeria (D4:15.3% and D5:15.0%) compared to Bangladesh (D4:4.1% and D5:3.9%) and Viet Nam (D4:5.4% and D5:5.2%).
Distribution of tests performed and test positivity across the AI-score deciles for the four countries. A Testing distribution by AI-score decile refers to the percentage of total tests performed within each AI-score decile. B Test positivity by AI-score decile shows the proportion of positive results in each AI-score decile. The decile labels (D1 to D10) represent AI-score deciles; D1–D10 for 0–0.99 in 0.1 intervals for qXR score and D1–D10 for 0–99 in 10-point intervals for CAD4TB score
In terms of positivity by AI-score decile (Fig. 3B), the two facility-based ACF sites exhibited higher rates than the community-based counterparts. In Bangladesh, positivity was higher in the high AI-score deciles of D7–D10 than in the other countries, with rates increasing from 12.3 to 70.0%. Zambia exhibited a similar pattern with a lower peak with rates, ranging from 13.6 to 65.1% for D7 and D10, respectively. In comparison, the respective positivity of D7 and D10 only rose from 4.8 to 28.7% in Nigeria and from 6.3 to 30.6% in Viet Nam.
Tests cost savings in the model output
All incremental screening and testing approaches resulted in additional savings except the indiscriminate pooling approach in Bangladesh and are presented for each country in Table 1. In Bangladesh, the CXR approach resulted in savings of 52.5% over baseline. Based on model parameters, these savings were realized at an AI threshold of 0.30–0.39, i.e., foregoing testing for D1–D3 and individually testing everyone in higher deciles. Indiscriminately pooling all persons in D4–D10 actually resulted in excess of 2.0% of tests over the CXR approach. Using AI to indicate individual testing for high AI-score deciles D9–D10 reversed this trend and increased cumulative savings to 61.5%.
The model assumes pool sizes of 4 with both pooling and individual testing sensitivity and specificity of 100% for a testing threshold resulting in missed cases of 4.4%, 4.7%, and 5.1% for Bangladesh, Nigeria and Viet Nam using qXR v3, respectively, and 3.3% for Zambia using CAD4TB v7. Incremental savings indicate the difference from the prior testing approach in the table (e.g., indiscriminate pooling approach compared to CXR approach or AI-guided pooling approach compared to indiscriminate pooling approach), whereas cumulative savings are calculated against the baseline approach.
In Zambia, the CXR approach yielded savings of 16.7% over baseline at an AI threshold of 0.3. Indiscriminately pooling all persons in D4 and higher produced incremental savings of 31.0% and cumulative savings over the baseline of 42.5%. Per AI guidance, testing reverted to an individual basis in D8–D10 to yield additional savings of 14.3% for cumulative savings of 50.8%.
The CXR approach in Nigeria showed an initial savings of 27.7% in testing at an AI threshold of 0.3 above which indiscriminate pooling reduced an incremental 37.8% in testing for a cumulative savings of 55.0% over baseline. There were diminishing returns from the AI-guided pooling approach as switching to individual testing in D10 led to incremental and cumulative savings over the baseline were only 5.4% and 57.5%, respectively.
In Viet Nam, the CXR approach reduced testing by 42.5% at an AI threshold of 0.4. Meanwhile, indiscriminate pooling above the AI threshold saved an incremental 27.7% in testing over the CXR approach for a cumulative savings of 58.4% over baseline. Similar to Nigeria, the AI-guided pooling approach indicated individual testing in D10 which added 7.3% in incremental test reductions for a cumulative savings of 61.5% (Fig. 4).
Tests performed under different approaches in the four countries. Legend. The model assumes pool sizes of 4 with both pooling and individual testing sensitivity and specificity of 100% for a testing threshold resulting in missed cases of 4.4%, 4.7%, and 5.1% for Bangladesh, Nigeria, and Viet Nam using qXR v3, respectively, and 3.3% for Zambia using CAD4TB v7. The percentages are reported with reference to the baseline approach for the respective countries
In Bangladesh, the number of tests needed declined from 24,079 to 9262 for savings of 12,403–14,817 tests. This translated to $98,852–$118,091 in crude costs or $26.87–$32.10 per person with TB diagnosed (Table 2). Alternatively, these savings could be redeployed for an expansion of mWRD access of 110–160% with existing public health resources. In Zambia, the number of tests dropped from 2353 to 1158 for savings of 393–1195 tests. This implies cost savings of $3132–$9524 or $11.47–$34.89 per person with TB for a theoretical expansion of mWRD access of 20–103%. Based on test volume reductions from 1021 to 434 in Nigeria, the total test and cost savings ranged from 283 to 587 and $2256–$4678 or $26.54–$55.04 per person with TB. This represented 38–135% in potentially greater access to mWRD. Lastly, in Viet Nam the number of tests needed fell from 5074 to 1955 for 2157–3119 tests saved corresponding to crude savings of $17,191–$24,858 or $37.78–$54.63 per person with TB. This represented a potential mWRD expansion of 74–160%.
Incremental savings indicate the difference from the prior case, whereas cumulative savings are calculated against the baseline approach. Crude cost savings are based on a cost of $7.97 per Xpert Ultra cartridge. Per-TB cost savings are based on a number of positive test results of 3679 in Bangladesh, 273 in Zambia, 85 in Nigeria, and 455 in Viet Nam. mWRD access expansion was calculated by dividing the number of cartridges saved by the number of cartridges used in each individual screening and testing approach.
The sensitivity analyses did not show a substantial change in the results. Across all different scenarios, the change in savings against the primary case (pool size of three with pooled sensitivity and specificity of 1) ranged from − 9.4% to + 7.6%. Testing in smaller pools increased the number of tests used. Reducing pooling sensitivity reduced test usage and so did increasing pooling specificity, which will result in an increase in missed cases and false positives, respectively, for the overall two-step hierarchical testing algorithm. (Additional file 1: Table S4) In Bangladesh, we compared savings between qXRv3 and CAD4TBv6 and found that differences were small (0.3%). CAD4TB scores resulted in 9200 tests in the AI-guided pooling approach compared to 9262 in qXRv3 against the baseline case of 24,079 tests. (Additional file 1: Table S5).
Discussion
Our modeling study results show that the combined use of computer-aided chest radiography and pooling may achieve compounding effects to achieve significant savings in diagnostic consumables that could be redeployed to increase the global coverage of mWRDs. We further found that the substantial heterogeneity in AI thresholds and the impact of AI scores on subsequent pooling across the different settings will require differentiated deployment of these two innovations in order to optimize the potential gains. However, while countries and settings may differ, it appears that the various screening and testing approaches particularly the AI-guided pooling approach may be able to achieve consistent results between the most advanced software platforms.
Across the different countries, settings, and approaches, the combination of using CXR with AI scores to inform decisions on pooling sputum produced cumulative savings on testing of 50.8–61.5% over baseline compared to universal testing of all individuals with signs of TB. In 2022, Bangladesh tested only 20% of people with TB with mWRDs as the first-line diagnostic test. While the gap was smaller in Nigeria, Zambia, and Vietnam, three in ten persons with TB were not diagnosed using molecular diagnostics [4]. Our results suggest that more than twice as many people could be tested for the same diagnostic test costs using CXR and pooling as compared to testing all presumptive individuals.
A major contributor to this potential capacity expansion was the use of AI and CXR. As the data of all countries originated from ACF campaigns rather than prevalence surveys, each dataset included an inherent level of preselection or pre-screening to raise the TB prevalence in the sample. This pre-filtering may have consisted of targeting highly vulnerable populations, such as contacts (Zambia), deploying in high-yield settings such as health facilities (Bangladesh), or having a previous binary read of the CXR by a potentially inexperienced human reader (Viet Nam) [17]. Despite these methods of preselection, our study once again highlighted the well-documented effectiveness of CXR for screening and triaging for TB [40,41,42,43]. Beyond that, we observed incremental savings by leveraging the AI’s quantitative output to optimize CXR interpretation and forego testing in lower AI-score deciles. This approach was particularly useful in settings with a high testing proportion in the lowest decile such as Bangladesh and Viet Nam, the first screening and testing approach generated already generated cumulative savings of 52.5% and 42.5%, respectively. This optimization of testing volumes through computer-aided chest radiography was also concordant with the available evidence [18, 30].
Our model suggested that in each country, pooling could be effective for generating additional savings in testing. However, our model also evinced differences across both pooling strategies based on the populations screened and the results of the CXR and AI. For instance, in the facility-based settings of Bangladesh and Zambia, 65–70% of individuals in the highest decile had bacteriologically confirmed TB compared to only 29–31% in Nigeria and Viet Nam’s community-based ACF cohort. This dichotomy was reflected by the pooling approach, as the AI-guided pooling, i.e., reversion to individual testing in high deciles, continued to generate substantial savings in the facilities, while the model exhibited substantial diminishing returns in the low-yield community setting. Interestingly, while indiscriminate pooling saved tests in three countries, in Bangladesh it actually increased testing unless an AI-guided pooling approach was used. In Bangladesh, where reads for both qXR and CAD4TB were available, the performance on both platforms was highly concordant. However, each required adjustment of the testing threshold, highlighting the need for end-user optimization based on the local epidemiology and platforms used. The approaches may be further optimized based on where sample volumes allow more pools. In one such additional approach, the AI-guided cohort pooling case resulted in up to 2.5% additional savings over the AI-guided pooling case. (Additional file 1: Table S5) Nevertheless, these findings inspire confidence in the high precision that characterizes many of today’s commercial AI solutions for TB [16].
This variability was encountered in different areas of our study and represented its key strength. For example, in contrast to the high savings achieved through the CXR approach in Bangladesh and Viet Nam, savings in Nigeria and Zambia were only 28% and 17%, respectively. Yet, both countries differed substantially in the additional gains from each incremental pooling approach. The testing distribution patterns also exhibited discordance, as all countries exhibited a U-shaped curve except Zambia, or in positivity by decile, whereby reversion to individual testing of the AI-guided pooling approach occurred in D8 for the Zambian dataset, in D9 for the data from Bangladesh and D10 in Nigeria and Viet Nam. Given the variation among the data we analyzed, any program implementing such an approach should consider individual results before deciding on a specific AI and pooling strategy. However, the results suggest that efficiency gains are likely across different settings. Particularly with respect to the optimal application of AI and its continuous outputs, there has been much discussion about using different AI threshold scores. However, the growing evidence base suggests variability in AI performance across demographic, clinical, or behavioral characteristics of the population, screening setting, or even radiography equipment, which underscores the well-documented need for local calibration and threshold setting [18, 19, 44]. Similarly, while general pooling can save costs, optimizing a pooling strategy will depend on the use of local data, capacities, and established practices.
Our study has a limitation in that modeled results are fraught with assumptions about performance and each situation will differ. AI technology changes rapidly and our data comes from different time periods with different screening approaches, but the results are generally similar in improving efficiency with AI. In the primary analysis, we assume perfect sensitivity and specificity for both pooling and individual testing, which is unlike real-world performance. While we attempt to mitigate this limitation in the sensitivity analysis by varying the pooling parameters, we do not model the overall performance of the diagnostic algorithm, e.g., the overall sensitivity and specificity of a combination of no testing, individual testing, and pooled testing in the AI-guided pooling case. This will also impact the overall number of missed cases in the algorithm, leading to continued transmission and potential mortality. False positive results would increase testing costs and workload. We do not expect a substantial impact on the number of tests given the superior performance of Xpert Ultra in both individual and pooled testing documented previously and encourage future prospective evaluations to factor in these considerations, in addition to other implementation challenges. None of these studies used culture to quantify how many people would have been missed due to Xpert Ultras’ imperfect sensitivity, but our analysis was focused on optimizing molecular testing. Importantly, our model assumes AI is available to interpret CXR images but does not have any other cost for the technology which has a cost, as do human readers. Costing studies on AI and CXR are urgently needed as programs consider adopting the technology and so are cost-effectiveness analyses that capture the complete costs of pooling interventions. Future work should also consider different scenarios (e.g., paucibacillary samples, children) which may have different results from pooled testing approaches. Lastly, although the data are based on real-world ACF activities they did not contain the daily distribution of people with presumptive TB and their abnormality scores. Hence, for this analysis, we assumed 100% completeness in pool sizes with four sputum specimens per pool. Pooling decisions based on abnormality scores such as the AI-guided pooling approach would require that these data be included in the laboratory order form, and on occasion require pools of two or three specimens, which might impact overall savings and complicated pooling rules may cause confusion in laboratories. However, we conducted this sensitivity analysis, which also showed that the impact on overall savings was small.
The real-world readiness for computer-aided chest radiography-informed pooling in real-time in programmatic settings can be challenging. Despite the positive reception of pooling by laboratory technicians for its time-saving properties [22], pooled testing for TB is not feasible in all laboratories. For example, though specificity issues have not been widely reported, the manipulation of several samples bears the risk of contamination. CXR and AI are also not readily available in most high TB-burden countries. Nevertheless, as pooling becomes established practice in more indications and access to AI improves, this type of pragmatic approach could mitigate commonly encountered cartridge shortages and provide more people with signs of TB access to molecular tests and should be evaluated prospectively to see how they work in real-world settings [39].
Conclusions
To achieve End TB Strategy goals, it is necessary to optimize the use of available tools. Integrating computer-aided chest radiography and pooling into TB screening and testing algorithms has the potential to substantially reduce diagnostic testing, thus freeing up constrained financial and human public health resources to save costs and extend access to more people in need of high-quality, rapid molecular testing for TB.
Availability of data and materials
All the data used for the analysis is available in the paper. The reproducible code and data for the analysis are available in a public GitHub repository at https://github.com/peptonefizz/Xpert-pooling-analysis [39].
Abbreviations
- ACF:
-
Active Case Finding
- AI:
-
Artificial Intelligence
- CXR:
-
Chest X-ray
- mWRD:
-
molecular WHO-recommended Rapid Diagnostic
- NAAT:
-
Nucleic Acid Amplification Test
- TB:
-
Tuberculosis
- WHO:
-
World Health Organization
References
Evans CA. GeneXpert—a game-changer for tuberculosis control? PLoS Med. 2011;8:e1001064.
Nikam C, Jagannath M, Narayanan MM, Ramanabhiraman V, Kazi M, Shetty A, et al. Rapid diagnosis of mycobacterium tuberculosis with truenat MTB: a near-care approach. PLoS One. 2013;8:1–7.
Branigan D. Tuberculosis Diagnostics Pipeline Report. 2023.
World Health Organization. Global Tuberculosis Report 2023. Geneva: Switzerland; 2023.
World Health Organization. WHO standard: universal access to rapid tuberculosis diagnostics. Geneva: World Health Organization; 2023.
Daniel TM. The history of tuberculosis. Respir Med. 2006;100:1862–70.
Albert H, Nathavitharana RR, Isaacs C, Pai M, Denkinger CM, Boehme CC. Development, roll-out and impact of Xpert MTB/RIF for tuberculosis: What lessons have we learnt and how can we do better? Eur Respir J. 2016;48:516–25.
Engel N, Ochodo EA, Karanja PW, Schmidt BM, Janssen R, Steingart KR, et al. Rapid molecular tests for tuberculosis and tuberculosis drug resistance: a qualitative evidence synthesis of recipient and provider views. Cochrane Database Syst Rev. 2022;4(4):CD014877. https://doi.org/10.1002/14651858.CD014877.pub2.
Stop TB Partnership. November 2023 Diagnostics, Medical Devices & Other Health Products Catalog. 2023.
Brümmer LE, Thompson RR, Malhotra A, Shrestha S, Kendall EA, Andrews JR, et al. Cost-effectiveness of lowcomplexity screening tests in community-based case-finding for tuberculosis. Clin Infect Dis. 2024;78:154–63.
Baik Y, Nakasolya O, Isooba D, Mukiibi J, Kitonsa PJ, Erisa KC, et al. Cost to perform door-to-door universal sputum screening for TB in a high-burden community. Int J Tuberc Lung Dis. 2023;27:195–201.
World Health Organization. WHO consolidated guidelines on tuberculosis: module 2: screening: systematic screening for tuberculosis disease. Geneva: World Health Organization; 2021.
Law I, Floyd K, Abukaraig EAB, Addo KK, Adetifa I, Alebachew Z, et al. National tuberculosis prevalence surveys in Africa, 2008–2016: an overview of results and lessons learned. Trop Med Int Heal. 2020;25:1308–27.
Onozaki I, Law I, Sismanidis C, Zignol M, Glaziou P, Floyd K. National tuberculosis prevalence surveys in Asia, 1990–2012: an overview of results and lessons learned. Trop Med Int Heal. 2015;20:1128–45.
Creswell J, Vo LNQ, Qin ZZ, Muyoyeta M, Tovar M, Wong EB, et al. Early user perspectives on using computer-aided detection software for interpreting chest X-ray images to enhance access and quality of care for persons with tuberculosis. BMC Glob Public Heal. 2023;1:30.
Gelaw SM, Kik SV, Ruhwald M, Ongarello S, Egzertegegne TS, Gorbacheva O, et al. Diagnostic accuracy of three computer-aided detection systems for detecting pulmonary tuberculosis on chest radiography when used for screening: Analysis of an international, multicenter migrants screening study. PLOS Glob Public Heal. 2023;3:e0000402.
Qin ZZ, Sander MS, Rai B, Titahong CN, Sudrungrot S, Laah SN, et al. Using artificial intelligence to read chest radiographs for tuberculosis detection: a multi-site evaluation of the diagnostic accuracy of three deep learning systems. Sci Rep. 2019;9:15000.
Qin ZZ, Ahmed S, Sarker MS, Paul K, Adel ASS, Naheyan T, et al. Tuberculosis detection from chest x-rays for triaging in a high tuberculosis-burden setting: an evaluation of five artificial intelligence algorithms. Lancet Digit Heal. 2021;3:e543-54.
Geric C, Qin ZZ, Denkinger CM, Kik SV, Marais B, Anjos A, et al. The rise of artificial intelligence reading of chest X-rays for enhanced TB diagnosis and elimination. Int J Tuberc Lung Dis. 2023;27:367–72.
Daniel EA, Esakialraj L BH, S A, Muthuramalingam K, Karunaianantham R, Karunakaran LP, et al. Pooled Testing Strategies for SARS-CoV-2 diagnosis: a comprehensive review. Diagn Microbiol Infect Dis. 2021;101:115432.
Ho J, Jelfs P, Nguyen PTB, Sintchenko V, Fox GJ, Marks GB. Pooling sputum samples to improve the feasibility of Xpert® MTB/RIF in systematic screening for tuberculosis. Int J Tuberc Lung Dis. 2017;21:503–8.
Vuchas C, Teyim P, Dang BF, Neh A, Keugni L, Che M, et al. Implementation of large-scale pooled testing to increase rapid molecular diagnostic test coverage for tuberculosis: a retrospective evaluation. Sci Rep. 2023;13:1–10.
Cuevas LE, Santos VS, Lima SVMA, Kontogianni K, Bimba JS, Iem V, et al. Systematic review of pooling sputum as an efficient method for xpert MTB/RIF tuberculosis testing during COVID-19 pandemic. Emerg Infect Dis. 2021;27:719–27.
Chakravorty S, Simmons AM, Rowneki M, Parmar H, Cao Y, Ryan J, et al. The new Xpert MTB/RIF ultra: improving detection of mycobacterium tuberculosis and resistance to rifampin in an assay suitable for point-of-care testing. mBio. 2017;8(4):e00812–17. https://doi.org/10.1128/mBio.00812-17.
Dos Santos PCP, Da Silva Santos A, De Oliveira RD, Da Silva BO, Soares TR, Martinez L, et al. Pooling sputum samples for efficient mass tuberculosis screening in prisons. Clin Infect Dis. 2022;74:2115–21.
Iem V, Bimba JS, Santos VS, Dominguez J, Creswell J, Somphavong S, et al. Pooling sputum testing to diagnose tuberculosis using xpert MTB/RIF and xpert ultra: a cost-effectiveness analysis. BMC Infect Dis. 2023;23:1–11.
Stop TB Partnership. The Global Plan to End TB 2023–2030. Geneva: Switzerland; 2022.
Ismail N, Nathanson CM, Zignol M, Kasaeva T. Achieving universal access to rapid tuberculosis diagnostics. BMJ Glob Heal. 2023;8:8–10.
Banu S, Haque F, Ahmed S, Sultana S, Rahman MM, Khatun R, et al. Social Enterprise Model (SEM) for private sector tuberculosis screening and care in Bangladesh. PLoS One. 2020;15:e0241437.
John S, Abdulkarim S, Usman S, Rahman MT, Creswell J. Comparing tuberculosis symptom screening to chest X-ray with artificial intelligence in an active case finding campaign in Northeast Nigeria. BMC Glob Public Heal. 2023;1:17.
Nguyen LH, Codlin AJ, Vo LNQ, Dao T, Tran D, Forse RJ, et al. An Evaluation of Programmatic Community-Based Chest X-ray Screening for Tuberculosis in Ho Chi Minh City Vietnam. Trop Med Infect Dis. 2020;5:185.
Vo LNQ, Codlin A, Ngo TD, Dao TP, Dong TTT, Mo HTL, et al. Early evaluation of an ultra-portable X-ray system for tuberculosis active case finding. Trop Med Infect Dis. 2021;6:163.
Muyoyeta M, Kasese NC, Milimo D, Mushanga I, Ndhlovu M, Kapata N, et al. Digital CXR with computer aided diagnosis versus symptom screen to define presumptive tuberculosis among household contacts and impact on tuberculosis diagnosis. BMC Infect Dis. 2017;17:1–8.
Stop TB Partnership, FIND. AI4HLTH. 2023. https://www.ai4hlth.org/. Accessed 10 May 2023.
Dorfman R. The detection of defective members of large populations. Ann Math Stat. 1943;14:436–40.
Abdurrahman ST, Mbanaso O, Lawson L, Oladimeji O, Blakiston M, Obasanya J, et al. Testing pooled sputum with Xpert MTB/RIF for diagnosis of pulmonary tuberculosis to increase affordability in low-income countries. J Clin Microbiol. 2015;53:2502–8.
Iem V, Chittamany P, Suthepmany S, Siphanthong S, Siphanthong P, Somphavong S, et al. Pooled testing of sputum with Xpert MTB/RIF and Xpert Ultra during tuberculosis active case finding campaigns in Lao People’s Democratic Republic. BMJ Glob Heal. 2022;7:1–8.
Bilder CR, Hitt BD, Biggerstaff BJ, Tebbs JM, Mcmahan CS. binGroup2: Statistical Tools for Infection Identification via Group Testing. 2018;XX:1–17.
Codlin AJ, Vo LNQ, Garg T, Banu S, Ahmed S, John S, et al. Xpert-pooling-analysis. Github. 2023.https://github.com/peptonefizz/Xpert-pooling-analysis.
Shazzadur Rahman AAM, Langley I, Galliez R, Kritski A, Tomeny E, Squire SB. Modelling the impact of chest X-ray and alternative triage approaches prior to seeking a tuberculosis diagnosis. BMC Infect Dis. 2019;19:1–11.
World Health Organization. Chest radiography in tuberculosis detection – summary of current WHO recommendations and guidance on programmatic approaches. Geneva; 2016.
van Cleeff MRA, Kivihya-Ndugga LE, Meme H, Odhiambo JA, Klatser PR. The role and performance of chest X-ray for the diagnosis of tuberculosis: a cost-effective analysis in Nairobi Kenya. BMC Infect Dis. 2005;5:1–9.
Creswell J, Qin ZZ, Gurung R, Lamichhane B, Yadav DK, Prasai MK, et al. The performance and yield of tuberculosis testing algorithms using microscopy, chest x-ray, and Xpert MTB/RIF. J Clin Tuberc Other Mycobact Dis. 2019;14:1–6.
Codlin AJ, Dao TP, Vo LNQ, Forse RJ, Van Truong V, Dang HM, et al. Independent evaluation of 12 artificial intelligence solutions for the detection of tuberculosis. Sci Rep. 2021;11:23895.
Acknowledgements
The authors would like to thank their affiliate institutions and NTP of the contributing countries. We also express our gratitude to public health staff and partners at primary and secondary care levels, and the communities and patients involved in the initial generation of the original data used for the secondary analysis of this study.
Funding
TW is supported by grants from: the Wellcome Trust, UK (209075/Z/17/Z); the Department of Health and Social Care (DHSC), the Foreign, Commonwealth & Development Office (FCDO), the Medical Research Council (MRC) and Wellcome, UK (Joint Global Health Trials, MR/V004832/1); the Medical Research Council (Public Health Intervention Development Award “PHIND”, APP2293); and the Medical Research Foundation (Dorothy Temple Cross International Collaboration Research Grant, MRF-131–0006-RG-KHOS-C0942).
Author information
Authors and Affiliations
Contributions
JC, LNQV, AJC and TG conceptualized the manuscript. The methodology was developed by AJC and TG. Formal analyses and visualization were conducted by LNQV and TG. Resources were contributed by SB, AJC, LNQV, MM, SJ and SA. JC, LNQV, and TG wrote the original draft, while review and editing was done by all authors. Supervision was provided by JC. JC and LNQV were responsible for coordination with the co-authors and submission of the article. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approvals were obtained from respective review committees for the original data collection in the four participating countries, i.e., in Bangladesh, the Research Review Committee and the Ethical Review Committee at icddr,b; Adamawa and Gombe State Research Ethical Committees in the Ministry of Health in Nigeria; the National Lung Hospital’s Institutional Review Board and the Scientific and Ethical Committee of the Ha Noi University of Public Health, and the Institutional Review Board of PNT Hospital in Viet Nam; University of Zambia Biomedical Research Ethics Committee in Zambia. All data furnished for this study were aggregated and contained no personally identifiable information and thus this modeling study did not require additional ethical approval.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
44263_2024_81_MOESM1_ESM.docx
Additional file 1: Table S1. Testing data for the four countries across the AI-score deciles. Table S2. Testing threshold information for the four countries across the AI-score deciles. Table S3. Testing combination information for the four countries across the AI-score deciles. Table S4. Results of the sensitivity analysis based on pool size of 3 and different sensitivity and specificity parameters of the pooling method. Table S5. Incremental and cumulative savings by country.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Codlin, A.J., Vo, L.N.Q., Garg, T. et al. Expanding molecular diagnostic coverage for tuberculosis by combining computer-aided chest radiography and sputum specimen pooling: a modeling study from four high-burden countries. BMC Global Public Health 2, 52 (2024). https://doi.org/10.1186/s44263-024-00081-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s44263-024-00081-2