Abstract
Background
Digital decision aids are becoming increasingly common in many areas of healthcare. These aids are designed to involve patients in medical decision making, with the aim of improving patient outcomes while decreasing healthcare burden. Previously developed contraceptive-based decision aids have been found to be effective at increasing women’s knowledge about reproductive health and contraception. Here, we sought to evaluate the effectiveness of a novel contraceptive-based decision aid at increasing women’s self-efficacy and knowledge about their reproductive health and contraceptive options, as well as their perceptions of their learning. This study was registered as a clinic trial at ClinicalTrials.gov (Contraception Decision Aid Use and Patient Outcomes, ID# NCT05177783) on 05/01/2022.
Methods
The Tuune® contraceptive decision aid’s effectiveness was evaluated by conducting an experiment in which 324 women were assigned to use the Tuune® decision aid or a control decision aid. Primary outcomes included reproductive health self-efficacy, reproductive health and contraceptive knowledge, and perceptions of learning. Secondary analyses examined whether prior experience using hormonal contraceptives moderated the relationship between decision aid and each outcome measure.
Results
Women assigned to use the Tuune® decision aid exhibited greater reproductive health self-efficacy, greater knowledge about reproductive health and contraception, and perceived having learned more than women assigned to use the control decision aid (ps ≤ .029). This pattern was also observed in women with previous contraceptive use experience, where women using Tuune® reported better outcomes than women using the control aid, regardless of their history of hormonal contraceptive use experience, although this interaction was not significant (p = .089).
Conclusions
Use of the Tuune® contraceptive-based decision aid improved each of the predicted outcomes relative to a control decision aid. This suggests that use of the Tuune® contraceptive-based decision aid is well poised to increase women’s confidence and knowledge about contraceptive use and may also reduce burden on healthcare systems.
Similar content being viewed by others
Healthcare systems throughout the world are overburdened, such that physicians often find themselves with more patients than they have time to see [1]. As a result, medical appointments are frequently delayed and brief, with patients waiting weeks to see a doctor, and with limited time available for doctors and patients to discuss medical options and treatments [2]. One study found that primary care physicians (PCPs) typically spend a median of five minutes discussing existing health concerns of the patient and one minute or less discussing other health concerns raised during the visit [3], suggesting that patient visits are often too brief to ensure adequate patient education and thorough discussion of treatment options. Indeed, most providers feel they do not have enough time to spend with their patients during primary care office visits [2], which ultimately leads to low patient satisfaction and health self-efficacy, poor health outcomes, and nonadherence to treatment plans [4].
One way to mitigate patient education problems arising from an overtaxed medical system is to adopt the use of patient decision aids, a practice that has been increasing in many areas of healthcare [5, 6]. Decision aids are educational tools designed to facilitate patient participation in health care decisions with doctors, allowing for the implementation of more personalized treatments. Use of these aids can replace traditional doctor's appointments or (as they are more commonly used) complement traditional doctor's appointments, with patients using the aids to learn more about their medical condition or treatment options, either before or after their appointment. Use of these aids has been found to increase the speed and quality of care provided by physicians [5], while also increasing patient education and self-efficacy, and decreasing patient decisional conflict and anxiety (Espinosa et al., unpublished data; [7, 8]).
Given the important role of contraceptive knowledge in choosing the right kind of contraception and reducing unintended pregnancy rates [9, 10], one treatment area in which decision aids are becoming increasingly common is in the area of contraceptive counseling and family planning [11]. Many contraceptive prescriptions are written by PCPs, including family practice physicians [12, 13], whose ability to stay informed about the hundreds of contraceptive options currently available on the market can be precluded by their need to provide treatment and counseling to a wide variety of patients with a wide variety of health concerns. Further, because the time needed to discuss each of the available treatment options often far exceeds patient appointment times, even the most knowledgeable physicians lack the time to adequately explain to women the range of options available to them. Accordingly, contraceptive decision aids can help patients meet educational needs that cannot be met in the context of a traditional physician’s visit. Indeed, research finds that use of decision aids yields improvements in patient contraceptive knowledge and reproductive health self-efficacy (e.g., [8, 14]), as well as improvements in contraceptive use continuity (see [11] for review).
Despite the growing use of contraceptive decision aids, many of the available aids have important limitations that restrict their use to specific types of settings or to patients looking for specific types of products. For example, most available contraceptive decision aids are designed to be used by women’s health care providers (e.g., [15]) or by patients in waiting rooms prior to appointments (e.g., [16]), rather than being used by patients outside of a healthcare setting. Others present women with a limited range of contraceptive options, such as those that focus on educating women about long-lasting reversible contraceptives (e.g., [17]) or those that only present women with information about the 10 most-common contraceptive options out of the hundreds available (e.g., [18]). Often, these aids do not make personalized contraceptive recommendations specific to each patient’s values and health history (e.g., [19]). Specifically lacking from the contraceptive health decision aid landscape are products that are designed to: (a) be used by patients of all demographics, (b) be used outside of a traditional healthcare setting, (c) provide personalized educational materials on a range of contraceptive options, and (d) provide personalized contraceptive recommendations based on a patient’s needs, values, and medical conditions.
Recently, a new contraceptive decision aid designed to address these gaps has been made available to clinicians and patients. The Tuune® contraceptive decision aid was designed to offer women personalized contraceptive recommendations based on their needs, values, medical conditions, and hormonal symptoms. While no details of the proprietary algorithm or tool have been published previously, the Tuune® contraceptive decision aid is regulated in the United States by the FDA as a Clinical Decision Support device and is a CE marked Class 1 Medical Device in the United Kingdom and European Union. The product has therefore passed the high level of scrutiny required for both, meeting and exceeding the appropriate safety, health, and environmental protection requirements. The Tuune® algorithm and tool were built based upon published results from peer-reviewed studies investigating the efficacy and side-effects of different contraceptive options and their suitability in the management of hormonal symptoms. As such, all recommendations made by the tool are evidence-based and where there is insufficient evidence to support a recommendation, the algorithm will return an “unknown” response as opposed to providing an unsubstantiated recommendation. To date, over 3000 women have used the Tuune® decision aid tool. The tool has implemented regulatory pathways and quality management systems for telemedicine as a decision support tool, ensuring user data is protected as personal health information. Unlike other decision aids, the Tuune® decision aid can be used either in concert with a traditional doctor’s visit (Tuune® for Medical Practices, used by clinicians in the United States) or by patients in their homes (currently being used in the United Kingdom), and includes significant, personalized educational information and guided walkthroughs of issues relating to 39 hormonal symptoms and conditions and over 250 contraceptive options. It then generates personalized contraceptive recommendations to women based on their responses to a series of targeted questions assessing their health, hormonal symptoms, lifestyle, and values. Despite rigorous safety and experience testing, the Tuune® decision aid has yet to be tested against an alternative decision aid to determine its effects on outcomes that are associated with women’s effective contraceptive use.
The current research
Here, we sought to examine whether use of the Tuune® decision aid (compared to a control decision aid) would increase women’s reproductive health self-efficacy and women’s reproductive health and contraceptive knowledge. These outcomes were assessed because each has been found to predict increased contraceptive use [20, 21] and continuity [22], both of which decrease women’s risk of unplanned pregnancy. We predicted that women who were assigned to use the Tuune® decision aid would (a) exhibit greater reproductive health self-efficacy, (b) report feeling more knowledgeable about reproductive health and contraceptive options, and (c) report more positive learning outcomes than women assigned to use a control decision aid. Exploratory follow-up analyses also examined the role of women’s prior experience using hormonal contraceptives on each of these outcomes.
Methods
Participants
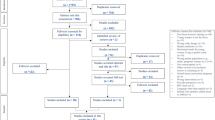
A power analysis was conducted using G*Power [23], based on effect sizes reported in past research investigating the impact of contraceptive-based decision aids on knowledge, self-efficacy, and contraception use among Latina adolescents [8]. Using the reported effect size of the difference in contraceptive knowledge between the app-use group and the control group from this study of d = 0.45, the power analysis revealed that we needed to obtain a sample size of N = 145 to achieve 85% power to detect an effect of this size, given statistical significance of α = 0.05. However, as this effect size is somewhat large, we also assessed the sample size needed to detect a smaller effect of d = 0.25. The power analysis revealed a sample size of 462 would be needed to detect an effect of d = 0.25. As such, we aimed to recruit at least 145, but up to 500, women for the current study to allow us to detect effects as small as d = 0.25, and to account for potentially greater heterogeneity of our target study population and to ensure sufficient power to conduct exploratory follow-up analyses within specific user segments. Participants were recruited through foot canvasing, advertising flyers, and university announcements at a midsized, private university in the Southern United States. To qualify, participants were required to be female, between the ages of 18–34, and not currently pregnant, breastfeeding, or seeking to become pregnant. Of those approached for participation in our study, we collected data from 324 women. Fourteen participants were excluded from data analysis after participation because they either did not comply with the study procedures or experienced technical issues during their session. The data analytic sample contained 310 women. See Table 1 for participant information reported by testing condition and Fig. 1 for a CONSORT Study Flow Diagram. Participants were compensated for their participation with nominal course credit and free access to the Tuune® decision aid.
Procedure and materials
All study procedures were approved by the local university Institutional Review Board (#2021–265). This study was registered as a clinic trial at ClinicalTrials.gov (Contraception Decision Aid Use and Patient Outcomes, ID# NCT05177783) and study predictions were preregistered using the Open Science Framework: https://osf.io/pg2c6.
Participants were assigned to one of two testing conditions (Tuune® condition vs. control condition) based on the date in which they participated in the study via block randomization with randomly selected block sizes. Specifically, participants who attended testing sessions during the first week (January 24, 2022 – January 28, 2022) and the final five weeks (March 21, 2022 – April 27, 2022) of data collection were assigned to the control condition (Block 1 n = 69; Block 3 n = 87), while those who attended sessions during the six weeks between the two control blocks (January 31, 2022—March 17, 2022) were assigned to the Tuune® condition (Block 2 n = 168).
All study sessions took place in-person, with participants completing sessions at individual computer terminals. To avoid any potential conflicts of interest, only research personnel without Tuune® affiliation were involved in data collection, analysis, and reporting. Participants began by providing informed consent, then answered questions about their personal and family health histories, as well as their knowledge about contraception and menstrual cycles. Participants were then directed to engage with one of two contraceptive decision aids: the experimental decision aid (Tuune®) or a fictitious control decision aid (called Nexxt), which was modelled after decision aids from another popular online birth control vendor and health assessment forms used in doctor’s offices, where many women receive contraceptive recommendations as a part of routine healthcare visits. Participants in both conditions responded to questions about hormone-related symptoms, menstrual cycles, and previous birth control use before being offered contraceptive recommendations. See Fig. 2A-D for screenshots taken from each decision aid for examples of the questions asked of participants and the recommendations given by the tools. Additionally, a full walk-through of the Nexxt decision aid control condition, along with all other study materials, are available on the Open Science Framework: https://osf.io/bnmx8/.
Examples of Screen Views from the Experimental Test and Control Condition Decision Aids. Note: Panels A & C are examples of question pages and panels B & D are examples of recommendation pages as viewed by the study participants using the digital decision aids. Panels A & B are from the experimental condition and panels C, D are from the control condition
Participants assigned to the Tuune® condition were redirected to the Tuune® website, (https://www.tuune.com/), where they completed the Tuune® contraceptive decision aid health assessment tool. This tool uses a proprietary algorithm to curate appropriate questions for each user based on their responses to earlier questions. Accordingly, of the 960 possible questions, each of our participants answered an average of 208 questions over an average of 35 min. The Tuune® digital decision aid tool asks participants questions about their medical history, history of birth control use, and experiences with menstrual cycle and hormonally-related symptoms. If women indicate any symptoms, the tool then asks them more detailed follow-up questions relating to each specific symptom, its severity, and its impact on daily activities. The Tuune® algorithm then collates information provided by the user and recommends a list of personalized birth control options from the over 250 available contraceptive products currently on the market. As part of their recommendation, women are also given access to educational resources that provide in-depth information about the recommended contraceptive options and about the hormonally-related symptoms they reported. After completing the Tuune® health assessment, participants returned to the survey to complete dependent measure questionnaires.
Similar to the Tuune® condition, participants in the control condition were redirected to a separate website where they completed a contraceptive-based decision aid tool called “Nexxt”, which was created for the purposes of this study. The full control decision aid tool stimuli are publicly available at: https://osf.io/bnmx8/. The control aid includes 42 questions regarding women’s menstrual history, sexual health history, birth control use, and general medical conditions. While the questions asked were similar between the control and experimental decision aid tools (see Fig. 2A and C), as is typical of other decision aids and standard physician intake forms, the control decision aid tool asked all women the same questions rather than personalizing the questions asked for each participant, as is done by the Tuune® decision aid tool. After engaging with the Nexxt decision aid, all participants in the control condition were given the same recommendation for a fictional birth control pill called “Xyla”, which they reviewed before returning to the survey to complete dependent measure questionnaires.
Following the experimental manipulation, participants responded to questions assessing their experiences with the decision aid they used, their reproductive health self-efficacy, reproductive health and contraceptive knowledge, and their perceptions of learning. During the debriefing process, women in the control condition were told that they were given a birth control recommendation for a product that does not exist as a part of the research study, and were given the option to access the Tuune® contraceptive decision aid tool after the study was completed at no cost. Women in both conditions were advised that their birth control recommendation had not been reviewed by a medical professional, and that they should not make changes to their contraceptive use without first consulting a medical professional. Following debriefing, participants were thanked, compensated, and dismissed.
Target outcome measures
Here, we sought to assess reproductive self-efficacy and knowledge about reproductive health and contraception. As well-validated, contemporary measures which assess these constructs are not available, we followed the standard for research in this area (see [20,21,22]) and designed measures which, while similar to existing measures, were modified to use contemporary language and to be focused on both reproductive health and contraceptive options. A full list of modified questions used in these measures can be found at: https://osf.io/bnmx8/. All items were responded to on a 7-point Likert scale (endpoints: 1 = Strongly Disagree, 7 = Strongly Agree).
Reproductive health self-efficacy
Reproductive health self-efficacy was measured using a modified version of the Menstrual Attitudes Questionnaire (MAQ) [24]. This modified MAQ is a 9-item measure, in which items were modified to assess menstrual cycle and hormonal health self-efficacy in a modern context. Appropriate items were reverse coded and a mean composite was computed, with higher scores indicating greater reproductive health self-efficacy (α = 0.69).
Reproductive health and contraceptive knowledge
Participant knowledge about reproductive health and contraception was measured using a modified version of the Health Literacy Instrument for Adults (HELIA) [25]. The modified version is a 6-item measure, and items were modified to assess participant’s knowledge about contraceptive options. The sum of the six items was computed, and each participant’s score was calculated using the following equation: Participant Score = (Participant Sum—6) / 36) * 100. Higher scores on this measure indicated greater reproductive health and contraceptive knowledge (α = 0.94).
Patient perceptions of learning
Participants’ perceptions of the educational quality of the decision aid was measured using a 4-item scale. A mean composite score for contraceptive education was computed, with higher scores indicating greater perceptions of educational attainment (α = 0.94).
Hormonal contraceptive use experience and demographic measures
Participants responded to items assessing their previous experiences with hormonal birth control, including whether or not they were currently using hormonal contraceptives (HCs), and whether they had ever used HCs in the past. Additionally, participants responded to standard demographic measures assessing age and racial/ethnic backgrounds.
Results
Target analyses: main effect of decision aid on participant outcome measures
We conducted a series of between-subjects analyses of variance analyses (ANOVAs) to investigate differences between women who used the Tuune® decision aid and women who used the control decision aid (i.e., Nexxt) in their (a) reproductive health self-efficacy, (b) reproductive health and contraceptive knowledge, and (c) perceptions of learning.
The results revealed a significant main effect of contraceptive decision aid on each of the three patient outcome measures. Specifically, women in the Tuune® condition exhibited greater reproductive health self-efficacy, F(1, 308) = 4.84, p = 0.029, and greater reproductive health and contraceptive knowledge, F(1, 307) = 62.76, p ≤ 0.001, compared to women in the control condition. Additionally, women who used the Tuune® decision aid also exhibited greater perceptions of learning compared to women in the control condition who used the control decision aid, F(1, 308) = 110.30, p ≤ 0.001. Together, these results indicate that use of the Tuune® decision aid led women to feel more in control and confident about their reproductive health, exhibit greater understanding of their contraceptive use and reproductive health, and feel more educated after using the Tuune® decision aid, compared to women who used of the control decision aid. See Table 2 for descriptive and inferential statistics and Fig. 3 for graphical depictions of results.
Secondary analyses: Does HC use experience influence the impact of decision aid use on participant outcomes?
Next, we tested if women’s prior HC use influenced the impact of the decision aid used on women’s reproductive health self-efficacy, knowledge, and perceptions of learning by conducting separate 2 (HC use experience: never used HCs vs. has used HCs) X 2 (decision aid used: Tuune® vs. control) between-subjects ANOVAs on each of the three target outcomes. Results revealed that while HC use experience did not impact the relationships between decision aid used and either reproductive health self-efficacy or perceptions of learning, HC use experience did impact the relationship between decision aid used and reproductive health and contraceptive knowledge. Specifically, results revealed a significant main effect of HC use experience on knowledge, F(1, 305) = 9.66, p = 0.002, in which women with previous HC use experience reported more knowledge compared to women with no previous HC use experience. Additionally, results revealed a significant main effect of decision aid used on knowledge, demonstrating that women who used the Tuune® decision aid reported that they were more knowledgeable compared to women who used the control decision aid (See Table 3 for descriptive and inferential statistics), as well as a marginally significant interaction between HC use experience and testing condition on reproductive health and contraceptive knowledge, F(1, 305) = 2.96, p = 0.086 (see Fig. 4 for interaction effect).
Probing this interaction using Bonferroni simple effects tests revealed that within the women who used the control decision aid, those with previous HC use experience reported more reproductive health and contraceptive knowledge compared to those with no previous HC use experience, p ≤ 0.001. Conversely, for those who used the Tuune® decision aid, prior HC use experience did not impact women’s reproductive health and contraceptive knowledge, p = 0.318.
Discussion
The use of patient decision aids to complement traditional physician appointments has been on the rise in many areas of healthcare (e.g., [5, 6]), including contraceptive counseling. Although there are a number of contraceptive decision aids available (see [11] for a review), none meet all needs, and many have limitations that restrict their use to specific types of settings or to patients looking for specific types of products. The recently developed Tuune® decision aid was designed to address these limitations, offering patients educational content as it relates to both contraceptive options and hormonal disorders, as well as personalized contraception recommendations based on a women’s needs, values, and medical conditions. The present research was designed to examine whether use of the Tuune® decision aid would lead to improvements in women’s reproductive and contraceptive self-efficacy and knowledge, two outcomes that have been shown to predict contraceptive use [20, 21] and continuity of use [22].
The results of the present study revealed that women assigned to use the Tuune® decision aid exhibited greater reproductive health self-efficacy, knowledge about reproductive health and contraception, and perceived having learned more than women assigned to use the control decision aid. Results also revealed that the impact of decision aid type (Tuune® vs. control aid) on women’s knowledge about reproductive health and contraception options was moderated by women’s previous HC use. Specifically, these results revealed that for women using the control decision aid, women with previous HC use experience reported higher levels of reproductive health and contraceptive knowledge than did women without previous HC use experience. However, for women using the Tuune® decision aid, there were no experience-based differences in women’s reproductive health and contraceptive knowledge, suggesting that use of the Tuune® decision aid leveled the playing field, leaving women with and without previous HC use experience equally knowledgeable about their reproductive health and contraceptive options, and more knowledgeable than women who had not used the Tuune® decision aid. Speculatively, these latter results suggest that using the Tuune® decision aid could result in more knowledge about reproductive health and contraception options, even when compared to a clinician visit.
Strengths, limitations, and future directions
While the current research has many strengths, it is not without its limitations. As described in the “Procedures and Materials” section, we did not utilize true random assignment to conditions in the current study, and instead, utilized a blocked assignment design. Despite scheduling for equally-sized blocks, we did not end up with two perfectly equal groups. That said, our experimental and control groups did not statistically differ with regards to age, racial distribution, previous HC use experience, or current HC use, suggesting that our two groups, while not perfectly equal in size, were well matched in key demographics and contraceptive experiences. Additionally, we were able to collect a sample that was racially representative of the United States, indicating that Tuune® is also effective at increasing reproductive knowledge and self-efficacy in minority populations. However, we relied upon a sample of undergraduate students, limiting the generalizability of the current results to college-aged women who have above a high school level of education. While other decision aids [8, 14], which have been designed to be used specifically in adolescent or minority populations, may be more effective within these populations, we have found Tuune® to be broadly effective across all demographic groups within the women who participated in the current study. However, as the women in our sample were all above a high school level of education and familiar with using and receiving information from computers, future research should investigate if Tuune® is equally effective for those without a high degree of technological literacy.
It is difficult to directly determine how Tuune® compares to other contraceptive based decision tools when it comes to increasing self-efficacy and knowledge. This is largely because past trials have relied primarily upon outcome measures that were created for, and are specific to, their own intervention or decision aid. The literature is currently lacking well-validated, standardized measures of reproductive health self-efficacy and knowledge that can be applied broadly to a range of decision aids. Once created, research conducted using such scales will be necessary to make comparisons between different decision aids to determine which aids are most effective, for whom they are most effective, and what the most beneficial features of these aids are for users. Until this time, it is important to use caution when comparing the effectiveness of the Tuune® decision aid to that of others for which similar outcomes have been measured, due to the use of relatively novel scales. Nonetheless, the current work is valuable in providing evidence that the Tuune® decision aid is effective in increasing women’s reproductive self-efficacy and knowledge.
It is also unclear for how long the effects of using decision aids lasts. Manlove and colleagues [26], for example, report that while users of a contraceptive-based decision aid showed increased knowledge compared to control participants after six weeks, that this difference had disappeared by six months. In light of these results, we aim to follow-up with the participants in the current study who consented to be contacted for future research, to determine if women retain knowledge acquired during the use of Tuune® over time.
Beyond utilizing different assessments of outcome measures, past studies have also used different control conditions when evaluating the efficacy of contraceptive-based decision aids (see [11]). While the control condition used in the current study provides a stronger test of Tuune®’s effectiveness than would control conditions in which participants do not use a decision aid at all, or view contraceptive product information only before responding to outcome measures, a control group of women who received contraceptive counseling during a standard clinician visit would have been a stronger test of Tuune®’s effectiveness and we plan to examine this in future studies. However, the use of such a control group in the current study would have presented additional limitations to the generalizability of the results (e.g., each clinician is likely to utilize a different process and give different recommendations during contraceptive counseling). Based on the methodology used in the current study, conclusions cannot be drawn about the relative impact of the Tuune® decision aid compared to a standard physician’s appointment on women’s reproductive and contraceptive self-efficacy and knowledge, although this is an important topic for future research to address.
Additionally, future research is also needed to examine whether use of the Tuune® decision aid increases rates of HC use and whether use of the contraceptives recommended by Tuune® is associated with lowers rates of side effects than is the use of contraceptives recommended in the context of a traditional clinician’s visit. While the Tuune® contraceptive decision aid is well-poised to reduce healthcare burden through its ability to improve patient education in the context of brief appointment times, if Tuune® is effective at increasing contraceptive use rates and decreasing contraceptive side effects, its potential utility to reduce healthcare burden within a traditional healthcare setting should be explored as well. That is, similar to other contraceptive-based decision aid tools which have been designed to be used in a doctor’s waiting room, Tuune® could be a beneficial tool, especially for PCPs seeking assistance in providing the best possible contraceptive recommendations to their patients.
Conclusions
In the current study, we found use of the Tuune® decision aid increased women’s reproductive health self-efficacy, knowledge about reproductive health and contraception, and women’s perceptions of learning, which has the potential to increase contraceptive use and decrease unplanned pregnancies. Tuune® is unique among other contraceptive-based decision aids, as it is designed to be used by diverse patients, both inside and outside of the traditional healthcare system, and it provides personally tailored education and recommendations of contraceptive options to women seeking contraceptive counseling. As such, use of the Tuune® contraceptive-based decision aid tool has the potential to reduce burden on healthcare systems, as well as improve patient outcomes, by empowering and educating women about their reproductive health and contraceptive options. These outcomes should better enable women to work with their PCPs to develop a birth control plan that they feel confident is the right option for them and thereby promote adherence to prescription regimens and successful contraceptive use.
Availability of data and materials
The dataset supporting the conclusions of this article and materials used in this study are available in the Open Science Framework repository, https://osf.io/bnmx8/
References
Grumbach K, Bodenheimer T. A primary care home for Americans. JAMA. 2002;288(7):889.
Linzer M, Bitton A, Tu S-P, Plews-Ogan M, Horowitz KR, Schwartz MD. The end of the 15–20 minute primary care visit. J Gen Intern Med. 2015;30(11):1584–6.
Tai-Seale M, McGuire TG, Zhang W. Time Allocation in Primary Care Office visits. Health Serv Res. 2007;42(5):1871–94.
Geraghty EM, Franks P, Kravitz RL. Primary care visit length, quality, and satisfaction for standardized patients with depression. J Gen Intern Med. 2007;22(12):1641–7.
Ankolekar A, Dekker A, Fijten R, Berlanga A. The benefits and challenges of using patient decision aids to support shared decision making in health care. JCO Clin Cancer Inform. 2018;2:1–10.
Stacey D, Légaré F, Lewis K, Barry MJ, Bennett CL, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;2017(4):CD001431.
Say R, Robson S, Thomson R. Helping pregnant women make better decisions: A systematic review of the benefits of patient decision aids in Obstetrics. BMJ Open. 2011;1(2):e000261.
Tebb KP, Rodriguez F, Pollack LM, Adams S, Rico R, Renteria R, et al. Improving contraceptive use among latina adolescents: A cluster-randomized controlled trial evaluating an mHealth application, health-E you/Salud ITU. Contraception. 2021;104(3):246–53.
Klima C. Unintended pregnancy consequences and solutions for a worldwide problem. J Nurse Midwifery. 1998;43(6):483–91.
Troutman M, Rafique S, Plowden TC. Are higher unintended pregnancy rates among minorities a result of disparate access to contraception? Contracept Reprod Med. 2020;5(1):16.
Goueth RC, Maki KG, Babatunde A, Eden KB, Darney BG. Effects of technology-based Contraceptive Decision AIDS: A systematic review and meta-analysis. Am J Obstet Gynecol. 2022;S0002–9378(22):00526–9.
Frost JJ. Trends in US women’s use of sexual and Reproductive Health Care Services, 1995–2002. Am J Public Health. 2008;98(10):1814–7.
Scholle SH, Chang JC, Harman J, McNeil M. Trends in women’s health services by type of physician seen: Data from the 1985 and 1997–98 NAMCS. Womens Health Issues. 2002;12(4):165–77.
Manlove J, Cook E, Whitfield B, Johnson M, Martínez-García G, Garrido M. Short-term impacts of pulse: an app-based teen pregnancy prevention program for black and latinx women. J Adolesc Health. 2020;66(2):224–32.
Koo HP, Wilson EK, Minnis AM. A computerized family planning counseling aid: a pilot study evaluation of smart choices. Perspect Sex Reprod Health. 2017;49(1):45–53.
Gilliam ML, Martins SL, Bartlett E, Mistretta SQ, Holl JL. Development and testing of an IOS waiting room “app” for contraceptive counseling in a Title X Family Planning Clinic. Am J Obstet Gynecol. 2014;211(5):481.e1-8.
Hebert LE, Hill BJ, Quinn M, Holl JL, Whitaker AK, Gilliam ML. Mobile contraceptive application use in a clinical setting in addition to standard contraceptive counseling: a randomized controlled trial. Contraception. 2018;98(4):281–7.
Sridhar A, Chen A, Forbes ER, Glik D. Mobile application for information on reversible contraception: A randomized controlled trial. Am J Obstet Gynecol. 2015;212(6):774.e1-7.
Wilson EK, Krieger KE, Koo HP, Minnis AM, Treiman K. Feasibility and acceptability of a computer-based tool to improve contraceptive counseling. Contraception. 2014;90(1):72–8.
Frost JJ, Lindberg LD, Finer LB. Young adults’ contraceptive knowledge, norms and attitudes: Associations with risk of unintended pregnancy. Perspect Sex Reprod Health. 2012;44(2):107–16.
Hamidi OP, Deimling T, Lehman E, Weisman C, Chuang C. High self-efficacy is associated with prescription contraceptive use. Womens Health Issues. 2018;28(6):509–13.
Hall KS, Ela E, Zochowski MK, Caldwell A, Moniz M, McAndrew L, et al. “I don’t know enough to feel comfortable using them:” women’s knowledge of and perceived barriers to long-acting reversible contraceptives on a college campus. Contraception. 2016;93(6):556–64.
Faul F, Erdfelder E, Lang AG, Buchner A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–91.
Brooks-Gunn J, Ruble DN. The Menstrual Attitude Questionnaire. Psychosom Med. 1980;42(5):503–12.
Tavousi M, Haeri-Mehrizi A, Rakhshani F, Rafiefar S, Soleymanian A, Sarbandi F, et al. Development and validation of a short and easy-to-use instrument for measuring health literacy: The Health Literacy Instrument for adults (Helia). BMC Public Health. 2020;20(1):656.
Manlove J, Whitfield B, Finocharo J, Cook E. Lessons learned from replicating a randomized control trial evaluation of an app-based Sexual Health Program. Int J Environ Res Public Health. 2021;18(6):3305.
Acknowledgements
We thank the dedicated research assistants who made this work possible.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SM, ME, SB, LJP, ER, SN, and SEH contributed to the conception and design of the work; ER, CR, and SN contributed to the design of the software used in the work and the acquisition of data through the decision aid; SM and ME collected in-person data; ME conducted data analysis; ME, SEH, and SM contributed to the interpretation of the data; SM, ME, SB, and SHE drafted and substantially revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All study procedures were approved by the Texas Christian University Institutional Review Board (#2021–265) and all participants provided informed consent prior to data collection. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
SAB, LJP, ER, CR, and SN are each current or former employees of Uniq Health Ltd, the parent company of Tuune and SEH is a member of their scientific advisory board. SM and ME do not have any competing interest to report. To avoid conflicts of interest, SM and ME collected all data, conducted data analysis, and wrote up the results of all statistical tests reported herein.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mengelkoch, S., Espinosa, M., Butler, S.A. et al. Tuuned in: use of an online contraceptive decision aid for women increases reproductive self-efficacy and knowledge; results of an experimental clinical trial. BMC Digit Health 1, 36 (2023). https://doi.org/10.1186/s44247-023-00034-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s44247-023-00034-z