Abstract
Background
Lung cancer (LC) is the most common solid tumor type in the intensive care unit (ICU). This study investigated the characteristics of LC patients admitted to the ICU, the major reasons for their admission, short-term mortality, and associated risk factors.
Methods
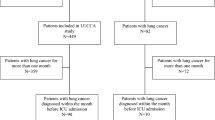
Patients with LC were retrospectively identified in the publicly available, large-scale, single-center database Medical Information Mart for Intensive Care (MIMIC) III. Demographic and clinical characteristics, including age, sex, smoking history, comorbidities, type of admission to ICU, major diagnoses, illness severity score as assessed by the Simplified Acute Physiology Score (SAPS) II and the Sequential Organ Failure Assessment (SOFA), ICU length of stay (LOS), use of mechanic ventilation (MV) or vasopressors, the existence of do-not-resuscitate (DNR) orders, and metastatic status were collected. The major reasons for ICU admission were analyzed in subgroups. The multivariate logistic regression analysis was used to determine the factors associated with the 28-day and 6-month mortality.
Results
A total of 1242 ICU admissions were included. Diseases of respiratory (42.7%), nervous (14.3%), and cardiovascular (11.9%) systems accounted for the top reasons for admission. Pneumonia/pneumonitis, respiratory failure, and sepsis were the primary reasons for ICU admission. The median survival was 2.93 (95% CI: 2.42–3.43) months. The 28-day inhospital and the 6-month mortality were 30.6% and 68.2%, respectively. Sepsis (63.9%), respiratory failure (47.0%), and pleural effusion (40.9%) accounted for the top three highest 28-day ICU mortality in all causes. An age ≥ 65 years, a SAPS II ≥ 37, a SOFA ≥ 3, metastasis, and MV use were independent risk factors for an inferior 28-day survival rate, while only metastatic status and SOFA score were associated with the 6-month mortality. SAPS II was accepatable and better than SOFA in predicting 28-day ICU [area under the curve (AUC): 0.714 and 0.658, respectively] or 28-day inhospital mortality (AUC: 0.717 and 0.660, respectively).
Conclusion
The 6-month prognosis for LC patients admitted to ICU was dismal. Multidisciplinary collaboration between intensivists and oncologists to identify high-risk patients and to determine a risk-benefit ratio of ICU treatment may improve survival prospects.
Similar content being viewed by others
Introduction
Lung cancer (LC) is the leading cause of cancer deaths, accounting for 18% of all cancer deaths worldwide [1]. Cancer patients may be admitted into the intensive care unit (ICU) because of malignancy, treatment-related complications, or exacerbation of underlying comorbidities [2,3,4,5]. LC has been reported as the most common type of solid cancer in all ICU admissions [6, 7]. However, this is a particular population that has been largely neglected. Whether intensive care improves survival and which type of LC patients might benefit from ICU intervention has not been fully elucidated. The prerequisite to addressing these issues is to fully understand the characteristics and prognostic factors for LC associated with ICU admissions. However, evidence on this topic is limited.
A trend of an increasing number of LC patients admitted to ICU was observed during the last two decades, and the incidence of ICU admission among LC patients varied from 1.5 to 31.3% [4, 8,9,10]. There exists a heterogeneity of triage decisions for ICU admissions due to a lack of consensus on admission criteria for cancer patients, which might be the major reason for the varied ICU incidence observed [11]. A previous study demonstrated that the choices of oncologists and intensivists regarding ICU admission and the aggressiveness of life support for cancer patients might differ because of how they judge the situation, oncologists from the perspective of cancer characteristics, and intensivists in terms of multiple organ failure [12]. However, it is not surprising that the number of LC patients admitted to ICU has increased due to recent advances in antitumor therapies, early screening programs, and general ICU management [7, 9, 10, 13]. Furthermore, the spectrum of critical treatment-related side effects has changed with the revolutionized therapies, for example, targeted and immunotherapy, which may also be related to an increased incidence of ICU admission [14].
Previous studies evaluating the characteristics of cancer patients admitted into ICU were mainly from the perspective of cancer treatment. To date, the largest-scale study of 49,373 LC patients based on the Surveillance, Epidemiology, and End Results (SEER)-Medicare registry demonstrated that only 35% of patients admitted to ICU for nonsurgical reasons were alive 6 months after discharge [15]. However, the ability to evaluate individual illness severity and causes of ICU admission for LC patients is limited in the SEER database analysis. Organ failure in the respiratory system is the main reason for ICU admission [4, 8, 16, 17]. Although a few studies revealed multiple risk factors associated with mortality, for example, the need for mechanical ventilation (MV) or vasopressors, poor performance status, metastasis at admission, and organ failures, the heterogeneity of this particular population made prognostic prediction difficult [15,16,17,18,19,20]. For LC patients admitted for postoperative care, the ICU mortality was relatively low. However, for those admitted for nonsurgical reasons, the mortality rate varied from 22 to 85% [4, 15,16,17,18,19, 21].
In the present study, we utilized a publicly accessible critical care database, Medical Information Mart for Intensive Care (MIMIC) III, to comprehensively evaluate the clinical characteristics, major reasons for ICU admission, short-term survival outcomes, and risk factors for LC patients [22]. The predictive performance of Sequential Organ Failure Assessment (SOFA) and Simplified Acute Physiology Score (SAPS) II was also investigated.
Methods
Study design and data source
This retrospective study was based on the MIMIC-III database, which collected data for 46,520 patients admitted to intensive care units at the Beth Israel Deaconess Medical Center (BIDMC) between 2001 and 2012 [22]. The data was available after we completed the online training course at the National Institutes of Health and obtained the certificate (record ID: 33161521). For the process of data mining, the data of patients with malignant neoplasm of respiratory and intrathoracic organs in the MIMIC-III database was the first extracted according to the method previously described [23]. Then, patients with LC were further identified based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes of 1622-1625 and 1628-1629. All patient identifiers in the MIMIC-III database were sealed in accordance with the Health Insurance Portability and Accountability Act (HIPAA) Safe Harbor provision.
Data collection
Demographic and clinical data for patients with LC admitted into the ICU were extracted. Data included age at ICU admission, sex, admission type, type of care unit, diagnosis, and illness severity as assessed by SOFA and SAPS II. Additional information about smoking history (codes: 305.1 or V15.82), the metastatic sites (196-198), and comorbidities, including hypertension (HTN, code: 401-405), hyperlipidemia (272), chronic obstructive pulmonary disease (COPD) and allied condition (490–496), heart failure (HF, 428), diabetes mellitus (DM, 250), and chronic kidney disease (CKD, 585), were all identified using ICD-9 codes. Data regarding ICU management, such as ICU length of stay (LOS), use of MV, prescription of vasopressor, and the existence of do-not-resuscitate (DNR) orders, were also collected. The major reasons for ICU admission were determined by reviewing the first diagnosis other than LC. Survival was analyzed, including the 28-day ICU mortality, 28-day inhospital mortality, and 6-month mortality after ICU admission. ICU admission was counted as individual, different cases, and we included each because the major reasons for admission may be different even for the same patient. There were no missing data for age, sex, admission type, and 28-day mortality. The remaining baseline variables with missing values (less than 5%) were not considered for the main analysis.
Statistical analysis
Descriptive statistics were used to summarize the characteristics of the study population. Continuous variables were presented as means and standard deviations (SD) or median (interquartile) as appropriate, and categorical variables were presented as absolute numbers and percentages. We used the Mann-Whitney test and one-way analysis for continuous variables and the χ2 test or Fisher’s exact test for categorical variables to compare the characteristics. Survival curves were estimated by the Kaplan-Meier method and compared using the log-rank test. Logistic regression analysis was used to identify the risk of mortality. The results of multivariate logistic regression analyses were conducted in a backward stepwise manner for factors in the univariate analyses with P < 0.20. Odds ratios (ORs) and 95% confidence intervals (CIs) were presented. Receiver operating characteristic (ROC) analysis with the calculation of area under the curve (AUC) was used to assess the accuracy of model predictions. Two-sided P-values < 0.05 were considered statistically significant. All statistical analyses were performed with SPSS (version 23.0) and R Statistics (version 4.2.0).
Results
Demographic and clinical characteristics
A total of 1242 ICU admission events were included in this study. The average age of LC patients admitted into the ICU was 68.2 ± 11.6 years, and 62.3% was ≥ 65 years. Male patients accounted for 56.0% of the population. Hypertension (49.8%), COPD (42.6%), hyperlipidemia (29.4%), DM (18.0%), HF (16.7%), and CKD (9.4%) were common comorbidities. The majority of patients (79.3%) were admitted as an emergency or urgently, and nearly half (49.2%) was initially admitted into the medical ICU (MICU). Totally, 655 patients (52.7%) had metastases (Table 1).
The median ICU LOS was 2.2 (1.3–4.7) days. The median SAPS II was 37.0 (30.0–45.0), and the median SOFA was 3.0 (1.0–5.0). Respectively, 36.9% of patients received MV, and 23.4% used vasopressors. The 28-day ICU and inhospital mortality were 31.9% and 30.6%, respectively, while the 6-month mortality was 68.2% (Table 1). During the observation period, 1010 (81.3%) died, and the overall population’s median survival was 2.93 (95% CI: 2.42–3.43) months.
When stratified by the ICU type and age, differences in demographic and clinical characteristics were observed. Specifically, the surgical or trauma/surgical ICU (SICU/TSICU) subgroup tended to be younger, had more smokers, was less severe, and had better short-term survival than the MICU and coronary care unit or cardiac surgery recovery unit (CCU/CSRU) subgroups. Comorbidities of COPD, HF, and CKD were more commonly seen in the MICU subgroup. Almost all patients (96.3%) were admitted to the MICU via the emergency department. Vasopressors were more frequently used in the CCU/CSRU subgroup. When stratified by age, older LC patients ≥ 65 years were more likely to have comorbidities, greater illness severity as assessed by the SAPS II and SOFA, and a poorer 28-day mortality. However, they had fewer tumor metastases than younger LC patients (Supplementary Tables 1 & 2).
Main reasons for ICU admission and clinical outcomes
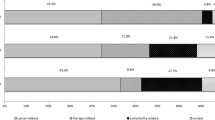
The main reasons for ICU admission were firstly categorized by body systems. The results showed that diseases of the respiratory (42.7%), nervous (14.3%), and cardiovascular (11.9%) systems accounted for the top reasons for ICU admission (Fig. 1A). In more detail, the 10 major reasons for ICU admission other than tumor metastasis were as follows: pneumonia/pneumonitis (13.2%), respiratory failure (12.2%), sepsis (4.9%), obstructive pulmonary disease (3.8%), pulmonary embolism and infarction (2.8%), kidney failure (2.6%), pneumothorax (2.4%), diseases of pericardium (2.2%), arrhythmia (2.1%), and HF (1.9%) (Fig. 1B). The most common metastatic sites for major admission reasons were the brain and spinal cord (9.8%), intrathoracic lymph nodes (3.3%), pleura (1.8%), and bone and bone marrow (1.6%) (Fig. 1C).
Leading first diagnoses for ICU admission and 28-day ICU mortality. A Distribution of first diagnosis categorized by body systems. B Top 15 causes other than secondary malignant neoplasms as the first diagnosis. C The most common metastatic sites as the first diagnosis. D 28-day ICU mortality for diseases of different body systems. E 28-day ICU mortality for leading diseases other than cancer-related metastasis. F 28-day ICU mortality for LC patients with different metastatic sites
When stratified by ICU type, respiratory failure and pnemumonia/pneumonitis were listed in top five diseases in all three types of ICU admissions. Diseases of the pericardium, arrhythmia and cardiac complications, and acute myocardial infarction were the other three primary diseases in the CCU/CSRU subgroup. For the MICU, sepsis, pulmonary embolism and infarction, and obstructive pulmonary diseases ranked in the top five reasons for admission. For SICU/TSICU, brain and spinal cord metastasis, intrathoracic lymph nodes metastasis, and cerebrovascular disease were the other most common first diagnoses (Fig. 2).
In terms of the 28-day ICU mortality rate, systemic infectious diseases (54.0%), digestive diseases (37.7%), and respiratory diseases (36.2%) had the highest mortality rate (Fig. 1D). In more detail, mortality for sepsis (63.9%), respiratory failure (47.0%), and pleural effusion (40.9%) ranked as the top three highest in all diseases (Fig. 1E). Patients with pleural metastasis had the highest 28-day ICU mortality of 63.6% among all metastatic sites (Fig. 1F).
The median overall survival for patients in the MICU (1.30, 95% CI: 0.92–1.68 months) was significantly shorter than that in the SICU (4.24, 2.73–5.76 months) or CCU (4.54, 3.23–5.84 months) (P < 0.001).
Risk factors and prediction models for short-term mortality
Comparison between the 28-day ICU survivors and non-survivors showed that survivors were younger and less likely to enter the MICU via emergency, without metastasis or HF. Not surprisingly, survivors also had fewer DNR orders, less MV and vasopressor use, shorter ICU LOS, and lower SAPS II and SOFA (Table 2). Similar differences were noted between the 28-day inhospital survivors and non-survivors (Supplementary Table 3). When comparing survivors and non-survivors at 6 months, the proportions of smokers, CKD, metastasis, emergency admission, MICU, and DNR were higher in the non-survivors. The non-survivors at 6 months also had greater SAPS II and SOFA and longer LOS than the survivors (Supplementary Table 4). The survival curves within 6 months following ICU admission also showed that patients with CKD, metastasis, nonelective admission, MICU admission, SAPS II ≥ 37, SOFA ≥ 3, and ICU LOS ≥ 2.2 d, MV, and DNR had decreased survival rates (Fig. 3).
The multivariate logistic analysis revealed that elder age (≥ 65 years), SAPS II ≥ 37, SOFA ≥ 3, metastatic status, and MV use were five independent risk factors for an inferior 28-day ICU and inhospital survival (Table 3, Supplementary Table 5). However, only metastatic status and SOFA were associated with the 6-month mortality (Table 4).
ROC analysis showed that SAPS II was better than SOFA in the prediction of 28-day ICU mortality (AUC: 0.714 and 0.658, respectively) or 28-day inhospital mortality (AUC: 0.717 and 0.660, respectively). However, the prediction performance of SOFA for the 6-month mortality was not satisfactory (AUC: 0.591) (Fig. 4).
Discussion
This study was the first to evaluate the clinical features and outcomes of LC patients admitted into an ICU through a large-scale intensive care database MIMIC-III. Nearly one-third of the population admitted to the ICU did not survive hospitalization, and approximately two-thirds did not survive 6 months. Predicting short-term survival based on conventional severity scores and cancer-related factors is still far from accurate. Pneumonia, respiratory failure, and sepsis ranked as the top three major causes of admission, while sepsis, respiratory failure, and pleural effusion had the highest 28-day ICU mortality.
The characteristics, disease spectrum, and hence the prognosis of LC patients admitted to ICU generally differed in the medical, surgical, and cardiac ICUs. Approximately half of the critical care patients were admitted into the MICU, presenting with more emergency episodes and higher proportions of COPD and CKD comorbidities than those in the surgical or cardiac ICUs. In terms of disease spectrum in the MICU group, sepsis was the highest cause of mortality, consistent with previous findings that sepsis was the top ICU admission diagnosis for LC, with a mortality rate of up to 45–60% [19, 24]. The incidence of severe sepsis in patients with LC was previously proved to be nearly 14 times than in the non-cancer population, and LC also had the highest inhospital mortality from severe sepsis of all the solid tumor types [25]. The frequent occurrence of sepsis is not unexpected in LC patients, given the immunocompromised condition from the malignancy itself, the side effects of treatment modalities such as chemotherapy or radiotherapy, and the impairment of normal leukocyte function [26]. Moreover, airway obstruction due to tumor mass may be one important factor associated with the incidence of complicated pneumonia, subsequent sepsis, and respiratory failure, which were also the most common diseases for ICU admission in the current study [27].
Our results that patients in the SICU group had a lowest 28-day mortality rate of around 20% among all ICU types was consistent with previous findings [4]. In this study, one-third of the patients in the surgical ICU were admitted electively, possibly for postoperative complications. In addition, the SICU subgroup was younger, had fewer chronic pulmonary comorbidities, and had lower severity scores at admission than the other two ICUs. All these factors may have contributed to the better outcome for the SICU subgroup. However, when it came to the 6-month survival, mortality still reached 61.5%. It is speculated that the leading major diagnosis of brain metastasis and cerebrovascular diseases in the SICU subgroup may partly explain this non-sustained survival benefit. Many anticancer agents have poor blood-brain and brain-tumor penetrability, leading to an average survival of fewer than 6 months for LC patients with brain metastasis [28].
Cardiovascular disease is often an underestimated issue for LC patients who may suffer from coexisting cardiovascular diseases and, in the course of cancer treatment, may experience various cardiovascular complications [29]. Data from an Austrian center revealed that 67% of non-small cell LC patients had at least one concomitant cardiovascular diseases, and 9.5% had documented cardiovascular complications [30]. Previous data indicated LC patients, irrespective of age and sex, had increased mortality related to cardiovascular diseases, including pericarditis, venous thromboembolic disease, HF, arrhythmias, and ischemic heart disease [31]. In this study population, 20% of LC patients entered the cardiac ICU, and the percentage of vasopressor use was the highest among all ICU types. Diseases of the pericardium, acute myocardial infarction, arrhythmia, and other cardiac complications frequently appeared as the main reasons for cardiac ICU admission. However, survival for this subgroup was better than that of the MICU group and even close to that of the SICU group, which indicated a potentially reversible critical condition caused by cardiac events after intensive care.
Conventional SOFA and SAPS II scores reflecting the severity of organ failures could predict the prognosis of critically ill patients. However, the prognosis of critically ill LC patients dependents on multiple cancer and non-cancer-related factors. Previous studies demonstrated that factors such as age, severity of organ failure, metastatic status of LC, and ventilation application all involved in the prediction of mortality, which is consistent with our findings [15, 32, 33]. In this study, while SAPS II and SOFA were both acceptable in predicting the 28-day mortality with SAPS II to be a superior predictor, the prediction of a 6-month survival was not satisfactory. When incorporating all factors including age, metastasis, and MV derived from the logistic regression model, it showed a slightly better predictive performance for the 28-day mortality (data not shown). However, it is of note that age and metastatic disease are already included in the SAPS II scoring system, and MV is included in the SOFA score. We speculate that these factors may put an extra weight in the prediction model, especially for metastatic status in the prediction of a 6-month mortality.
Whether intensive care improved survival rates for LC patients admitted into ICU is still debatable, although the incidence of cancer patients receiving ICU care is increasing over time [20, 32]. Previous large observational studies show that the percentage of patients with LC admitted to an ICU who survived hospitalization and were alive at 6 months did not improve from 1992 to 2005 [15]. Another recent study also did not observe any continuous improvement in outcomes for LC in ICU from 2007 to 2018 [32]. The current study’s ICU and the 6-month mortality rate in appeared to align with previous studies [32, 34]. Conversely, improvements after intensivist involvement for LC ICU patients were noted in some studies [5, 35]. Notably, the proportions of critical patients with metastatic diseases are increasing over time, which may reduce the survival benefit from intensive care [32]. Triage for LC patients who may benefit from ICU care has not been clearly defined. ICU admission is associated with meaningful survival for patients with good performance status and nonrecurrent/progressive disease [34]. Although cancer-related treatment was not reviewed in this study, we speculate that progressive disease should not be an absolute contradiction for ICU admission nowadays. For treatment-naïve LC patients in a critical condition, previous studies revealed that anticancer therapies, for example, epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) or even chemotherapy for selected patients, could be beneficial [2]. With ICU admissions linked to targeted therapy and immunotherapy increased, the survival benefit from a well-tolerated and efficacious modality is more likely to achieve [32].
The present study has several limitations. First, detailed information on the types of LC, performance status, clinical stage, anticancer therapy in ICU, and their impact on survival were not reviewed due to the retrospective nature of the study. Second, the prediction model was not validated externally. Third, the MIMIC-III dataset only collected data until 2012, which might not fully represent the current conditions of LC admitted into the ICU.
In conclusion, the 6-month prognosis for LC admitted to ICU was still dismal, although approximately one-third of LC patients in ICU survived the first 28 days. Multidisciplinary collaboration is necessary between intensivists and oncologists to identify high-risk patients, establish an early mortality prediction, and consider the risk-benefit ratio of antitumor and intensive treatment. From our findings, the conventional illness severity score is valuable in predicting the 28-day survival but not for the 6-month survival. Since metastasis is an important factor associated with the 6-month survival, ICU intervention in patients without subsequent antitumor therapy combating metastatic progression may have limited value. An early prediction of mortality would guide clinicians to allocate medical resources well and optimize treatment behavior. In addition, respiratory infection and sepsis as the most common LC-related ICU admission reasons warrant further investigation.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Chen YF, Lin JW, Ho CC, et al. Outcomes of cancer therapy administered to treatment-naïve lung cancer patients in the intensive care unit. J Cancer. 2017;8(11):1995–2003.
Lai CC, Ho CH, Chen CM, et al. Risk factors and mortality of adults with lung cancer admitted to the intensive care unit. J Thorac Dis. 2018;10(7):4118–26.
Park J, Kim WJ, Hong JY, et al. Clinical outcomes in patients with lung cancer admitted to intensive care units. Ann Transl Med. 2021;9(10):836.
Soubani AO, Ruckdeschel JC. The outcome of medical intensive care for lung cancer patients: the case for optimism. J Thorac Oncol. 2011;6(3):633–8.
Kress JP, Christenson J, Pohlman AS, et al. Outcomes of critically ill cancer patients in a university hospital setting. Am J Respir Crit Care Med. 1999;160(6):1957–61.
Soares M, Caruso P, Silva E, et al. Characteristics and outcomes of patients with cancer requiring admission to intensive care units: a prospective multicenter study. Crit Care Med. 2010;38(1):9–15.
Puxty K, Grant CH, McLoone P, et al. Factors associated with intensive care admission in patients with lung cancer: a population-based observational study of 26, 731 patients. BMC Pulm Med. 2020;20(1):36.
Sauer CM, Dong J, Celi LA, et al. Improved survival of cancer patients admitted to the intensive care unit between 2002 and 2011 at a U.S. teaching hospital. Cancer Res Treat. 2019;51(3):973–81.
Vigneron C, Charpentier J, Valade S, et al. Patterns of ICU admissions and outcomes in patients with solid malignancies over the revolution of cancer treatment. Ann Intensive Care. 2021;11(1):182.
Koutsoukou A. Admission of critically ill patients with cancer to the ICU: many uncertainties remain. ESMO Open. 2017;2(4):e000105.
Nassar AP Jr, Dettino ALA, Amendola CP, et al. Oncologists' and intensivists' attitudes toward the care of critically ill patients with cancer. J Intensive Care Med. 2019;34(10):811–7.
Taccone FS, Artigas AA, Sprung CL, et al. Characteristics and outcomes of cancer patients in European ICUs. Crit Care. 2009;13(1):R15.
Kroschinsky F, Stölzel F, von Bonin S, et al. New drugs, new toxicities: severe side effects of modern targeted and immunotherapy of cancer and their management. Crit Care. 2017;21(1):89.
Slatore CG, Cecere LM, Letourneau JL, et al. Intensive care unit outcomes among patients with lung cancer in the surveillance, epidemiology, and end results-medicare registry. J Clin Oncol. 2012;30(14):1686–91.
Toffart AC, Minet C, Raynard B, et al. Use of intensive care in patients with nonresectable lung cancer. Chest. 2011;139(1):101–8.
Adam AK, Soubani AO. Outcome and prognostic factors of lung cancer patients admitted to the medical intensive care unit. Eur Respir J. 2008;31(1):47–53.
Roques S, Parrot A, Lavole A, et al. Six-month prognosis of patients with lung cancer admitted to the intensive care unit. Intensive Care Med. 2009;35(12):2044–50.
Soares M, Darmon M, Salluh JIF, et al. Prognosis of lung cancer patients with life-threatening complications. Chest. 2007;131(3):840–6.
Angus DC, Shorr AF, White A, et al. Critical care delivery in the United States: distribution of services and compliance with leapfrog recommendations. Crit Care Med. 2006;34(4):1016–24.
Lin YC, Tsai YH, Huang CC, et al. Outcome of lung cancer patients with acute respiratory failure requiring mechanical ventilation. Respir Med. 2004;98(1):43–51.
Johnson AE, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data. 2016;3:160035.
Kurniati AP, Hall G, Hogg D, et al. Process mining in oncology using the MIMIC-III dataset. J Phys Conf Ser. 2018;971:012008.
Bonomi MR, Smith CB, Mhango G, et al. Outcomes of elderly patients with stage IIIB-IV non-small cell lung cancer admitted to the intensive care unit. Lung Cancer. 2012;77(3):600–4.
Williams MD, Braun LA, Cooper LM, et al. Hospitalized cancer patients with severe sepsis: analysis of incidence, mortality, and associated costs of care. Crit Care. 2004;8(5):R291–8.
Conlon C. Sepsis in immunocompromised hosts. J R Coll Physicians Lond. 2000;34(6):533–6.
Valvani A, Martin A, Devarajan A, et al. Postobstructive pneumonia in lung cancer. Ann Transl Med. 2019;7(15):357.
Healey N. Better treatments for lung cancer that spreads to the brain. Nature. 2020;587(7834):S14–s15.
Zaborowska-Szmit M, Krzakowski M, Kowalski DM, et al. Cardiovascular complications of systemic therapy in non-small-cell lung cancer. J Clin Med. 2020;9(5):1268.
Kocher F, Fiegl M, Mian M, et al. Cardiovascular comorbidities and events in NSCLC: often underestimated but worth considering. Clin Lung Cancer. 2015;16(4):305–12.
Strongman H, Gadd S, Matthews A, et al. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: a population-based cohort study using multiple linked UK electronic health records databases. Lancet. 2019;394(10203):1041–54.
Vigneron C, Charpentier J, Wislez M, et al. Short-term and long-term outcomes of patients with lung cancer and life-threatening complications. Chest. 2021;160(4):1560–4.
Kim YJ, Kim MJ, Cho YJ, et al. Who should be admitted to the intensive care unit? The outcome of intensive care unit admission in stage IIIB-IV lung cancer patients. Med Oncol. 2014;31(3):847.
Soares M, Toffart AC, Timsit JF, et al. Intensive care in patients with lung cancer: a multinational study. Ann Oncol. 2014;25(9):1829–35.
Song JH, Kim S, Lee HW, et al. Effect of intensivist involvement on clinical outcomes in patients with advanced lung cancer admitted to the intensive care unit. PLoS One. 2019;14(2):e0210951.
Acknowledgements
None.
Funding
This study was funded by the nurture projects for research of Shanghai Chest Hospital (No. 2020YNJCM12).
Author information
Authors and Affiliations
Contributions
Conceptualization, JQ; data curation, JQ and LH; formal analysis, JQ, RQ, and YS; funding acquisition, JQ; investigation, RQ and LH; methodology, LH, YS, HY, and WN; software, LH, YS, HY, and BZ; supervision, JQ and BH; validation, BZ and WN; writing — original draft, JQ and RQ; and writing — review and editing, YL and BH. The authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Consent for publications
All authors agreed to this publication.
Ethics approval and consent to participate
The study was approved by the ethics committee of the hospital. Patient informed consent was waived by the ethics committee because the database was de-identified and publicly available. The study protocol conformed to the Helsinki Declaration.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table 1.
Patients’ characteristics and clinical outcomes categorized by the ICU type. Supplementary Table 2. Patients’ characteristics and clinical outcomes categorized by age group. Supplementary Table 3. Comparison of in-hospital 28-day survivors and non-survivors. Supplementary Table 4. Comparison of 6-month survivors and non-survivors. Supplementary Table 5. Univariable and multivariable logistic regression analyses for the 28-day in-hospital mortality.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qian, J., Qin, R., Hong, L. et al. Characteristics and clinical outcomes of patients with lung cancer requiring ICU admission: a retrospective analysis based on the MIMIC-III database. Emerg Cancer Care 2, 1 (2023). https://doi.org/10.1186/s44201-022-00017-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s44201-022-00017-2