Abstract
Background
Pectus excavatum (PE), a congenital deformity of the chest wall, can lead to cardiac compression and related symptoms. PE surgical repair can improve cardiac function. Intraoperative transesophageal echocardiography (TEE) has been successfully employed to assess intraoperative hemodynamic variations in patients undergoing PE repair. FloTrac/Vigileo™ system (Edwards Life-sciences Irvine, CA) (FT/V) is a minimally invasive cardiac output monitoring system. This retrospective study aimed to assess hemodynamic changes in surgical repair of PE using FT/V and concordance with parameters measured by TEE.
Results
N=19 patients submitted to PE repair via Ravitch or Nuss technique were enrolled. Intraoperative cardiac assessments simultaneously obtained via TEE and FT/V system were investigated. The agreement between TEE-derived cardiac output (CO-TEE) and FT/V system parameter (COAP) was evaluated. The relationship between COTEE and COAP was analyzed for all data using linear regression analysis. A significant correlation between COAP and COTEE values (R = 0.65, p < 0.001) was found. Bland-Altman analysis of COAP and COTEE showed a bias of 0.13 L/min and a limit of agreement of − 2.33 to 2.58 L/min, with a percentage error of 48%. Intraoperative measurements by TEE and FT/V both showed a significant increase in CO after surgical correction of PE (p < 0.005).
Conclusions
FT/V system compared to TEE in hemodynamic monitoring during PE surgery yielded clinically unacceptable results due to a high percentage error. After surgical correction of PE, CO, measured by TEE and FT/V, significantly improved.
Similar content being viewed by others
Introduction
Pectus excavatum (PE) is a congenital abnormality characterized by a depression of the anterior chest wall as a result of dorsal deviation of the sternum and of the third to seventh rib or costal cartilage. Pectus excavatum affects approximately 1 in 400 children. Males are afflicted approximately four times more often than females [1]. The two main surgical techniques for PE correction are the modified Ravitch procedure and the Nuss procedure [2], also called minimally invasive repair of PE (MIRPE). The etiology of PE remains unknown. Currently, the underlying pathogenesis of PE is thought to involve overgrowth in the costochondral region of the ribs [3]. Clinical presentation of PE is various: patients can be asymptomatic or affected by exercise limitations or by pain. Most patients are worried about their physical appearance and undergo surgery for esthetic reasons [4].
The cardiopulmonary consequences of these deformities have been widely debated. Specifically, PE is characterized by a reduction of the sterno-vertebral distance and a leftward displacement and rotation of the heart with compression of the right chambers, thus resulting in limitation of diastolic filling and stroke volume [5, 6]. Patients can have documented cardiac compression [7, 8], and almost 80% can show related symptoms [9]. PE surgical repair showed in both adult and pediatric patients an improvement in exercise cardiopulmonary function and exercise tolerance [10,11,12].
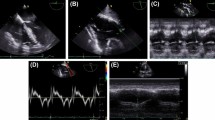
Intraoperative transesophageal echocardiography (TEE) has been successfully employed to assess cardiac function improvements in patients undergoing PE repair [13,14,15]. TEE allows an accurate analysis, overcoming transthoracic echocardiography limitations due to the abnormal anatomy of the deformed anterior chest wall. TEE requires an adequately trained operator.
FloTrac/Vigileo™ system (Edwards Life-sciences Irvine, CA) (FT/V) is a minimally invasive cardiac output monitoring system useful in a wide variety of medical situations and allows continuous assessment of cardiac output (CO), stroke volume (SV), and stroke volume variation (SVV). At present, no data regarding the use of intraoperative monitoring via fourth-generation FloTrac/Vigileo™ system (FT/V) to assess hemodynamic changes in surgical repair of PE have been reported.
The first aim of this study was to compare intraoperative cardiac output (CO) measurements acquired using the FT/V system by arterial pressure waveform (COAP) and CO measured by transesophageal echocardiography (COTEE) during PE surgery repair. Secondary aims were measurements and assessment of CO changes resulting from the surgical repair.
Methods
This retrospective study, was approved by the Bioethics Committee of Sapienza University of Rome (no. 6181_2020). All patients submitted to PE repair via modified Ravitch or Nuss technique from January to October 2020 were included in the study. Demographic data, medical history, comorbidities, Haller Index (derived from dividing the transverse diameter of the chest by the anterior-posterior diameter on a simple computerized tomography scan) [16], operative time, surgical approach (Ravitch or Nuss technique), and intraoperative data were collected from the electronic medical record system.
As a standard in this institution, patients received an intraoperative cardiac assessment simultaneously with TEE and minimally invasive monitoring via the FT/V system. Written informed consent was obtained from the patients.
Before anesthesia induction, a 20-G cannula was inserted into the radial artery and then connected to the FloTrac pressure transducer that preprocesses and sends a signal to both the cardiovascular monitor (for real-time waveform display) and to the Vigileo monitor. The FT/V algorithm of hemodynamic calculation has been described in detail [17]. This system samples the arterial waveform at 100 times per second (100 Hz) and calculates pressure wave standard deviation (SD) over a 20-s interval. The system calculates the arterial pressure using arterial pulsatility, resistance, and patient-specific vascular compliance determined from an internal demographic data base (age, sex, height, and weight) and mean arterial pressure using a conversion factor “χ.” This factor corresponds to the vascular tone and is calculated through a multivariate polynomial function including pulse rate, body surface area, aortic compliance, mean arterial pressure, skewness, and kurtosis of the arterial pressure. CO was recorded by FT/V before and after PE surgical correction.
The transesophageal probe was inserted after tracheal intubation and the cardiac examination was conducted by an expert operator with an Esaote TM MyLabTM 30 GOLD—CardioVascular (Esaote Italia, Firenze, Italia) before and after surgical correction of the deformity. The preoperative and postoperative TEE measurements were made in apnea by disconnecting the patient from the ventilator. TEE parameters were analyzed and recorded before and after PE surgical correction: right ventricular outflow tract distal diameter (RVOT), left ventricular outflow tract diameter (LVOT), velocity-time integral (VTI) of left ventricular outflow tract systolic flow. All measurements were made according to the recommendations for echocardiographic quantification published by the American Society of Echocardiography [18]:
RVOT: This parameter was measured in midesophageal right ventricular inflow outflow view, transducer angle 20° to 70°. The RVOT diameter was measured 0.5 to 1.0 cm under pulmonary valve at end-diastole and end-systole.
LVOT: This parameter was measured in midesophageal left ventricular outflow tract view, transducer angle 120° to 140°, as the diameter of the left ventricular outflow tract 0.5-1 cm under aortic valve in systole.
LVOT VTI: Pulsed Doppler TEE was performed using a deep trans-gastric approach in order to obtain a deep long-axis view of the left ventricle and placing the sample volume just under the aortic valve, taking care to obtain a smooth Doppler profile, calculating the velocity time integral of the Doppler signal directed across LVOT.
The LVOT area was calculated from the diameter assuming a circular geometry. LVOT VTI was used to estimate SV as it corresponds to the column of blood that moves through the LV outflow tract during each systole. Cardiac output was calculated from the left ventricular outflow tract area, the velocity-time integral of the blood flow profile, and heart rate.
Stroke Volume (SV) = LVOT VTI × cross-sectional area of the left ventricular outflow tract
CO: SV multiplied by heart rate.
Statistical analysis
All results were expressed as means ± SD. The relationship between COTEE and COAP was analyzed for all data using linear regression analysis. The agreement between COTEE and COAP values was assessed by Bland-Altman analysis [19]. Bias (mean difference between COTEE and COAP) represents the systemic error between the 2 methods. Precision (SD of the bias) represents the random error or variability between the different techniques. The limits of agreement, calculated as bias ±2 SD, define the range in which 95% of the differences between the methods are expected to lie. The percentage error was calculated as the ratio of 2 SD of the bias to the mean CO; this value was considered clinically acceptable if below 30% [20]. The t test for paired observation was used to compare the measured values in each patient before and after the surgical correction. P values < 0.05 were considered significant. Data were analyzed using the SPSS v25.0 software (SPSS Inc., Chicago, IL, USA).
Results
Nineteen patients (4 women and 15 men) were included in the study. 38 acceptable pairs of measurements of cardiac output were analyzed: a pre-correction and a post-repair measurement for each patient obtained via TEE and FT/V. Preoperative reported electrocardiography alterations were: right bundle branch block in four patients, incomplete right bundle branch block in five patients, right atrial enlargement in one patient, left axis deviation in one patient, right axis deviation in three patients, left posterior fascicular block in two patients and left ventricular hypertrophy in one patient. No patient was affected by valvular disease. Patients received a moderate intravenous fluid administration (5–6 mL/kg/h), and no significant variations in fluid balance were reported.
The demographic data are represented in Table 1.
Analysis of the overall relationship between COTEE and COAP (Fig. 1) showed a significant correlation between COTEE and COAP (R = 0.65, p < 0.001). The Bland-Altman plot displays the limits of agreement between COTEE and COAP (Fig. 2), revealing a bias of 0.13 L/min and a limit of agreement of − 2.33 to 2.58 L/min. The percentage error of all data was 48%. Comparisons of CO determinations were made are shown in Table 2.
The mean values of the COTEE and COAP before and after surgical correction of PE are summarized in Table 3. The mean values of RVOT before and after correction were 20.21±5.15 and 24.73±4.05, p=0.001.
Discussion
The main findings of this study indicate that overall COAP yielded clinically unacceptable results during PE repair surgery, although they showed a significant correlation with COTEE. The mean values of the COTEE and COAP significantly improved after surgical correction of PE.
The “gold standard” method for measuring CO in the clinical setting is considered thermodilution using a pulmonary artery catheter (PAC) [21,22,23]. However, because the risk of PAC insertion-related risks cannot be justified in non-cardiac surgery, less invasive methods are commonly employed.
Pulse contour devices showed to be less dependable compared to Doppler-derived CO because they miss compensating for circulatory modifications in peripheral resistance. FT/V showed greater bias in both low and high systemic vascular resistance (SVR) states compared to thermodilution [20]. Despite the fourth-generation algorithm can adjust for acute modifications in SVR, its precision, accuracy, and trending ability are still clinically unacceptable [24].
The estimations made with FT/V showed a considerable bias and a wide range of levels of agreement compared to PAC [25,26,27,28]. Form analysis of arterial wave algorithms is founded on features of the arterial system, such as impedance, peripheral vascular resistance, and compliance. Aortic impedance is necessary to calculate SV and varies considerably from patient to patient. These interindividual discrepancies in aortic impedance may participate to inaccuracies in calculating the CO when calibration is founded only upon demographic data.
When repeated hemodynamic variations occur, like during surgery for PE repair, the k value may be delineated at a time in which vascular peripheral resistance, arterial compliance, or impedance may not be the same as the moment of estimation [29]. The results of this study, are according to previous reports comparing FT/V and TEE, showing that COAP values measured by the Flotrac/Vigileo system were not clinically acceptable [28,29,30,31]. Concha et al. during laparoscopic colon surgery reported considerable variations between CO measurements obtained with TEE and FT/V; bias was 1.17 and limits of agreement − 2.02 and 4.37, and the percentage error was 40% [28]. During surgery for abdominal aortic aneurysm, COAP assessments demonstrated to be not clinically acceptable because of extensive changes during aortic clamping and declamping (bias of 0.12 L/min and limits of agreement 1.66 to 1.90 L/min, with a percentage error of 41%) [30].
Although FT/V has failed to show that it is comparable to TEE for cardiac monitoring during surgical repair of PE, this tool can be helpful for intraoperative management. The use of this minimally invasive system can allow even less experienced personnel to monitor cardiac changes during PE surgical correction. It was also shown that hemodynamic measures vary significantly and almost instantaneously following surgical decompression, and the FT/V system has the advantage to allow continuous monitoring of these parameters. During PE surgery, major complications can occur. They include bleeding due to possible perforation of the heart and large vessels, bleeding of the chest wall, right ventricular compression, hypotension, and arrhythmias, which can be promptly identified and treated thanks to the aid of continuous hemodynamic monitoring [32].
This study has some limitations. Although thermodilution was not used as a gold standard reference because it would require a PAC, which would represent an additional unjustifiable risk in these patients, CO measured by TEE was previously reported as clinically acceptable and is a well-validated tool in reporting hemodynamic changes in PE repair surgery [33]. In addition, COTEE takes some minutes to calculate CO. As a result, CO measurements by FT/V and TEE were not exactly reported at the same timepoint. Another limit is that TEE is an operator-dependent technique. However, when a good determination of the aortic valve area and proper alignment of the ultrasound beam and the LVOT are obtained, there is an agreement between TEE and PAC [33]. An additional possible limitation of the study is that the results could have been conditioned by outlier data because we investigated CO estimations obtained by only 19 patients. Finally, the limit of the retrospective nature of this study could be overcome with future prospective trials.
In conclusion, the FT/V system compared to TEE in hemodynamic monitoring during PE surgery was not clinically acceptable due to a high percentage error. Nevertheless, FT/V was able to monitor and detect an intraoperative increase of hemodynamic parameters after correction.
Availability of data and materials
The data that support the findings of this study are available upon reasonable request.
Abbreviations
- CO:
-
Cardiac output
- COAP:
-
Cardiac output measured by FloTrac/Vigileo™ system by arterial pressure waveform
- COTEE:
-
Cardiac output measured by transesophageal echocardiography
- FT/V:
-
FloTrac/Vigileo™ system
- LVOT:
-
Left ventricular outflow tract diameter
- MIRPE:
-
Minimally invasive repair of pectus excavatum
- PAC:
-
Pulmonary artery catheter
- PE:
-
Pectus excavatum
- RVOT:
-
Right ventricular outflow tract distal diameter
- SD:
-
Standard deviation
- SV:
-
Stroke volume
- SVR:
-
Systemic vascular resistance
- SVV:
-
Stroke volume variation
- TEE:
-
Transesophageal echocardiography
- VTI:
-
Velocity-time integral
References
Fonkalsrud EW (2003) Current management of pectus excavatum. World J Surg 27(5):502–508. https://doi.org/10.1007/s00268-003-7025-5
Frantz FW (2011) Indications and guidelines for pectus excavatum repair. Curr Opin Pediatr 23(4):486–491. https://doi.org/10.1097/MOP.0b013e32834881c4
Dean C, Etienne D, Hindson D, Matusz P, Shane Tubbs R, Loukas M (2012) Pectus excavatum (funnel chest): A historical and current prospective. Surg Radiol Anat 34(7):573–579. https://doi.org/10.1007/s00276-012-0938-7
Huddleston CB (2004) Pectus excavatum. Semin Thorac Cardiovasc Surg 16(3):225–232. https://doi.org/10.1053/j.semtcvs.2004.08.003
Töpper A, Polleichtner S, Zagrosek A, Prothmann M, Traber J, Schwenke C, von Knobelsdorff-Brenkenhoff F, Schaarschmidt K, Schulz-Menger J (2016) Impact of surgical correction of pectus excavatum on cardiac function: Insights on the right ventricle. A cardiovascular magnetic resonance study. Interact Cardiovasc Thorac Surg 22(1):38–46. https://doi.org/10.1093/icvts/ivv286
Jaroszewski DE (2017) Physiologic implications of pectus excavatum. J Thorac Cardiovasc Surg 153(1):218–219. https://doi.org/10.1016/j.jtcvs.2016.09.045
Abu-Tair T, Turial S, Hess M, Wiethoff CM, Staatz G, Lollert A, Kampmann C (2018) Impact of Pectus Excavatum on Cardiopulmonary Function. Ann Thorac Surg 105(2):455–460. https://doi.org/10.1016/j.athoracsur.2017.09.037
Lollert A, Emrich T, Eichstädt J, Kampmann C, Abu-Tair T, Turial S, Düber C, Kreitner KF, Staatz G (2018) Differences in myocardial strain between pectus excavatum patients and healthy subjects assessed by cardiac MRI: a pilot study. Eur Radiol 28(3):1276–1284. https://doi.org/10.1007/s00330-017-5042-2
Jaroszewski DE, Ewais MM, Chao C et al (2016) Success of Minimally Invasive Pectus Excavatum Procedures ( Modified Nuss ) in in Adult Patients (≥30 Years). Ann Thorac Surg 102(3):993–1003. https://doi.org/10.1016/j.athoracsur.2016.03.105
Neviere R, Montaigne D, Benhamed L, Catto M, Edme JL, Matran R, Wurtz A (2011) Cardiopulmonary response following surgical repair of pectus excavatum in adult patients. Eur J Cardio-thoracic Surg 40:77–82. https://doi.org/10.1016/j.ejcts.2011.03.045
Keefe JO, Byrne R, Montgomery M et al (2013) Longer term effects of closed repair of pectus excavatum on cardiopulmonary status. J Pediatr Surg 48(5):1049–1054. https://doi.org/10.1016/j.jpedsurg.2013.02.024
Quigley PM, Hailer JA, Jelus KL, Loughlin GM (1996) Cardiorespiratory function before and after corrective surgery in pectus excavatum. J Pediatr 128(5):638–643. https://doi.org/10.1016/S0022-3476(96)80128-4
Krueger T, Chassot P, Christodoulou M et al (2010) Cardiac Function Assessed by Transesophageal Echocardiography During Pectus Excavatum Repair. Ann Thorac Surg 89(1):240–243. https://doi.org/10.1016/j.athoracsur.2009.06.126
Chao C, Jaroszewski DE et al (2015) Surgical repair of pectus excavatum relieves right heart chamber compression and improves cardiac output in adult patients d an intraoperative transesophageal echocardiographic study. Am J Surg 210(6):1118–1125. https://doi.org/10.1016/j.amjsurg.2015.07.006
Chao C, Jaroszewski D, Gotway M et al (2018) Effects of Pectus Excavatum Repair on Right and Left Ventricular Strain. Ann Thorac Surg 105(1):294–301. https://doi.org/10.1016/j.athoracsur.2017.08.017
Haller JA Jr, Kramer SS, Lietman S (1987) Use of CT scans in selection of patients for pectus excavatum surgery: a preliminary report. J Pediatr Surg 22(10):904–906. https://doi.org/10.1016/S0022-3468(87)80585-7
Grensemann J (2018) Cardiac Output Monitoring by Pulse Contour Analysis , the Technical Basics of Less-invasive Techniques. Front Med (Lausanne) 5:1–6. https://doi.org/10.3389/fmed.2018.00064
Hahn RT, Abraham T, Adams MS, Bruce CJ, Glas KE, Lang RM, Reeves ST, Shanewise JS, Siu SC, Stewart W, Picard MH, American Society of Echocardiography, Society of Cardiovascular Anesthesiologists (2014) Guidelines for Performing a Comprehensive Transesophageal Echocardiographic Examination : Recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. Anesth Analg 118(1):1–68. https://doi.org/10.1213/ANE.0000000000000016
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet (London, England) 1:307–310
Critchley LA, Critchley JA (1999) A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J Clin Monit Comput 15(2):85–91. https://doi.org/10.1023/a:1009982611386
Swan HJ, Ganz W, Forrester J et al (1970) Catheterization of the heart in man with use of a flow-directed balloon-tipped catheter. N Engl J Med 283(9):447–451. https://doi.org/10.1056/NEJM197008272830902
Branthwaite MA, Bradley RD (1968) Measurement of cardiac output by thermal dilution in man. J Appl Physiol 24(3):434–438. https://doi.org/10.1152/jappl.1968.24.3.434
Gómez CM, Palazzo MG (1998) Pulmonary artery catheterization in anaesthesia and intensive care. Br J Anaesth 81(6):945–956. https://doi.org/10.1093/bja/81.6.945
Kusaka Y, Ohchi F, Minami T (2019) Evaluation of the Fourth-Generation FloTrac/Vigileo System in Comparison With the Intermittent Bolus Thermodilution Method in Patients Undergoing Cardiac Surgery. J Cardiothorac Vasc Anesth 33(4):953–960. https://doi.org/10.1053/j.jvca.2018.06.017
Opdam HI, Wan L, Bellomo R (2007) A pilot assessment of the FloTrac cardiac output monitoring system. Intensive Care Med 33(2):344–349. https://doi.org/10.1007/s00134-006-0410-4
Sander M, Spies CD, Grubitzsch H, Foer A, Müller M, von Heymann C (2006) Comparison of uncalibrated arterial waveform analysis in cardiac surgery patients with thermodilution cardiac output measurements. Crit Care 10(6):R164. https://doi.org/10.1186/cc5103
Biais M, Nouette-Gaulain K, Cottenceau V et al (2008) Cardiac output measurement in patients undergoing liver transplantation: pulmonary artery catheter versus uncalibrated arterial pressure waveform analysis. Anesth Analg 106(5):1480–1486. https://doi.org/10.1213/ane.0b013e318168b309
Concha MR, Mertz VF, Cortínez LI, González KA, Butte JM (2009) Pulse contour analysis and transesophageal echocardiography: a comparison of measurements of cardiac output during laparoscopic colon surgery. Anesth Analg 109(1):114–118. https://doi.org/10.1213/ane.0b013e3181a491b8
Meng L, Tran NP, Alexander BS et al (2011) The impact of phenylephrine, ephedrine, and increased preload on third-generation Vigileo-FloTrac and esophageal doppler cardiac output measurements. Anesth Analg 113(4):751–757. https://doi.org/10.1213/ANE.0b013e31822649fb
Kusaka Y, Yoshitani K, Irie T, Inatomi Y, Shinzawa M, Ohnishi Y (2012) Clinical comparison of an echocardiograph-derived versus pulse counter-derived cardiac output measurement in abdominal aortic aneurysm surgery. J Cardiothorac Vasc Anesth 26(2):223–226. https://doi.org/10.1053/j.jvca.2011.07.011
Phan TD, Kluger R, Wan C (2016) Minimally invasive cardiac output monitoring: agreement of oesophageal Doppler, LiDCOrapidTM and Vigileo FloTracTM monitors in non-cardiac surgery. Anaesth Intensive Care 44(3):382–390. https://doi.org/10.1177/0310057X1604400313
Mavi J, Moore D (2014) Anesthesia and analgesia for pectus excavatum surgery. Anesth Clin Mar 32(1):75–84. https://doi.org/10.1016/j.anclin.2013.10.006
Darmon PL, Hillel Z, Mogtader A, Mindich B, Thys D (1994) Cardiac output by transesophageal echocardiography using continuous-wave Doppler across the aortic valve. Anesthesiology 80(4):796–805; discussion 25A. https://doi.org/10.1097/00000542-199404000-00011
Funding
The author received no specific funding for this article.
Author information
Authors and Affiliations
Contributions
Domenico Massullo, Silvia Fiorelli: conceptualization, methodology, and writing—original draft preparation. Gelsomina Capua, Cecilia Menna: data curation and writing—original draft preparation. Elisabetta Giorni, Ettore Riva, Elisabetta Agostini, Fabrizio D’Andrea, Elisa Massullo: visualization and investigation. Monica Rocco, Claudio Andreetti: supervision. Valentina Peritore: software and validation. Silvia Fiorelli, Monica Rocco, Cecilia Menna: writing—reviewing and editing. The authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Bioethics Committee of Sapienza University of Rome (RIF.6181_2020)
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fiorelli, S., Capua, G., Menna, C. et al. Intraoperative cardiac function assessment by transesophageal echocardiography versus FloTrac/Vigileo™ system during pectus excavatum surgical repair. J Anesth Analg Crit Care 1, 21 (2021). https://doi.org/10.1186/s44158-021-00025-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s44158-021-00025-4