Abstract
Micro- and nanoplastics (MNPs) are ubiquitous environmental pollutants representing a concern for human health. MNPs have been detected in human placentas, indicating that during pregnancy maternal exposure may lead to placental transfer and foetal exposure, with potential for adverse effects on early-life development. However, a comprehensive risk assessment (RA) framework, specific to early-life is lacking. Here, we propose a novel roadmap to assist the development of an early-life health RA of MNPs. This roadmap is designed based on established chemical, mixture, particle, and MNP assessment strategies aligned with standard RA components (problem formulation, hazard identification, hazard characterisation, exposure assessment, risk characterisation). We systematically work through these stages to identify what is needed to progress a RA for the early-life impacts of MNPs, including what information is missing, and what may be used in the interim. While challenges such as complex physicochemical properties of MNPs, limited toxicity data at relevant exposure levels, and uncertainties related to characterising complex exposures have been described elsewhere, our work discusses how these challenges specifically impact early-life stages such as the significance of MNP presence in biological samples and factors influencing bioaccumulation and placental transfer. Additionally, we introduce the development of new technology readiness levels for methods used in the detection of MNPs in complex matrices. Importantly, this review integrates a broad scope of relevant information into one comprehensive document, providing a unified resource. We highlight specific requirements and areas for targeted research, including the development of dose-response relationships specific to early-life stages and novel strategies for assessing bioaccumulation and placental transfer of MNPs. By addressing these gaps, our roadmap aims to advance the development of a robust framework, ultimately enhancing the understanding and mitigation of risks associated with early-life exposure to MNPs.
Similar content being viewed by others
Introduction
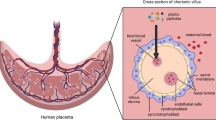
Micro- and nanoplastics (MNPs) are widespread environmental pollutants to which humans are unavoidably exposed through air, food and water [1]. Microplastics (MPs) can be defined as particles smaller than 5 mm and nanoplastics (NPs) less than 1 μm [2, 3], and can be further categorised as primary MNPs, purposefully manufactured, and secondary which result from environmental degradation of larger plastic debris [1, 4]. Although research on the effects of MNPs on human health is still in its infancy, there is emerging evidence that exposure to MNPs may pose a health risk [1, 5, 6]. Increasingly, there are concerns that maternal exposure to MNPs during pregnancy results in foetal exposure [2, 3], which holds significance as the developing foetus and children exhibit greater vulnerability than adults due to rapid growth and development [7]. MNPs have been detected in the human placenta [8,9,10,11,12], and in vivo and in vitro studies indicate that exposures may lead to placental dysfunction and foetal damage [2, 3, 13].
There has been extensive development in the field of human-health risk assessment (RA) of MNPs, including recent publications from the World Health Organization (WHO) addressing implications of dietary and inhalation exposure of humans to MNPs [1], as well as the identification of four clear paradigms that need to be addressed for adequate assessment of human health risks of MNPs [2]. However, there is an urgent need to specifically assess the potential health risks of MNP exposure for the developing foetus and resulting child (collectively referred to from here-on to as early-life), as it is believed that the lifelong health of a person can be critically affected through in utero exposures [14]. However due to limited hazard and exposure data, the impact of MNP exposure on early-life remain largely unexplored. We build upon the existing work on MNP RA to provide a roadmap to support development of a comprehensive RA framework specifically addressing early-life health, including outlining the factors which need to be considered for early-life RA and identifying knowledge gaps that need to be addressed. We have focused on reproductive toxicity, in particular developmental effects, of MNPs during early-life stages, and incorporated a structured methodological approach that systematically addresses all stages of a traditional RA, identifying specific research areas crucial for closing existing knowledge gaps. For pragmatic classification purposes and focus on early-life health, the development of the RA roadmap primarily uses examples of developmental toxicity. This is defined as adverse effects induced during pregnancy that may manifest, for example, as structural abnormalities, altered growth, and functional impairments in the developing foetus or resulting child [15]. Adverse impacts may be observed at cellular and molecular levels, and a comprehensive RA should try to integrate clinical, molecular, and inflammatory markers to evaluate potential effects on early-life health. Our approach builds upon established strategies used in RA for chemicals, plastics, mixtures, and particles in general, and MNPs specifically. The document is organised into sections covering the key components involved in developing the framework. The methods section outlines the development process for the roadmap; we then methodically work through the recognised stages of RA: problem formulation, hazard identification, hazard characterisation, exposure assessment and risk characterisation. Through the development of a roadmap to achieve a RA we outline key research areas crucial for closing knowledge gaps and ultimately establish a robust framework for the RA of MNPs affecting early-life health.
Methods
The development of the roadmap was facilitated by an evidence mapping exercise of the existing RA approaches to MNPs and included a literature review to identify the knowledge regarding exposure and hazards associated with MNPs, identify knowledge gaps and outline key research areas crucial for addressing and closing these. Existing standards and guidance for MNP-specific RA approaches were identified and screened. Based on this screening, missing elements required for development of the roadmap were identified. To fill these gaps general RA approaches for chemicals, mixtures, and nanomaterials (NMs) were identified and assessed for their potential to be adopted or adapted for MNPs. Key resources used can be found in Supplementary Materials (SM) Table S1.1.
PubMed [16] and Web of Science [17] were queried to identify the potentially relevant peer-reviewed literature. We searched review articles and primary research papers that identified MNP hazards and evidence of maternal exposure, respectively. No publishing date limit was used, and the searches were originally conducted between January and March 2022 to allow drafting of the RA strategy, with further supporting literature subsequently obtained during strategy development; an updated search was also performed in May 2024 to ensure most recent advances were accounted for. The search terms used are detailed in SM Tables S1.2-1.5. RA methods require reasonably robust datasets on exposure routes and concentrations to characterise risk. However, due to knowledge gaps associated with MNPs, current RAs provide insufficient guidance to inform exposure characterisation of MNPs at this stage. To identify information needed for exposure assessments of MNPs, an additional literature screening exercise was undertaken focused on identifying studies reporting evidence of MNPs in biological systems, factors influencing MNP exposure, and any useful data for exposure quantification. The search terms used for this are described in the SM.
Approach: a roadmap towards risk assessment
The methods undertaken enabled identification of data gaps regarding exposure and hazards associated with MNPs. This knowledge gap analysis then allowed formulation of a proposed framework for a RA strategy, extensively informed by the World Health Organisation (WHO) “Human Health Risk Assessment Toolkit: Chemical Hazards” [18] and the International Council of Chemical Associations (ICCA) “Guidance on Chemical Risk Assessment” [19]. The literature review provided contextual information to inform the subsequent sections. The proposed adaptable framework accounts for current data deficiencies while presenting a roadmap for advancing the MNP risk analysis as new evidence emerges.
Problem formulation
Problem formulation, as outlined by the WHO [18], defines the objectives, approach and scope of the HHRA, including the risk management goals and acceptable uncertainty that will drive the analysis (Fig. 1).
Risk assessment objectives
The HHRA aims to estimate the risk of potential developmental and reproductive toxicity and health effects of MNPs during early-life.
Statement of the problem
MNPs have distinct properties when compared to chemicals, including morphology, small particle size, large surface area, and potential to act as vectors for microorganisms and environmental pollutants [20]. MNPs can also contain a variety of chemicals (see Sect. 3.2.1.3) and weathering processes (e.g., ultraviolet irradiation, mechanical or thermal stress) can further alter the properties and composition of MNPs over time [22].
This results in a wide array of potentially hazardous properties that should be analysed in RAs, and those with an early-life scope require exposure data specific to the placenta and developing foetus, rather than comprehensive environmental presence. However, only limited information is available due to ethical and practical constraints on obtaining such samples [23]. Additionally required, but currently insufficient data, are exposure routes, toxicokinetics, and full toxicity profiles for MNPs. This knowledge gap, compounded by the distinct and diverse properties of MNPs creates difficulties in evaluating potential risks. Potentially hazardous properties include the polymer type, additives, and non-intentionally added substances (NIAS) (e.g., impurities, by-products, and breakdown products), as well as particle-specific properties including size, morphology, surface charge, and hydrophobicity [5, 24, 25]. The absorption of unknown environmental chemicals, such as heavy metals, antibiotics, and persistent organic pollutants, pose a hazard [5, 26], which is heightened by an understanding that concomitant exposure of MNPs with adsorbed heavy metals via the gastrointestinal track (GIT), for example, can increase bioaccumulation of these heavy metals [27]. This issue is further complicated by the characteristics of microplastics, which influence their bioaccumulation and effects, and is highlighted as an area requiring improved research focus [28]. Additional factors such as weathering, protein coronas and microbial biofilms can further influence hazards [13, 24, 29, 30]. Specific details on identified hazards are provided in Hazard Identification (Sect. 3.2).
Risk management goals
Risk management goals of an RA may include informing policymakers and the plastic value chain (including suppliers, brands, etc.) on mitigating measures to reduce exposure to MNPs from currently underappreciated sources, such as unintended generation from the routine use of plastic food contact materials [31,32,33]. Beyond just raising awareness of these exposure sources, policymakers could incentivise best practices aimed at reducing MNP generation; for example, by stipulating new technical standards to minimise abrasion from regular plastics usage. These goals are informed by qualitative statements and recommendations for risk management, through to quantitative guidance based on risk estimates. Common outputs from a human health hazard assessment are a Derived No Effect Level (DNEL) or a Derived Minimal Effect Level (DMEL) [34], which are then compared to actual or estimated human exposure levels. The quotient of effect level-to-exposure level, also known as risk characterisation ratio (RCR), provides quantitative information on risk, with values larger than 1 indicating the Margin of Safety, and values below 1, where exposures exceed effect levels, showing risk and the need for reducing exposure. However, in cases where a DNEL/DMEL cannot be established, a qualitative approach may be adopted, and risk characterisation is achieved through justification rather than calculating an RCR. A thorough qualitative assessment should determine the conditions necessary for the safe utilisation of MNPs by employing risk management measures (RMMs) that are suitable and proportional. A Weight of Evidence (WoE) approach [35] should also be considered, when multiple data sources of varying quality and related scientific uncertainty are available (see 3.5.1).
A quantitative approach is preferred for MNP RA; however, dose-response toxicity data are generally not available, and robust quantitative approaches typically require toxicity testing across multiple doses. This data scarcity necessitates the initial adoption of a qualitative approach for a MNP RA. In the future, as the evidence base expands, a semi-quantitative approach may be taken, where qualitative descriptors and quantitative estimates are combined to characterise risk in cases where a full quantitative assessment is not (yet) feasible. Ultimately, robust quantitative methods, utilising data from hazard and exposure assessments, should be used. A comparison of the three different approaches (qualitative, semi-quantitative, and quantitative) is provided in SM Table S2.1.
Acceptable degree of uncertainty
Existing RA approaches state [18, 19] that uncertainties and potential impacts on the derived risk should be identified and documented at each stage of the RA, aligning with the acceptable degree of uncertainty defined in the Problem Formulation [18, 36, 37]. This includes data quality uncertainties and variations in contact/exposure rates between species and populations, to ensure risk is not under or overestimated [36, 38]; concerns have already been raised regarding the quality and reliability of data available when attempts were made to assign a human health-based threshold value on MPs found in drinking water [39]. We identified two further key areas to reduce uncertainty in MNP RA: (1) aligning MNP exposures with mixture toxicity concepts, and (2) improving current methods to characterise and quantify MNPs in complex matrices.
Since MNPs contain mixtures of polymers, additives, impurities, and adsorbed pollutants [40], a mixtures RA approach is relevant. The Environmental Protection Agency (EPA) [41] identified that merging assumptions for chemical mixtures and individual chemicals can minimise uncertainty, a potentially valuable tool for MNPs. The European Food Safety Authority (EFSA) [37] suggests for RA of combined exposure to multiple chemicals using whole mixture approaches (WMAs) or component-based approaches (CBAs). In a WMA, substances are grouped as a single unit. This is useful when the composition is partially known, or characterisation is difficult. On the other hand, CBA requires defined mixtures with known exposures, making its use currently limited for MNP RA. EFSA also recommends basing the assessment on the most hazardous ingredient [37].
The Mixture Assessment Factor (MAF) approach, suggested as a tool to account for potential mixture risks during chemical RAs [42], could potentially be adapted for use in a MNP RA. The MAF is a generic factor applied to safe exposure levels determined for individual substances, lowering them to account for data gaps and additive effects between chemicals. Potential strategies for using the MAF include conducting chemical analysis to determine composition, estimating hazards through read-across or quantitative structure–activity relationships (QSARs), applying default conservative MAFs, separating by toxic modes of action, simplifying via multivariate statistics, empirically deriving MAFs from toxicity tests, and developing predictive interaction models [37, 43]. However, it is important to consider that MNPs involve complex interactions, including agonistic, additive, synergistic, and antagonistic effects. Therefore, the MAF approach may be overly simplistic to account for these nuances.
Grouping approaches to address the complex mixture of heterogeneous particles have been extensively developed and implemented in various regulatory and scientific contexts [44] . These methods are crucial for assessing risks from combined chemical exposures, particularly when components share common mechanisms or pathways. Criteria for grouping can include regulatory requirements, such as those in European pesticide regulations [44, 45], and scientific considerations like structural, physicochemical, metabolic, and toxicological factors [44] . EFSA employs a tiered approach that incorporates organ/system-level effects, phenomenological effects, modes of action (MOA), and mechanisms of action to enhance RA precision [37]. Starting with dose addition and hazard index (HI) estimation, specific risk assessment options are applied sequentially or in parallel when unacceptable risks are identified. These include reference point index (RPI), modified RPI (mRPI), and MAF approaches, focusing on specific effects, uncertainty factors, and vulnerable populations [44]. Read-across methods can be used to predict toxicity based on structurally similar chemicals, filling data gaps and supporting comprehensive assessments [44, 45]. The implementation of these approaches is bolstered by ongoing developments in new approach methodologies (NAMs), integrated testing and assessment (IATA), and improved data sharing and RA tools, for chemicals [44, 45] and for nanomaterials [46]. A pressing issue related to uncertainty is the suitability of methods for characterising and quantifying MNPs in complex matrices. Documenting methodological advances through technology readiness levels (TRLs) could help to gain confidence in the methods used and therefore reduce uncertainty. TRLs provide a structured framework indicating a technology’s maturity from initial concepts to fully operational systems, facilitating comparison and highlighting the progression and reliability of different methods [47, 48]. For example, a technique may be at high risk for false positives and false negatives, potentially leading to over- or underestimation of MNP abundance [49]. While TRLs are not a solution for all types of uncertainty, they could reduce uncertainties related to technological maturity and readiness.
A revised TRL strategy for MNP characterisation tools is proposed in Table 1, which adapts TRLs to identify, characterise, and quantify MNPs in human tissues, and a flowchart on how to assign TRLs is provided in Fig. 2; aligning with quantification in human tissues would allow for development of robust methods that provide empirical data to understand direct in utero exposure. However, for quantification of full maternal exposure, methods for quantification in all matrices would be required and linked to exposure modelling techniques, e.g. drinking water, air, food etc. Understanding method maturity as it relates to MNPs identification, characterisation, and quantification in human tissue supports a better understanding and reduction of data uncertainties as it allows accurate communication of method status, informs development of appropriate methods, and supports risk analysis. The specific application of the techniques can influence a TRL ranking; for example, fluorescence microscopy may be assigned a high TRL for studying model nanoparticle uptake and toxicity in exposure studies, yet have lower applicability for detecting real-world MNPs in human tissue. Similarly, electron microscopy (EM) could receive a high TRL for measuring particle size and size distribution, but a low TRL for chemical characterisation. Furthermore, some emerging analytical techniques that combine microscopy and spectroscopy to measure almost all the various physicochemical properties noted in the following section could currently receive low TRL scores, as they are not yet extensively tested in in vivo human studies, however, are promising for future development. Examples include correlated scanning electron microscopy (SEM) + Raman microscopy, or atomic force microscopy (AFM) + infrared (IR) spectroscopy (i.e. photo-induced force microscopy (PIFM)). A low TRL ranking does not imply these analytical techniques cannot properly assess MNPs, it solely indicates the level of broad development and adaptation to date. More information on promising techniques is outlined in the review by Mandemaker and Meirer [50]. The considerations presented here serve as a tool to evaluate the advancement of these promising MNP detection and micro-spectroscopy techniques in this relatively young research field. It is crucial to highlight that, beyond the analytical method itself, a fundamental factor is ensuring the plastic contamination-free preparation and concentration of the sample prior to MNP analysis.
Hazard identification
Hazard identification is used to determine whether exposure to a substance has the potential to cause adverse effects in a population of concern [18]. It informs subsequent hazard characterisation and exposure assessment, and the general steps are outlined in Fig. 3. If unknown, then data gathering is required to better identify the hazard. Concurrent hazard and exposure assessment are recommended due to interdependence [19]; hazard data informs toxicity drivers needing exposure evaluation, and predicted exposures influence RA priority tier allocation.
To assess early-life health risks associated with MNP exposure, the first step requires characterising properties and identifying which of these may cause adverse effects (Table 2). An additional complexity at this stage is that, as noted before, weathering of MNPs can influence all properties outlined here (polymer type excluded), as well as facilitating the release of MNP-associated chemicals [24].
Data on plastic-related chemicals from industrial, scientific, and regulatory data sources can aid hazard identification, such as PlasticMap [77], Plastic Health Map [78], databases of Chemicals associated with Plastic Packaging (CPPdb) [79, 80], European Chemicals Agency (ECHA) [81, 82], the Registry of Toxic Effects of Chemical Substances (RTECS) [83], the NORMAN network database [84], the Danish Environmental Protection Agency [85], as well as peer-reviewed literature containing in vitro and in vivo toxicity studies.
Existing RA strategies recommend a tiered approach to screen and prioritise substances for assessment (see Sect. 3.2.1) [18, 19]. The goal is to optimise screening while minimising resource usage. This information can also inform the risk characterisation stage (Sect. 3.5). For MNPs, the proposed tier structure considers polymer, chemical, and particulate hazards, however, this can be adapted as new hazard data emerges.
Although it has been shown that detectable levels of harmful contaminants, such as BPA, can be found in up to 84% of microplastics extracted from seafood [86], it is important to note that focusing solely on contaminant exposure from MNPs does not account for other significant exposure routes unrelated to plastics. Based on consumption estimates from EFSA and assuming complete release from MPs, the contribution of MNPs to overall chemical exposure is relatively small compared to other sources [87, 88]. This could lead to incorrect assumptions that exposure to these contaminants is negligible when based only on MNP exposure. However, understanding MNP hazards associated with MNP exposure is still crucial for the regulation of plastics, as they can contribute to environmental chemical contamination, highlighting the necessity of comprehending their leaching potential and overall contribution to total exposure and associated risks.
Tier allocation of MNPs based on polymers, particles and chemicals of concern
Ideally, the proposed tiering framework would group MNPs based on known hazardous properties such as reproductive and developmental toxicity, mutagenicity, bioaccumulation, and physicochemical hazards. This framework aims to eventually assign point values to hazardous characteristics and exposure which can be used to generate a priority score, and can also be used to inform semi-quantitative RA (see Sect. 3.5.3). Points would be assigned based on the severity and relevance of each hazard. For instance, a human health hazard, such as known endocrine-disrupting activity, might be assigned more points than a hazard linked to concerns relating specifically to polymer physicochemical properties. However, due to significant data limitations and uncertainties, it is not yet feasible to develop a defined, hazard-weighted point allocation system. This system can be developed and evolve alongside future research and development. Figure 4 provides an example of how tiering may look, which will evolve with the addition of numerical values to specific hazards as more information becomes available.
To avoid overlooking potential risk, points should also be allocated to account for uncertainty and data gaps when hazard and exposure data is lacking, although currently this may allocate all MNPs to the highest tier. This highlights the need for further research to refine a point allocation system. The ICCA [19] guidance prioritisation utilises exposure potential, yet has limitations for MNPs as quantitative exposure and dose-toxicity data are lacking. While available data indicates high doses are required for toxicity [3, 89], with substantial data gaps this cannot be assumed for all polymers. Thus, toxic effects from minimal exposures for unevaluated MNPs cannot be ruled out.
Another consideration within the tiering approach is whether detecting MNPs in the placenta should automatically assign them to a high priority tier. While placental presence indicates a potential for bioaccumulation, it may not prove to be an inherent hazard or risk without more data on toxicity and effects. Currently, research has identified polyethylene (PE), polystyrene (PS), polypropylene (PP), polyethylene terephthalate (PET) and polyvinyl chloride (PVC) in placental samples [9,10,11,12]. However, the argument could be made that MNPs of all polymer types of a certain size have the potential to cross the placental barrier, which would group all polymers into the highest tier. Therefore, a more balanced approach should also consider placental detection alongside factors such as polymer hazards, additives and particle morphology.
MNPs with the highest priority scores (≥ 5) based on available evidence would be allocated to Tier 1 and are the highest priority for assessment. Assignment to Tier 1 does not necessarily mean these MNPs present the absolute highest risk, as exposure levels also contribute to overall risk. However, allocating them to the top tier based on significant concern identified from the hazard evidence ensures these substances undergo a prioritised and comprehensive assessment. Certain identified hazards associated with reproductive and developmental toxicity should automatically allocate MNPs to the highest priority for RA; for example, if contains known endocrine disruptors, as maternal exposure is associated with negative impacts on foetal growth and neurological development [90]. While the level of chemical exposures specifically linked to MNPs needs to be contrasted with known exposures from other sources (as previously discussed), due to the potential for serious health impacts, the initial allocation to high priority is warranted. This priority status may be revisited if it is established that environmental exposures from other sources are already significantly high.
MNPs receiving a score of 3–4 would be allocated to Tier 2, indicating medium priority for assessment with less data requirements compared to Tier 1. Scores of 1–2 assign MNPs to Tier 3, designating low priority for assessment. A score of zero means no hazard or exposure has been identified, so RA may not currently be necessary. The tier system and MNPs should undergo periodic reviews to account for new information that may necessitate a new tier designation and ensure that the framework remains adaptive and responsive to emerging scientific data. Examples of information that can support hazard identification and tier allocation will be provided in the following sections.
Polymers as a hazard
In defining hazards posed by polymer chemistry, it can be difficult to distinguish this with particle specific hazards, as polymer chemistry will affect particle properties, such as size, shape, surface chemistry etc. Conversely, there is potential for polymer-specific effects which may be relevant for a MNP RA. For example, when linked to the adsorption of environmental pollutants, as different types of polymers, such as PE and PP, exhibit varying capacities to adsorb environmental pollutants, which could influence the overall toxicity of MNPs and potential health impacts following exposure [57]. Or that PVC and PU demonstrate higher toxicity in vitro compared to PET and HDPE [91]. It is suggested that there may be polymer-specific effects on placental functioning [24], and certain polymers are known to specifically release toxic monomers, such as from styrene [92,93,94] and from PUR, which utilises carcinogenic monomers and toxic additives, and PVC, which contains more hazardous additives compared to other plastics [95]. Therefore, there is potential to rank polymer hazards based on the presence of these toxic monomers [96]. However, MNPs in the environment, and those that humans are exposed to, have undergone various degrees of weathering and ageing. MNPs may follow different degradation pathways [97], with weathering seemingly affecting polymer composition [98]. A recent article explores the influence of environmental stressors on the degradation of plastic particles and other particles [99]. Thus, the toxicity of pure polymers is limited as a reliable indicator in hazard assessments, and an improved understanding of MNP ageing is necessary. Environmental prevalence of polymers should also be considered for tiering, for example there are certain polymers that make up the greatest proportion in sediment and the water column (marine and freshwater), such as PE, PET, PA, PP, PS, PVC, PVA and PU [91, 100], however the relevance of environmental prevalence will increase as dose-dependent toxicity data improves.
Given this, it would be pragmatic to explore existing and forthcoming mechanisms that may allow RA of these separately. For example, RA models are available for assessment of nanoparticles, albeit linked to occupational exposures [96], While for polymers, the European Commission (EC) plan to categorise polymers under REACH into ‘polymers of low concern’ (PLC) or ‘Polymers Requiring Registration’ (PRR), based on various polymer properties including molecular weight, reactive functional groups, and polymer surface activity [30]. However, only 5.5% of the estimated 200,000 polymers on the EU market will be classified as PRR under these criteria [101]. Moreover, The PRR criteria have key gaps in assessing polymer hazards, they do not consider polymers’ tendency to generate environmental MNPs, anionic and amphoteric polymers, impurities and stability additives, or high production and widespread use polymers that heavily contribute to plastic pollution. Additionally, the PRR does not address metal content or binding affinities, critical factors in determining toxicity and extent of contaminant accumulation. Fundamentally, polymers utilised in large quantities and contributing significantly to plastic pollution would not subject to registration requirements under the proposed scheme, despite their disproportionate impact. Furthermore, the exclusion of various polymer subclasses, such as polyesters and surface-active polymers, lacks sufficient justification [102].
Methods are under development that may aid polymer hazard tiering based on mechanical and physical properties. For example, the MicroPlastic Index [103] looks at theoretical particle size and energy required for MNP formation.
Particles of concern
Particle characteristics such as size, shape, surface properties, and concentration can influence the toxicity of MNPs. Smaller MNPs demonstrate increased reactivity [65] and can bypass biological barriers including the placental barrier [104]. All current data on translocation is based on PS particles which indicate a size-dependent maternal-to-foetal translocation, where smaller particles are transferred more readily than bigger particles [3, 13, 24]. More information related to hazards specifically associated with size is needed, such as size-dependent toxicity data.
Shape-dependent toxicity occurs with NMs, such as carbon nanotubes and asbestos fibres [105], however, the effect of MNP shape on toxicity is understudied. Most MNP toxicity research uses PS spheres, yet fragments and fibres are reported to dominate in placental samples [11, 106]. Fibres also demonstrate increased accumulation and more severe intestinal toxicity in zebrafish models compared to spherical particles [63]. Until more information becomes available, hypothetical models, such as the high aspect ratio nanoparticle (HARN) model, which explores the shape-dependent toxicity of nanoparticles with a high ratio of length to width [107], may be applied to identify potential hazards of MNPs.
MNPs can undergo surface modifications due to biological processes, such as the attachment of microorganisms, which secrete biofilms or extracellular substances, and environmental factors, such as ultraviolet irradiation [29]. This creates uncertainty when assessing hazard, as surface properties influenced placental transport of MNPs in an ex vivo model; increased transport of carboxylated PS MNPs across the placental barrier and greater accumulation of amine modified PS MNPs in placental tissue were observed [84, 85] and serum proteins facilitated differential transplacental transport as well, preferentially transporting plain, then carboxylated, then amine modified MNPs [108, 109].
Concentration-dependent MNP toxicity has been observed in various testing models, although most use unrealistically high doses that may not correspond to real life exposures [29, 63, 110]. However, the durability and slow degradation of MNPs allows for bioaccumulation in organisms, increasing exposure over time [62]. Without definitive real-world exposure or bioaccumulation data, it is prudent to take a conservative approach and assume that higher internal concentrations in organisms, in particular in respective gastrointestinal tracts, are possible through gradual accumulation in the body.
MNP-associated chemicals of concern
Plastics contain a complex mixture of intentionally added substances (IAS) such as plasticisers, stabilisers, antioxidants, flame-retardants, fillers, and colorants. The final product also contains non-intentionally added substances (NIAS) such as impurities, reaction by-products, and breakdown products of polymerisation and compounding [70]. Over 4,700 IASs have been identified in plastic food packaging [79] and over 13,000 chemicals are associated with plastics manufacturing worldwide [32]. Many of these chemicals are not covalently bound to the polymer matrix and can transfer into food or the environment, with up to 2,000 found to migrate or be extractable from food-contact plastics [111]. This results in continuous exposure to complex chemical mixtures [112].
A key issue is that many of these chemicals are hazardous; carcinogenic, mutagenic, toxic for development, persistent, bioaccumulative, or endocrine disrupting [113]. Over 3,200 have been identified as substances of potential concern, yet many have not been assessed for hazards, and most have received little regulatory attention [32]. Chemical profiling of MNPs has been used to assess the effects of these chemicals on gene expression in placental cells, data which can aid hazard characterisation [24]. MNPs also adsorb and accumulate contaminants when released into the environment, including Persistent Organic Pollutants (POPs), heavy metals and antibiotics, which can transfer to organisms [5, 26]. Numerous physical and chemical interactions influence contaminant sorption, which must be considered alongside environmental properties such as pH, temperature, and salinity contributing to research gaps that need to be addressed [28]. These factors add complexity to RA, as hazards of individual chemicals as well as their mixture toxicities are often unclear.
Hazard characterisation
Hazard characterisation, as outlined in chemical RAs [18, 19], describes the potential of the substance to cause adverse health outcomes following exposure. This involves linking available guidance values (see Sect. 3.4.5) to contact rates and exposure rates.
The WHO [18] define contact rates as the mass or volume of the medium in contact with the body, and exposure rate the concentration of a substance in an exposure medium multiplied by the rate at which a person inhales, ingests or has dermal contact with that medium, divided by a representative body weight. Contact and exposure estimates require reference values to quantify risks relative to safe levels. As guidance values do not currently exist for MNPs, this limits progression to Risk Characterisation based on guidance from chemical RAs. Figure 5 outlines the information that can be utilised to progress through the RA.
In the absence of guidance values for MNPs, hazard testing is necessary to obtain dose-response data. Alternatively, a Mode of Action (MOA) approach could qualitatively or quantitatively assess the ability of MNPs to induce adverse effects from exposure [18, 19]. Adverse Outcome Pathways (AOPs) relevant to MNPs are being developed, for example, based on the toxicity mechanisms of chemical additives [114]. This is achieved using molecular in vivo and in vitro toxicity databases alongside deep learning models. These efforts aim to propose AOPs pertinent to MNP pollution, providing insights into the toxicity mechanisms, such as neurotoxicity, inflammation, lipid metabolism, and cancer pathways, of a broad range of environmental chemicals, such as plastic additives, helping to address previously identified research gaps [114]. However, due to the lack of data on the direct or indirect effects of MNPs on humans and limited information on molecular initiating events (MIEs) and key events required to derive an AOP or MOA for any specific type of MPs, these techniques are currently limited. For example, the physicochemical characteristics of the particle need to be considered, and it is difficult to identify a MIE and determine whether it is triggered by the biomolecular coating or polymer surface chemistry [115]. However, developments in these areas will help define MOA and allow for a better RA.
Existing guidance may be relevant for chemical additives and contaminants identified during Hazard Identification, especially for high priority substances. These values could be applicable, however must be carefully evaluated for relevance to early-life. For example, workplace exposure limits such as 8-hour time-weighted averages (TWAs), designed for healthy adult workers [116], may lack relevance for early-life RAs, which require consideration of continuous exposures and vulnerable developmental stages [117]. In contrast, Tolerable Daily Intake (TDI) values estimate the maximum safe daily exposure level over a lifetime based on toxicity data and uncertainty factors [118]. As such, TDIs like EFSA’s 0.2 ng/kg bodyweight standard for Bisphenol A (BPA) could prove more useful in evaluating MNP risk [119].
For early-life effects, we must consider how guidance values translate to actual contact and exposure rates for the developing foetus. Although it may be preferential to monitor pregnant mothers (i.e., blood sampling), given this would provide a holistic picture, there would be increased uncertainty and dependence on fate modelling to estimate foetal exposure. For example, physiology-based pharmacokinetic (PBPK) modelling is a simulation technique that incorporates blood flow and tissue composition of organs to define pharmacokinetics, and is already used to predict foetal drug exposure during pregnancy [120]. Moreover, PBPK models originally developed for assessment of chemical distribution are now being adapted to MNPs [121], aiming to reduce uncertainty between external exposure and internal dosimetry, enhancing HHRA accuracy. Therefore PBPK modelling may be useful to predict foetal exposure rates to MNPs, and the potential transfer of chemicals to the foetus during pregnancy, based on the simulated maternal-foetal pharmacokinetics [81]. In comparison to maternal blood sampling, placental exposure monitoring may have both advantages and disadvantages. It can provide valuable information on bioaccumulation and placental transfer kinetics across the full pregnancy, rather than transient maternal blood levels. This can strengthen modelling of long-term foetal exposures. However, variability introduced through non-standardised sampling and storage may compromise data quality and limit reproducibility between studies [23]. Additionally, placental sampling only occurs at delivery, meaning opportunities for risk mitigation are reduced for that pregnancy, whereas maternal blood tests can be acted on during gestation. Along with barriers surrounding ethical approval and practical constraints around tissue collection [23], these factors highlight current limitations associated with placental sampling for high-volume exposure screening. Thus, maternal blood monitoring may present a more standardised and feasible approach for estimating exposure rates. An integrated strategy harnessing both methodologies could provide the most effective for hazard characterisation. To address limitations in studying reproductive effects in mammals, such as limited particle characterisation, and the use of single polymer types, standard guidelines such as OECD 421, 422, and 443 contain key points that should be included when studying reproductive effects in mammals, such as fertility and foetal effects [39]. This can enhance the reliability, relevance, and comparability of data on MNP’s reproductive toxicity.
Various clinical, molecular, and inflammatory markers should also be integrated to gauge the potential impact on early-life; for example, birth weight, a fundamental clinical parameter, holds great significance in this assessment. Low birth weight (less than 2,500 g) is associated with increased neonatal mortality, developmental delays, and chronic health conditions, while high birth weight (above 4,000 g) may indicate maternal health issues like gestational diabetes, posing risks to both mother and child [122]. Beyond clinical measures, molecular markers like mitochondrial and telomere targets and epigenetic modifications can reveal genetic predispositions to conditions, such as preeclampsia, that can affect birth outcomes and future health [123]. Inflammatory markers like cytokines and C-reactive protein provide insights into maternal-foetal inflammation, which can trigger a cascade of events that may adversely affect birth outcomes. Elevated inflammatory markers may predict risks for conditions like preterm birth [124].
Structured testing strategies like the Grouping and Read-across of Nanomaterials and Nanoforms (GRACIOUS) framework for nanomaterials [46] and Organisation for Economic Cooperation and Development (OECD) guidance for chemical mixtures [36] are recommended to fill data gaps. These strategies utilise in silico, in chemico, and in vitro testing first to reduce in vivo needs and allow grouping by physicochemical properties or common mechanisms/adverse outcome pathways (AOPs). Incorporating early-life hazard assessment tools, such as in vitro and ex vivo placental models [3] facilitates implementation of a structured approach to evaluate the early-life health effects of MNPs.
Exposure assessment
The exposure assessment (Fig. 6) begins with how the population of concern (early-life) may come into contact with MNPs, the characteristics of MNPs the population is exposed to, and how much and how long exposure is likely to occur [18]. We have also considered factors associated with MNPs that may increase exposure, e.g., size of MNPs influencing barrier permeability. The following sections consider information that may inform each stage and exposure considerations are also presented in Fig. 7. When data on MNPs is missing, information from extensively studied particles may serve as a model for filling data gaps and defining relevant exposure metrics for an MNP RA.
To streamline the exposure assessment, prioritisation of data becomes crucial. While endpoints related to pregnant mothers can aid in determining contact and exposure rates for the placenta, they may not be indicative of contact and exposure rates in the developing foetus.
Maternal, placental and foetal routes of MNP exposure
Data indicates the presence of MNPs in human placenta samples [8,9,10,11,12], as well as in meconium and amniotic fluid [9, 10, 125], indicating that particles can pass through the placenta rather than being retained, and that foetal exposure is possible. However, other studies have failed to detect MNPs, or highlighted concerns that despite stringent controls, that sample contamination may occur [9, 126]. Overall, current evidence (see SM Table 3.1) only provides snapshot data at birth rather than exposure throughout gestation. Understanding exposure pathways is essential to managing risks associated with MNPs. However, a lack of sampling and analysis methods, at a suitable level of sensitivity (refer to TRLs, Sect. 3.1.7) hinders assessing total (the absolute amount) and relative (exposure level in proportion to a reference value) exposures, present a major barrier to performing a RA. While the widespread presence of MNPs in the environment is acknowledged, what reaches the placenta and developing foetus is, at present, unknown.
Recent reviews [127,128,129] report on human exposure sources and pathways. They indicate a high prevalence of MNPs in indoor environments, particularly in settled dust and from synthetic textiles, and that outdoor air contains MNPs from tire abrasion, atmospheric fallout, and dust, with both indoor and outdoor air contributing to exposure through inhalation [127, 129]. While information on the effects of MNPs inhalation in humans is limited, MNPs have been detected in bronchoalveolar lavage fluid (BALF) [130] and sputum in adults [131]. MNPs in the lung have the potential to then enter the bloodstream, as observed with other particles [132,133,134] and animal models have demonstrated maternal lung to foetal translocation of MNPs [60]. Larger inhaled particles (above 5 µm diameter) are likely to become trapped within lung mucus and undergo mucociliary clearance [135], which may result in increased ingestion.
Ingestion is the most studied route to date, with MNPs reaching the gastrointestinal system through consumed food, drinks, food packaging and mucociliary clearance [127]. The presence of MNPs in human and animal faeces [136,137,138] provides evidence that intake has occurred, dermal exposure occurs through contact with dust, personal care products, textiles, and food contact materials [139]. However, dermal translocation/absorption capabilities are currently not known, but probable, considering data on NMs indicate that particles ≤ 4 nm in diameter can penetrate intact skin, which increases to 45 nm for damaged skin [140]. Therefore skin conditions such as eczema, of which 60% of cases appear within the first year of life, may also increase uptake as the skin barrier is compromised [141]. In addition, some personal care products, such as cosmetics, contain nanosized ingredients designed to increase dermal penetration and may also cause skin damage [142], both of which may increase dermal uptake of MNPs. However, here remains considerable unknown factors regarding potential dermal exposure routes and consequent absorption kinetics of MNPs. Another consideration when estimating exposure is social determinants of health [143], as increased body burden of plasticisers has been observed in pregnant women in minority racial/ethnic groups, low-income areas and those with lower educational attainment [7].
Meconium-stained amniotic fluid (MSAF) occurs in up to 25% of pregnancies, more often in Black and South Asian populations [144]. Up to 10% of newborns experience meconium aspiration syndrome in which they inhale MSAF in utero or during delivery, with ethnicity potentially influencing exposure extent [145]; this creates a potential for additional inhalation exposure if MNPs are present in the amniotic fluid. MNPs have also been detected in breastmilk [146], and infant formula [147] indicating a postnatal route of early-life exposure. Quantifying this presents a unique challenge given differences in rates of breast feeding versus formula use, and the use of plastic baby-bottles and milk-storage bags [10]. Child-specific behaviours such as crawling, increased hand-to-mouth activity, mouthing on plastic toys and surfaces [148] can also raise early-life MNP exposures. The breathing zone is also closer to the ground for infants and children [148], increasing exposure to dust containing MNPs; additionally, greater relative ingestion and inhalation rates per unit body weight when compared to adults, and may lead to increased uptake [7, 149].
Characteristics and quantity of MNPs reaching the placenta and/or foetus
Accurate evaluation of MNP risks necessitates quantifying exposure concentrations and hazardous profiles of MNPs reaching the mother, placenta and foetus. The current methods used for MNP monitoring were designed for other materials, such as NMs, and have been adapted for MNPs with varying success. Advancing spectroscopic techniques is required to better characterise MNP exposures in terms of number, size and shape [104]. Also relevant are methods providing data on polymer type, mass, concentrations in source media (food, water, soil, air) [138], and identifying MNP forms in these media to predict relevant exposure characteristics and quantities.
Evaluating in utero exposure to MNPs and associated chemicals is critical. MNP and MNP-associated chemicals, including endocrine disruptors, have been detected in human amniotic fluid [125, 150]; this is of concern, as the continual ingestion and excretion of amniotic fluid by the foetus creates the potential for recurrent exposure [151]. It is also critical to recognise the potential for indirect foetal harm caused by placental damage resulting from the translocation or accumulation of particles, as is observed with NMs [152]. Quantifying MNP exposure levels in fluids surrounding the foetus during gestation is therefore critical to ascertain uptake potential and enhance risk prediction considering placental toxicity concerns that may manifest in the developing foetus.
As previously highlighted, maternal exposure may be used to estimate placental uptake and foetal exposure using PBPK modelling [24, 81]. While at present no research has quantified MNPs in maternal blood, data obtained from adult females [153] could fill this gap. Maternal exposure may also be estimated using environmental data with inhalation, and ingestion estimates. However, the OECD [36] highlights that measured exposure data is preferred over modelled exposure data. Underscoring the significance of this preference is the widely varying range of adult ingestion estimates, from 258 particles/day [154] to as much as 5 g per week [155]. The 5 g figure, though widely reported, was later found to have significant methodological errors in the analysis [156], with authors concluding that the calculations, which used data consolidated from different measurements, led to an overestimation of the mass of ingested MPs. The corrected analysis indicates that the actual ingestion rate is substantially lower, while also highlighting issues with using modelled data.
Characteristics of MNPs, such as size, may also influence MNP exposure. For example, it is understood that MNPs larger than 150 μm pass through the GIT to be excreted and EFSA [157] estimate up to 90% of ingested MNPs are excreted in faeces; indicating that the majority of MNPs humans are exposed to pass through the GIT. However, MNPs smaller than 150 μm may translocate across the gut epithelium [157] and MNPs up to 10 μm via immune cells into lymphatic tissue [67]. However, MNPs smaller than 1.5 μm may penetrate deeply into organs [157].
Examples of research that may be used to inform characteristic and quantity are summarised SM Table S3.1. However, it is important to note that variations in the sensitivity, selectivity, sample processing, instrumentation, and data analysis inherent to each detection technique can substantially influence the uncertainty around measured MNP concentrations and characterisation; it is hoped that monitoring technological advances of these systems via TRL assignment will help to reduce this uncertainty. Faecal excretion data from maternal stool samples and adult populations (see study details in SM Table S3.2) [4, 137, 158] may inform ingestion quantities and allow the characterisation of MNP properties that humans are exposed to. However, this would not factor in systematic absorption and accumulation. There are also multiple factors that could reduce excretion, which should be considered when determining exposure (see Sect. 3.4.3).
Intake estimations compared against excretion data may provide insights into quantities that persists within the body. However, it is important to note that excretion of MNPs does not indicate that no hazard is present, as leaching of chemicals and/or contaminants prior to excretion must also be considered. It is possible to estimate leaching using in chemico approaches, according to literature available. This supports method selections, and decisions on choice of suitable biological simulant fluid, to address both exposure routes and biological compartments [159,160,161,162,163,164,165]. However, in context to this release it will be important to consider whether effects by different components can be antagonistic, additive or synergistic, as discussed earlier, with uncertainty consideration pertaining to mixture effects of known toxicants.
Factors that could increase exposure and how these may apply to MNPs
While the majority of MNPs are believed to pass through the GIT [157], there are factors which may decrease excretion rates, such as an increase in GIT permeability (leaky gut) due to compromised integrity of the intestinal barrier or improved translocation across biological barriers. This may allow substances to cross into the bloodstream that would otherwise be restricted, increasing contact and exposure rates. Some of these factors are outlined in Table 3, and include MNP and NM exposure, certain health conditions, and dysbiosis. Although assessing the effects of heightened barrier permeability presents difficulties, it remains crucial to consider these factors during exposure estimation. Neglecting to do so could lead to an underestimation of exposure.
Duration and timing of MNP exposure
Duration of exposure. The duration and frequency of exposure is critical to exposure assessment and should identify exposure as single, cumulative, short, medium or long-term [151]. MNPs detected in meconium indicate particles are excreted by the foetus, however bioaccumulation and gestational age may influence exposure levels [29, 190]. Recurrent foetal ingestion and exhalation of MNPs within amniotic fluid could also lead to chronic exposures [151].
Bioaccumulation. MNPs have been found to accumulate in tissues and organs in rats (21 nm) [60], mice (0.1 and 2 μm) [191], zebrafish (4–40 μm) [63] and marine organisms (10 μm − 5 mm) [62]. PS nanoparticles (21 nm) accumulated in rat foetal tissues including the liver, lungs, heart, kidney, and brain following maternal respiratory exposure [60]. Probabilistic lifetime exposure models can provide estimations that MNPs can irreversibly accumulate in humans [190], however, the ability of MNPs to accumulate in the placenta and/or foetus needs attention for accurate exposures. The accumulation of MNPs in maternal organs allows for re-introduction into the circulatory system, as deposited particulates may leach from tissues back into the bloodstream [67], increasing the potential for translocation across the placenta to foetal tissues.
Gestational age. The timing of exposure to environmental contaminants is extremely important in predicting foetal susceptibility and the risk associated with that exposure. Organogenesis may be affected by early embryonic exposures, while later embryonic exposures influence organ system maturation [192]. Major windows of vulnerability exist in utero and throughout early-life, when the human brain is uniquely susceptible to chemical toxicities [192]. The impact of certain drugs on the foetus also varies with gestational age, owing to organ development stages and vulnerability fluctuations throughout pregnancy [193], a factor also to consider when assessing MNP associated contaminants.
Currently, there is limited evidence on how gestational age influences MNP translocation. However, murine animal models indicate an increased early pregnancy exposure risk for some NMs, with reduced placental transfer as pregnancy progresses [194, 195]. However, distinct variations exist in placental anatomy and function when comparing mouse and human models [196], and caution should be taken when extrapolating data from mice to humans. Carbon black particles were found in the same abundance in term and prenatal human placentas, which is suggested to indicate a maximal transfer from maternal blood to the foetus, occurring in the first and second trimester [197], however, may also indicate continuous replacement. To understand MNPs exposure and identify critical pregnancy stages, it is important to comprehend how gestational age affects foetal exposure and potential hazard during windows of vulnerability.
Guideline values for MNP exposure
A further challenge in quantifying MNP exposure is dose metrics and the most suitable toxicological endpoints on which to base guidelines and evaluate the risks linked to exposure [67]. Spectroscopic techniques for monitoring MNP exposure from environmental samples often report concentrations per volume sampled (e.g., particles/m3), however, in vitro toxicological studies frequently express MNP doses as mass concentrations (µg/mL) [104], which does not provide information on MNP size or number, limiting comparison to environmental levels. Recently, the Barchiesi model has been proposed for MP characterisation that better aligns with the limitations in analytical detection methods used for characterising MNPs in bulk samples of mixed particles [198]. The model accounts for toxicologically-relevant metrics of particle volume and surface area and allows for assessment of microplastic mixtures without the calibration that is required by other similar models. Bridging dose metrics between environmental or source (e.g. within food) sampling and toxicological impact is essential to evaluate potential MNP risks, and is discussed by Koelmans and Redondo-Hasselerharm [199].
The usual metric for chemical exposure and expression of guideline values is mass-based, as per the previous example for BPA [119]. However, this has long been considered inappropriate for particle assessment [200], and overlooks the crucial consideration of polymer content. There is a need for a comprehensive assessment that incorporates other properties such as surface area or particle number or shape which may bear more relevance for MNP hazards and as such should be considered when guideline values are established. Given the multi-faceted hazard profile present in MNPs, with risks associated with chemical release and with particle properties, it is possible that multiple dose-metrics will be relevant.
Examining existing in vivo and in vitro studies, environmental data, and knowledge from other well-researched particles may provide educated interim judgments regarding exposures, with potential for read across. Complexity arises, as a robust assessment requires data on the physicochemical properties, for example, polymers found, whereas exposure data often groups MNPs, limiting polymer-specific understanding behaviours and associated hazards.
Risk characterisation
To characterise risk to early-life, associated with MNPs, hazard and exposure data are combined to justify appropriate RMMs. Depending on the goals established in Problem Formulation, Risk Characterisation will differ depending on the qualitative, semi-quantitative or quantitative approach adopted (Fig. 8). No matter the approach, expert judgment is required to accurately characterise risk, with transparency and appropriate justification for conclusions drawn [201]. As current evidence indicates that a quantitative approach is not yet feasible based on current data availability, this section will focus on using WoE with a qualitative or semi-qualitative approach to characterise risk. WoE consolidates varied evidence sources, making it most relevant for these approaches where conclusions or risk rankings need to be informed.
Weight of evidence
Determining the strength of evidence and overall weight to assign different data underlies the interpretation, judgment and conclusions made throughout the risk characterisation process. A WoE approach [35] is recommended for MNP RA as there are likely to be multiple sources of data available with varying quality. A comprehensive evaluation of these sources is required to reach an informed conclusion on risk, along with an acceptable level of uncertainty being defined during the Problem Formulation. Since current data on MNP hazards and exposures varies substantially, selectively synthesising the strongest evidence reduces the likelihood of over or underestimating the risks. The transparency and systematic documentation entailed in a WoE strategy also aligns with clearly defining the RA scope and objectives during problem formulation.
Qualitative WoE provides a framework to systematically assess the available evidence and strengthen risk conclusions. Semi-quantitative WoE can aid integrating scored hazard data with exposure estimations to support ranking. Used appropriately, WoE enables developing evidence-based risk conclusions from disparate data with known confidence levels. This approach can therefore strengthen MNP risk characterisation given present data constraints.
Qualitative approach
The ECHA Guidance on Information Requirements and Chemical Safety Assessment [201] note that for qualitative RAs, risk characterisation is completed in the absence of dose-response (DNEL/DMEL) data for the human health hazard endpoints. This is achieved using a systematic, documented approach for justification rather than calculating a RCR, which is the goal for a quantitative RA. In the absence of quantitative dose-response data for MNPs, risk characterisation would rely on a qualitative approach. This involves a systematic collection and analysis of available information on the environmental and health impacts of MNPs. Key considerations should include those highlighted here, such as their interaction with biological systems, persistence in different environmental contexts, and potential for bioaccumulation and toxicity, including their mechanism of action. Expert judgement and studies on similar pollutants will be integral to this process, guiding the development of protective measures and the identification of priorities for further research [201].
Additionally, for chemicals with no dose-response data, ECHA [201] recommend the use of hazard control banding that reflect the severity of the hazard. For instance, MNPs could be grouped into high, moderate or low risk bands based on available toxicity data, considering physicochemical properties and exposure scenarios. MNPs demonstrating developmental, endocrine or carcinogenic effects would likely warrant placement in the high concern band, while those causing minimal toxicity, such as irritation, could be assigned to lower bands. With limited quantitative dose-response data currently, systematically grouping MNPs by severity of potential effects enables prioritisation of RMMs, with more stringent control measures required for higher risk bands. The absence of data should also be considered at this stage, as risk mitigation should involve improving research for such MNPs. An example of qualitative hazard banding is presented in Table 4.
As the evidence base of MNP toxicity increases over time, this qualitative categorisation approach could transition towards semi-quantitative methods, then eventually to quantitative methods. However, it provides a reasonable interim risk mitigation plan given current data limitations. Uncertainty is greater with qualitative approaches, so clearly documenting the rationale behind risk band assignments and conclusions is essential, along with the WoE approach (Sect. 3.5.1). This allows a rational prioritisation and risk management aligned with hazard levels, which should be periodically reviewed and refined as more data becomes available.
Semi-quantitative approach
The semi-quantitative approach can expand upon the qualitative approach by assigning a numerical value that can help inform hazard banding, for example, using the tiering process outlined in Sect. 3.2.1 (Fig. 4), in which a priority score is calculated based on hazardous properties. Exposure potential should also be factored in, when possible, to assign risk scores, however as previously discussed, without dose-response data, the impact of exposure would likely remain qualitative. An example of hazard banding for semi-quantitative MNP risk characterisation is presented in Table 5.
Overall, this allows integration of available hazard data with exposure assessments to reach a semi-quantitative risk characterisation. Again, as data quality and availability improve, the assessment would shift towards more robust quantitative methods. However, this provides a feasible target to progress towards in the interim.
For a meaningful semi-qualitative approach, clear specification of terminology is critical; exposure potential ratings like high, medium or low require explicit thresholds. While insights from more characterised surrogate particles may be informative, directly generalising risks to MNPs is likely inappropriate given their high variability in properties and behaviours, ultimately necessitating dedicated research efforts. Additionally, criteria for what constitutes ‘adequate’ study of MNP hazards should be specified, with sufficiency thresholds for data volume to enable hazard classification at each banding level. Using unambiguous language and defining key terms supports consistent interpretation and application of the MNP RA framework.
Hazard banding can be challenging when there are imbalances between the availability of hazard and exposure data. If robust hazard data is available but exposure data is lacking, conservative exposure estimates may be utilised to derive an initial risk ranking [19], such as information outlined in Sect. 3.4. Alternatively, if exposure estimates are adequate but hazard data is limited, read-across techniques and modelling methods may provide interim toxicity information by extrapolating from similar materials or predicting activity. Uncertainty factors should also be incorporated when either hazard or exposure data availability is limited [18, 19], and preliminary hazard bands adjusted iteratively as new data becomes available.
Risk management measures
Policymakers are beginning to address MNP emissions through various RMMs aimed to reduce preventable releases (Table 6), such as restricting MNPs intentionally added to products, improved labelling to allow consumers to make more informed choices, preventing environmental leakage, and capturing emitted MNPs before they reach water bodies [202,203,204]. However, there are limitations to control options due to the ubiquitous spread in the environment, making complete exposure elimination impossible. Therefore, high hazard banding and associated control measures may not always be actionable, particularly secondary sources like tyre and textile wear, which likely represent the majority share of environmental MNP pollution in OECD countries [202]. Effective risk reduction relies on improving the scientific ability to measure MNP emissions, prioritise major sources based on risk levels, and promote evidence-based solutions that address top priorities. As highlighted in Sect. 3.2.1.1, Methods such as the MicroPlastic Index may also be utilised for the reduction of MNP generation, aiding selection or redesign of polymers [103].
Document risk characterisation results
Documentation of risk characterisation need not vary from other clearly established RAs. The methodology should be clearly described, justifying the qualitative or semi-qualitative approach adopted, based on data availability and acceptable uncertainty, as defined during the problem formulation. This should detail the steps achieved to identify of hazard and exposures, uncertainties, limitations and assumptions to provide transparency in the conclusions made. Documentation should also cover the information utilised, weighting decisions, calculations, models and integration methods to demonstrate scientific rigor and enable reproducibility. Suitable RMMs linked to the characterised risk levels need reporting. Recommendations for further data requirements and analyses to refine the assessment by reducing key uncertainties will facilitate iterative improvements. It should also explicitly state when the RA should be reviewed, if this is a set date, (i.e. annually), or when new data becomes available, or as testing methods improve [18, 40].
Future directions
Through the development of a roadmap to enable robust early-life MNP RA framework we have highlighted numerous research requirements that must be addressed. Key data gaps exist across all the assessment stages, as summarised in Table 7. Obtaining dose-response data is critical for establishing guideline values, requiring development of standardised reference MNPs and non-animal approaches. Exposure characterisation necessitates analytical techniques with greater sensitivity and specificity as well as fate modelling to address their variability. Identifying determinants of placental transfer and foetal bioaccumulation are needed, as well as associations between polymer characteristics and toxicity. Expanded absorption, distribution, metabolism, excretion (ADME) and toxicokinetic data through in vitro and in silico approaches can aid absorption and transformation predictions. Identification of early developmental effects and windows of heightened susceptibility are key.
Targeted, collaborative research initiatives focused on addressing current data gaps are essential to translating existing practices for assessing MNPs. Integrating perspectives across polymer science, nanotechnology, analytical chemistry, toxicology, and RA fields will facilitate a comprehensive characterisation of hazards and exposures. Advancing MNP RA tools can enable scientifically supported regulation and material innovation to mitigate risks to early-life.
Conclusions
This review outlines a proposed foundation for RA of MNPs relevant to early-life health. Leveraging established approaches for chemicals, particles, and mixtures, we present a framework aligned with existing RA components. Significant knowledge gaps and complexities related to the distinct properties of MNPs are highlighted, centred on exposure characterisation, hazard data, and early-life impacts. While current limitations preclude a comprehensive assessment, targeted research efforts focused on addressing key data needs hold promise for translating practices to evaluate MNPs. Cross-disciplinary engagement is required to generate the evidence base necessary for understanding and mitigating risks during early-life and advancement of a robust early-life MNP RA. Overall, this review calls attention to critical research directions needed to elucidate the impacts of MNPs on this highly vulnerable population.
Abbreviations
- ADME:
-
Absorption, Distribution, Metabolism, Excretion
- AFM:
-
Atomic Force Microscopy
- AOP:
-
Adverse Outcome Pathway
- AURORA:
-
Actionable eUropean ROadmap for early-life health Risk Assessment of micro- and nanoplastics
- BALF:
-
Bronchoalveolar Lavage Fluid
- BPA:
-
Bisphenol A
- CBA:
-
Component-Based Approach
- DNEL:
-
Derived No Effect Level
- DMEL:
-
Derived Minimal Effect Level
- ECHA:
-
European Chemicals Agency
- EFSA:
-
European Food Safety Authority
- EM:
-
Electron Microscopy
- EPA:
-
Environmental Protection Agency
- FTIR:
-
Fourier-Transform Infrared [Microspectroscopy]
- GIT:
-
Gastrointestinal Tract
- GRACIOUS:
-
Grouping, Read-Across, Characterisation and Integrated Approaches for Environmental and Health Safety
- HARN:
-
High Aspect Ratio Nanoparticle
- HDPE:
-
High-density Polyethylene
- HHRA:
-
Human Health Risk Assessment
- IAS:
-
Intentionally Added Substances
- IBD:
-
Inflammatory Bowel Disease
- ICCA:
-
International Council of Chemical Associations
- IR:
-
Infrared
- LDPE:
-
Low-density Polyethylene
- LPS:
-
Lipopolysaccharide
- MAF:
-
Mixture Assessment Factor
- MIE:
-
Molecular Initiating Events
- MNP:
-
Micro- and Nanoplastics
- MOA:
-
Mode of Action
- MP:
-
Microplastics
- MSAF:
-
Meconium-Stained Amniotic Fluid
- NM:
-
Nanomaterial
- NP:
-
Nanoplastics
- NIAS:
-
Non-intentionally added substances
- OECD:
-
Organisation for Economic Cooperation and Development
- PA:
-
Polyamide
- PBPK:
-
Physiology Based Pharmacokinetics
- PC:
-
Polycarbonate
- PCB:
-
Polychlorinated biphenyl
- PCP:
-
Polychloroprene
- PE:
-
Polyethylene
- PET:
-
Polyethylene Terephthalate
- PIFM:
-
Photo-Induced Force Microscopy
- PLC:
-
Polymers of Low Concern
- PM:
-
Particulate Matter
- POPs:
-
Persistent Organic Pollutants
- PP:
-
Polypropylene
- PTFE:
-
Polytetrafluoroethylene
- PRR:
-
Polymers Requiring Registration
- PS:
-
Polystyrene
- PBS:
-
Polybutylene succinate
- PU:
-
Polyurethane
- PVC:
-
Polyvinyl Chloride
- PMMA:
-
Polymethyl methacrylate
- QSAR:
-
Quantitative structure–activity relationship
- RA:
-
Risk Assessment
- RCR:
-
Risk Characterisation Ratio
- R&D:
-
Research and Development
- RMM:
-
Risk Management Measures
- RTECS:
-
Registry of Toxic Effects of Chemical Substances
- SEM:
-
Scanning Electron Microscopy
- SOP:
-
Standard Operating Procedure
- SiO2:
-
Silicon dioxide
- TDI:
-
Tolerable Daily Intake
- TiO2:
-
Titanium dioxide
- TRL:
-
Technology Readiness Level
- TWA:
-
Time Weighted Average
- WHO:
-
World Health Organisation
- WMA:
-
Whole Mixture Approach
- WoE:
-
Weight of Evidence
References
Gouin T, Boobis A, Cassee F, Koelmans A, Price S, Wagener S et al. Dietary and inhalation exposure to nano- and microplastic particles and potential implications for human health2022.
Noventa S, Boyles MSP, Seifert A, Belluco S, Jiménez AS, Johnston HJ, et al. Paradigms to assess the human health risks of nano- and microplastics. Microplastics Nanoplastics. 2021;1(1):9.
Dusza HM, van Boxel J, van Duursen MBM, Forsberg MM, Legler J, Vahakangas KH. Experimental human placental models for studying uptake, transport and toxicity of micro- and nanoplastics. Sci Total Environ. 2023;860:160403.
Sathicq MB, Sabatino R, Corno G, Di Cesare A. Are microplastic particles a hotspot for the spread and the persistence of antibiotic resistance in aquatic systems? Environ Pollut. 2021;279:116896.
Yee MS, Hii LW, Looi CK, Lim WM, Wong SF, Kok YY et al. Impact of Microplastics and nanoplastics on Human Health. Nanomaterials (Basel). 2021;11(2).
Vethaak AD, Legler J. Microplastics and human health. Science. 2021;371(6530):672–4.
Sripada K, Wierzbicka A, Abass K, Grimalt JO, Erbe A, Rollin HB, et al. A children’s Health Perspective on Nano- and microplastics. Environ Health Perspect. 2022;130(1):15001.
Ragusa A, Matta M, Cristiano L, Matassa R, Battaglione E, Svelato A, et al. Deeply in Plasticenta: Presence of Microplastics in the Intracellular compartment of human placentas. Int J Environ Res Public Health. 2022;19(18):11593.
Braun T, Ehrlich L, Henrich W, Koeppel S, Lomako I, Schwabl P, et al. Detection of Microplastic in Human Placenta and Meconium in a clinical setting. Pharmaceutics. 2021;13(7):921.
Liu S, Guo J, Liu X, Yang R, Wang H, Sun Y, et al. Detection of various microplastics in placentas, meconium, infant feces, breastmilk and infant formula: a pilot prospective study. Sci Total Environ. 2023;854:158699.
Zhu L, Zhu J, Zuo R, Xu Q, Qian Y, An L. Identification of microplastics in human placenta using laser direct infrared spectroscopy. Sci Total Environ. 2023;856(Pt 1):159060.
Ragusa A, Svelato A, Santacroce C, Catalano P, Notarstefano V, Carnevali O, et al. Plasticenta: first evidence of microplastics in human placenta. Environ Int. 2021;146:106274.
Medley EA, Spratlen MJ, Yan B, Herbstman JB, Deyssenroth MA. A systematic review of the placental translocation of Micro- and nanoplastics. Curr Environ Health Rep. 2023;10(2):99–111.
Zurub RE, Cariaco Y, Wade MG, Bainbridge SA. Microplastics exposure: implications for human fertility, pregnancy and child health. Front Endocrinol (Lausanne). 2023;14:1330396.
United States Environmental Protection Agency. Guidelines for Developmental Toxicity Risk Assessment 1991 [Available: https://www.epa.gov/risk/guidelines-developmental-toxicity-risk-assessment [Accessed 19th January 2024].
PubMed [Internet]. 2005. https://pubmed.ncbi.nlm.nih.gov/.
Web of Science [Internet]. 2022. https://www.webofscience.com/.
World Health Organization (WHO). WHO human health risk assessment toolkit: chemical hazards 2021 [Available: https://www.who.int/publications/i/item/9789240035720 [Accessed 16th January 2024].
International Council of Chemical Association (ICCA). ICCA Guidance on Chemical Risk Assessment. Int Council Chem Associations Global Prod Strategy. 2011;116:128.
Wang J, Guo X, Xue J. Biofilm-developed Microplastics as vectors of pollutants in aquatic environments. Environ Sci Technol. 2021;55(19):12780–90.
RealTimeBoard I. 2022. www.miro.com/app/.
Campanale C, Massarelli C, Savino I, Locaputo V, Uricchio VF. A detailed review study on potential effects of Microplastics and additives of concern on Human Health. Int J Environ Res Public Health. 2020;17(4).
Burton GJ, Sebire NJ, Myatt L, Tannetta D, Wang YL, Sadovsky Y, et al. Optimising sample collection for placental research. Placenta. 2014;35(1):9–22.
Dusza HM, Katrukha EA, Nijmeijer SM, Akhmanova A, Vethaak AD, Walker DI, et al. Uptake, Transport, and toxicity of Pristine and Weathered Micro- and nanoplastics in human placenta cells. Environ Health Perspect. 2022;130(9):97006.
Wieland S, Balmes A, Bender J, Kitzinger J, Meyer F, Ramsperger AF, et al. From properties to toxicity: comparing microplastics to other airborne microparticles. J Hazard Mater. 2022;428:128151.
Amelia TSM, Khalik WMAWM, Ong MC, Shao YT, Pan H-J, Bhubalan K. Marine microplastics as vectors of major ocean pollutants and its hazards to the marine ecosystem and humans. Progress Earth Planet Sci. 2021;8(1):12.
Mittal N, Tiwari N, Singh D, Tripathi P, Sharma S. Toxicological impacts of microplastics on human health: a bibliometric analysis. Environmental Science and Pollution Research; 2023.
Rafa N, Ahmed B, Zohora F, Bakya J, Ahmed S, Ahmed SF, et al. Microplastics as carriers of toxic pollutants: source, transport, and toxicological effects. Environ Pollut. 2024;343:123190.
Xu JL, Lin X, Wang JJ, Gowen AA. A review of potential human health impacts of micro- and nanoplastics exposure. Sci Total Environ. 2022;851(Pt 1):158111.
Groh KJ, Arp HPH, MacLeod M, Wang Z. Assessing and managing environmental hazards of polymers: historical development, science advances and policy options. Environ Sci Process Impacts. 2023;25(1):10–25.
Altunışık A. Prevalence of microplastics in commercially sold soft drinks and human risk assessment. J Environ Manage. 2023;336:117720.
United Nations Environment Programme. Chemicals in Plastics: A Technical Report 2023 [Available: https://www.unep.org/resources/report/chemicals-plastics-technical-report [Accessed 4th December 2023].
Zhu J, Dong X, Zhao N, Jiang S, Jin H. Microplastics in polystyrene-made food containers from China: abundance, shape, size, and human intake. Environ Sci Pollut Res. 2023;30(14):40084–93.
Ahn YS, Jeong KS, Kim KS. Cancer morbidity of professional emergency responders in Korea. 9 ed2012.
Committee ES, Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen HK, et al. Guidance on the use of the weight of evidence approach in scientific assessments. EFSA J. 2017;15(8):e04971.
Organisation for Economic Co-operation and Development (OECD). Considerations for Assessing the Risks of Combined Exposure to Multiple Chemicals, Series on Testing and Assessment No. 296 Environment, Health and Safety Division, Environment Directorate.2018 [Available: https://www.oecd.org/chemicalsafety/risk-assessment/considerations-for-assessing-the-risks-of-combined-exposure-to-multiple-chemicals.pdf [Accessed 16th January 2024].
EFSA Scientific Committee, More SJ, Bampidis V, Benford D, Bennekou SH, Bragard C, et al. Guidance on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals. EFSA J. 2019;17(3):e05634.
National Institute of Technology and Evaluation. Risk Assessment on Chemicals: For Better Understanding 2022 [Available: https://www.safe.nite.go.jp/english [Accessed 16th January 2024].
Coffin S, Bouwmeester H, Brander S, Damdimopoulou P, Gouin T, Hermabessiere L, et al. Development and application of a health-based framework for informing regulatory action in relation to exposure of microplastic particles in California drinking water. Microplastics Nanoplastics. 2022;2(1):12.
European Chemicals Agency (ECHA). Practical Guide 15: How to undertake a qualitative human health assessment and document it in a chemical safety report 2012 [Available: https://echa.europa.eu/documents/10162/13655/pg_15_qualitative-human_health_assessment_documenting_en.pdf/26a645d4-a81e-4223-8ca9-20162ae74e72 [Accessed 16th January 2024].
Putzrath RM. Reducing uncertainty of risk estimates for mixtures of chemicals within regulatory constraints. Regul Toxicol Pharmacol. 2000;31(1):44–52.
Backhaus T. The mixture assessment or allocation factor: conceptual background, estimation algorithms and a case study example. Environ Sci Europe. 2023;35(1):55.
U.S. Environmental Protection Agency. Guidelines for the Health Risk Assessment of Chemical Mixtures 1986 [Available: https://www.epa.gov/risk/guidelines-health-risk-assessment-chemical-mixtures [Accessed 7th February 2024].
Nikolopoulou D, Ntzani E, Kyriakopoulou K, Anagnostopoulos C, Machera K. Priorities and challenges in Methodology for Human Health Risk Assessment from Combined exposure to multiple chemicals. Toxics. 2023;11(5).
Backhaus T, Bergman P, Faust M, Molander L, Slunge D. Future chemical risk management: Accounting for combination effects and assessing chemicals in groups. SOU 2019:45. Swedish Government Official Reports2019 [Available: https://www.government.se/legal-documents/2019/11/sou-201945/ [Accessed 17th May 2024].
Stone V, Gottardo S, Bleeker EAJ, Braakhuis H, Dekkers S, Fernandes T, et al. A framework for grouping and read-across of nanomaterials- supporting innovation and risk assessment. Nano Today. 2020;35:100941.
Bruno I, Lobo G, Covino BV, Donarelli A, Marchetti V, Panni AS et al. Technology readiness revisited. Proceedings of the 13th International Conference on Theory and Practice of Electronic Governance. 2020:369 – 80.
European Commission D-GRI, De Rose A, Olivieri N, Strazza C, Tawil-Jamault D, Buna M et al. Technology readiness level: guidance principles for renewable energy technologies: annexes: Publications Office; 2017 [Available: https://data.europa.eu/doi/10.2777/863818 [Accessed 7th February 2024].
Vanavermaete D, Lusher A, Strand J, Abad E, Farré M, Kallenbach E, et al. Plastics in biota: technological readiness level of current methodologies. Microplastics Nanoplastics. 2024;4(1):6.
Mandemaker LDB, Meirer F. Spectro-microscopic techniques for studying nanoplastics in the Environment and in Organisms. Angew Chem Int Ed Engl. 2023;62(2):e202210494.
Buchner GA, Stepputat KJ, Zimmermann AW, Schomäcker R. Specifying technology readiness levels for the Chemical Industry. Ind Eng Chem Res. 2019;58(17):6957–69.
Rier SC, Vreemann S, Nijhof WH, van Driel V, van der Bilt IAC. Interventional cardiac magnetic resonance imaging: current applications, technology readiness level, and future perspectives. Ther Adv Cardiovasc Dis. 2022;16:17539447221119624.
Liu S, Shi J, Wang J, Dai Y, Li H, Li J, et al. Interactions between Microplastics and Heavy metals in aquatic environments: a review. Front Microbiol. 2021;12:652520.
Matthews S, Mai L, Jeong CB, Lee JS, Zeng EY, Xu EG. Key mechanisms of micro- and nanoplastic (MNP) toxicity across taxonomic groups. Comp Biochem Physiol C Toxicol Pharmacol. 2021;247:109056.
Cartwright L, Poulsen MS, Nielsen HM, Pojana G, Knudsen LE, Saunders M, et al. In vitro placental model optimization for nanoparticle transport studies. Int J Nanomed. 2012;7:497–510.
Madhusudana Rao B. Microplastics in the aquatic environment: implications for post-harvest fish quality. Indian J Fisheries. 2019;66(1).
Rochman CM, Hoh E, Hentschel BT, Kaye S. Long-term field measurement of sorption of organic contaminants to five types of plastic pellets: implications for plastic marine debris. Environ Sci Technol. 2013;47(3):1646–54.
Makhdoumi P, Hossini H, Pirsaheb M. A review of microplastic pollution in commercial fish for human consumption. Rev Environ Health. 2023;38(1):97–109.
Wick P, Malek A, Manser P, Meili D, Maeder-Althaus X, Diener L, et al. Barrier capacity of human placenta for nanosized materials. Environ Health Perspect. 2010;118(3):432–6.
Fournier SB, D’Errico JN, Adler DS, Kollontzi S, Goedken MJ, Fabris L, et al. Nanopolystyrene translocation and fetal deposition after acute lung exposure during late-stage pregnancy. Part Fibre Toxicol. 2020;17(1):55.
Krause S, Baranov V, Nel HA, Drummond JD, Kukkola A, Hoellein T et al. Gathering at the top? Environmental controls of microplastic uptake and biomagnification in freshwater food webs. Environ Pollut. 2021;268(Pt A):115750.
Miller ME, Hamann M, Kroon FJ. Bioaccumulation and biomagnification of microplastics in marine organisms: a review and meta-analysis of current data. PLoS ONE. 2020;15(10):e0240792.
Qiao R, Deng Y, Zhang S, Wolosker MB, Zhu Q, Ren H, et al. Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere. 2019;236:124334.
Andrady AL. The plastic in microplastics: a review. Mar Pollut Bull. 2017;119(1):12–22.
Pathan SI, Arfaioli P, Bardelli T, Ceccherini MT, Nannipieri P, Pietramellara G. Soil Pollution from Micro- and nanoplastic debris: a hidden and unknown Biohazard. Sustainability. 2020;12(18):7255.
Yang X, Man YB, Wong MH, Owen RB, Chow KL. Environmental health impacts of microplastics exposure on structural organization levels in the human body. Sci Total Environ. 2022;825:154025.
Wright SL, Kelly FJ. Plastic and human health: a Micro Issue? Environ Sci Technol. 2017;51(12):6634–47.
Billings A, Jones KC, Pereira MG, Spurgeon DJ. Plasticisers in the terrestrial environment: sources, occurrence and fate. Environ Chem. 2021;18(3):111–30.
Sridharan S, Kumar M, Saha M, Kirkham MB, Singh L, Bolan NS. The polymers and their additives in particulate plastics: what makes them hazardous to the fauna? Sci Total Environ. 2022;824:153828.
Muncke J. Exposure to endocrine disrupting compounds via the food chain: is packaging a relevant source? Sci Total Environ. 2009;407(16):4549–59.
Dong H, Chen Y, Wang J, Zhang Y, Zhang P, Li X, et al. Interactions of microplastics and antibiotic resistance genes and their effects on the aquaculture environments. J Hazard Mater. 2021;403:123961.
Zhang F, Man YB, Mo WY, Man KY, Wong MH. Direct and indirect effects of microplastics on bivalves, with a focus on edible species: a mini-review. Crit Rev Environ Sci Technol. 2019;50(20):2109–43.
Lupo C, Angot JL. [Public health issues associated with seafood consumption]. Bull Acad Natl Med. 2020;204(9):1017–33.
Gruber MM, Hirschmugl B, Berger N, Holter M, Radulovic S, Leitinger G, et al. Plasma proteins facilitates placental transfer of polystyrene particles. J Nanobiotechnol. 2020;18(1):128.
Siepmann J, Faham A, Clas SD, Boyd BJ, Jannin V, Bernkop-Schnurch A, et al. Lipids and polymers in pharmaceutical technology: lifelong companions. Int J Pharm. 2019;558:128–42.
Kirchkeszner C, Petrovics N, Tábi T, Magyar N, Kovács J, Szabó BS, et al. Swelling as a promoter of migration of plastic additives in the interaction of fatty food simulants with polylactic acid- and polypropylene-based plastics. Food Control. 2022;132:108354.
Wiesinger H, Wang Z, Hellweg S. Deep dive into Plastic monomers, additives, and Processing Aids. Environ Sci Technol. 2021;55(13):9339–51.
Minderoo Foundation Limited. The Plastic Health Map 2024 [Available: https://www.minderoo.org/plastic-health-map [Accessed 4th January 2024].
Groh KJ, Backhaus T, Carney-Almroth B, Geueke B, Inostroza PA, Lennquist A, et al. Overview of known plastic packaging-associated chemicals and their hazards. Sci Total Environ. 2019;651(Pt 2):3253–68.
Groh KJ, Geueke B, Martin O, Maffini M, Muncke J. Overview of intentionally used food contact chemicals and their hazards. Environ Int. 2021;150:106225.
Thépaut E, Brochot C, Chardon K, Personne S, Zeman FA. Pregnancy-PBPK models: how are biochemical and physiological processes integrated? Comput Toxicol. 2023;27:100282.
European Chemicals Agency (ECHA). Information on Chemicals 2023 [Available: https://echa.europa.eu/information-on-chemicals [Accessed 16th January 2024].
Registry of Toxic Effects of Chemical Substances (RTECS) [Internet]. 2023. https://www.ccohs.ca/products/rtecs/.
NORMAN Database System [Internet]. 2023. https://www.norman-network.net/.
Danish Environmental Protection Agency. Chemicals 2023 [Available: https://eng.mst.dk/chemicals [Accessed 4th December 2023].
Montero V, Chinchilla Y, Gómez L, Flores A, Medaglia A, Guillén R, et al. Human health risk assessment for consumption of microplastics and plasticizing substances through marine species. Environ Res. 2023;237:116843.
Pironti C, Ricciardi M, Motta O, Miele Y, Proto A, Montano L. Microplastics in the environment: intake through the Food web, human exposure and Toxicological effects. Toxics [Internet] 2021; 9(9).
Gallo F, Fossi C, Weber R, Santillo D, Sousa J, Ingram I, et al. Marine litter plastics and microplastics and their toxic chemicals components: the need for urgent preventive measures. Environ Sci Eur. 2018;30(1):13.
Mattioda V, Benedetti V, Tessarolo C, Oberto F, Favole A, Gallo M et al. Pro-inflammatory and cytotoxic effects of Polystyrene Microplastics on Human and murine intestinal cell lines. Biomolecules. 2023;13(1).
Street ME, Bernasconi S. Endocrine-disrupting chemicals in human fetal growth. Int J Mol Sci. 2020;21(4).
Zimmermann L, Dierkes G, Ternes TA, Volker C, Wagner M. Benchmarking the in Vitro Toxicity and Chemical composition of Plastic Consumer products. Environ Sci Technol. 2019;53(19):11467–77.
Arora R, Manila. In: Singh J, Kaushik RD, Chawla M, editors. Chapter 29 - styrene: risk assessment, environmental, and health hazard. Hazardous Gases: Academic; 2021. pp. 363–74.
Pilevar Z, Bahrami A, Beikzadeh S, Hosseini H, Jafari SM. Migration of styrene monomer from polystyrene packaging materials into foods: characterization and safety evaluation. Trends Food Sci Technol. 2019;91:248–61.
Zhang Y, Paul T, Brehm J, Völkl M, Jérôme V, Freitag R, et al. Role of residual monomers in the Manifestation of (Cyto)toxicity by Polystyrene Microplastic Model particles. Environ Sci Technol. 2023;57(27):9925–33.
Yuan Z, Nag R, Cummins E. Human health concerns regarding microplastics in the aquatic environment - from marine to food systems. Sci Total Environ. 2022;823:153730.
Franken R, Heringa MB, Oosterwijk T, Dal Maso M, Fransman W, Kanerva T, et al. Ranking of human risk assessment models for manufactured nanomaterials along the Cooper stage-gate innovation funnel using stakeholder criteria. NanoImpact. 2020;17:100191.
Phan S, Padilla-Gamiño JL, Luscombe CK. The effect of weathering environments on microplastic chemical identification with Raman and IR spectroscopy: part I. polyethylene and polypropylene. Polym Test. 2022;116:107752.
Enyoh CE, Duru CE, Ovuoraye PE, Wang Q. Evaluation of nanoplastics toxicity to the human placenta in systems. J Hazard Mater. 2023;446:130600.
Wohlleben W, Bossa N, Mitrano DM, Scott K. Everything falls apart: how solids degrade and release nanomaterials, composite fragments, and microplastics. NanoImpact. 2024;34:100510.
Bratovcic A. Degradation of micro-and nano-plastics by photocatalytic methods. J Nanosci Nanotechnol Appl. 2019;3:206.
European Commission Directorate-General for Environment, Bougas K, Corden C, Crookes M, Federici G, Fisk P. Scientific and technical support for the development of criteria to identify and group polymers for registration/evaluation under REACH and their impact assessment – Final report: Publications Office; 2020 [Available: https://data.europa.eu/doi/10.2779/890644 [Accessed 7th February 2024].
Almroth BC, Groh K, Walker TR, Bergmann M, Allen S, Nerin C et al. Statement on the Registration of Polymers under REACH and List of Signatures in Support International Panel on Chemical Pollution2021 [Available: https://www.ipcp.ch/activities/polymer-statment [Accessed 25th January 2024].
Boersma A, Grigoriadi K, Nooijens MGA, Henke S, Kooter IM, Parker LA et al. Microplastic Index: How Predict Microplastics Formation? Polym. 2023;15(9).
Kannan K, Vimalkumar K. A review of human exposure to Microplastics and insights into Microplastics as Obesogens. Front Endocrinol (Lausanne). 2021;12:724989.
Donaldson K, Poland CA, Murphy FA, MacFarlane M, Chernova T, Schinwald A. Pulmonary toxicity of carbon nanotubes and asbestos - similarities and differences. Adv Drug Deliv Rev. 2013;65(15):2078–86.
Amereh F, Amjadi N, Mohseni-Bandpei A, Isazadeh S, Mehrabi Y, Eslami A et al. Placental plastics in young women from general population correlate with reduced foetal growth in IUGR pregnancies. Environ Pollut. 2022;314.
Murphy F, Dekkers S, Braakhuis H, Ma-Hock L, Johnston H, Janer G, et al. An integrated approach to testing and assessment of high aspect ratio nanomaterials and its application for grouping based on a common mesothelioma hazard. NanoImpact. 2021;22:100314.
Grafmueller S, Manser P, Diener L, Diener PA, Maeder-Althaus X, Maurizi L, et al. Bidirectional transfer study of polystyrene nanoparticles across the placental barrier in an ex vivo human placental perfusion model. Environ Health Perspect. 2015;123(12):1280–6.
Grafmueller S, Manser P, Diener L, Maurizi L, Diener PA, Hofmann H, et al. Transfer studies of polystyrene nanoparticles in the ex vivo human placenta perfusion model: key sources of artifacts. Sci Technol Adv Mater. 2015;16(4):044602.
Stock V, Bohmert L, Lisicki E, Block R, Cara-Carmona J, Pack LK, et al. Uptake and effects of orally ingested polystyrene microplastic particles in vitro and in vivo. Arch Toxicol. 2019;93(7):1817–33.
Geueke B, Groh KJ, Maffini MV, Martin OV, Boucher JM, Chiang YT, et al. Systematic evidence on migrating and extractable food contact chemicals: most chemicals detected in food contact materials are not listed for use. Crit Rev Food Sci Nutr. 2023;63(28):9425–35.
Muncke J, Andersson AM, Backhaus T, Boucher JM, Carney Almroth B, Castillo Castillo A, et al. Impacts of food contact chemicals on human health: a consensus statement. Environ Health. 2020;19(1):25.
Zimmermann L, Scheringer M, Geueke B, Boucher JM, Parkinson LV, Groh KJ, et al. Implementing the EU Chemicals Strategy for sustainability: the case of food contact chemicals of concern. J Hazard Mater. 2022;437:129167.
Jeong J, Choi J. Development of AOP relevant to microplastics based on toxicity mechanisms of chemical additives using ToxCast™ and deep learning models combined approach. Environ Int. 2020;137:105557.
Jones LR, Wright SJ, Gant TW. A critical review of microplastics toxicity and potential adverse outcome pathway in human gastrointestinal tract following oral exposure. Toxicol Lett. 2023;385:51–60.
Health and Safety Executive (HSE). EH40/2005 Workplace exposure limits. Containing the list of workplace exposure limits for use with the Control of Substances Hazardous to Health Regulations 2002: TSO (The Stationery Office); 2020 [Fourth:[Available: https://www.hse.gov.uk/pubns/books/eh40.htm [Accessed 4th December 2023].
European Agency for Safety and Health at Work (EU-OSHA). Occupational exposure limit values 2022 [Available: https://oshwiki.osha.europa.eu/en/themes/occupational-exposure-limit-values [Accessed 25th January 2024].
European Commission. TDI - Tolerable Daily Intake 2024 [Available: https://ec.europa.eu/health/scientific_committees/opinions_layman/en/phthalates-school-supplies/glossary/tuv/tdi-tolerable-daily-intake.htm [Accessed 18th January 2024].
Gundert-Remy U, Bodin J, Bosetti C, FitzGerald R, Hanberg A, Hass U, et al. Bisphenol A (BPA) hazard assessment protocol. EFSA Supporting Publications. 2017;14(12):1354E.
Balhara A, Kumar AR, Unadkat JD. Predicting Human fetal drug exposure through maternal-fetal PBPK modeling and in Vitro or Ex vivo studies. J Clin Pharmacol. 2022;62(Suppl 1Suppl 1):S94–114.
Wardani I, Hazimah Mohamed Nor N, Wright SL, Kooter IM, Koelmans AA. Nano- and microplastic PBK modeling in the context of human exposure and risk assessment. Environ Int. 2024;186:108504.
Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311(6998):171–4.
Martens DS, Sleurs H, Dockx Y, Rasking L, Plusquin M, Nawrot TS. Association of Newborn Telomere length with blood pressure in Childhood. JAMA Netw Open. 2022;5(8):e2225521.
Ragsdale HB, Kuzawa CW, Borja JB, Avila JL, McDade TW. Regulation of inflammation during gestation and birth outcomes: inflammatory cytokine balance predicts birth weight and length. Am J Hum Biol. 2019;31(3):e23245.
Halfar J, Cabanova K, Vavra K, Delongova P, Motyka O, Spacek R, et al. Microplastics and additives in patients with preterm birth: the first evidence of their presence in both human amniotic fluid and placenta. Chemosphere. 2023;343:140301.
Li Z, Wang J, Gao X, Du J, Sui H, Wu J, et al. Investigation of Microplastics in Meconium by Fourier Transform Infrared Microspectroscopy. Toxics. 2023;11(4):310.
Yang T, Wang J. Exposure sources and pathways of micro- and nanoplastics in the environment, with emphasis on potential effects in humans: a systematic review. Integr Environ Assess Manag. 2023;19(6):1422–32.
Dang F, Wang Q, Huang Y, Wang Y, Xing B. Key knowledge gaps for one health approach to mitigate nanoplastic risks. Eco Environ Health. 2022;1(1):11–22.
Dewika M, Markandan K, Irfan NA, Mohd Abdah MAA, Ruwaida JN, Sara YY, et al. Review of microplastics in the indoor environment: distribution, human exposure and potential health impacts. Chemosphere. 2023;324:138270.
Baeza-Martinez C, Olmos S, Gonzalez-Pleiter M, Lopez-Castellanos J, Garcia-Pachon E, Masia-Canuto M, et al. First evidence of microplastics isolated in European citizens’ lower airway. J Hazard Mater. 2022;438:129439.
Huang S, Huang X, Bi R, Guo Q, Yu X, Zeng Q, et al. Detection and analysis of Microplastics in Human Sputum. Environ Sci Technol. 2022;56(4):2476–86.
Li W, Lin G, Xiao Z, Zhang Y, Li B, Zhou Y, et al. A review of respirable fine particulate matter (PM(2.5))-induced brain damage. Front Mol Neurosci. 2022;15:967174.
Nakane H. Translocation of particles deposited in the respiratory system: a systematic review and statistical analysis. Environ Health Prev Med. 2012;17(4):263–74.
Nemmar A, Hoet PH, Vanquickenborne B, Dinsdale D, Thomeer M, Hoylaerts MF, et al. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002;105(4):411–4.
Gasperi J, Wright SL, Dris R, Collard F, Mandin C, Guerrouache M, et al. Microplastics in air: are we breathing it in? Curr Opin Environ Sci Health. 2018;1:1–5.
Prata JC, Dias-Pereira P. Microplastics in Terrestrial Domestic animals and Human Health: implications for Food Security and Food Safety and their role as sentinels. Animals. 2023;13(4).
Zhang N, Li YB, He HR, Zhang JF, Ma GS. You are what you eat: Microplastics in the feces of young men living in Beijing. Sci Total Environ. 2021;767:144345.
Zarus GM, Muianga C, Hunter CM, Pappas RS. A review of data for quantifying human exposures to micro and nanoplastics and potential health risks. Sci Total Environ. 2021;756:144010.
Rahman A, Sarkar A, Yadav OP, Achari G, Slobodnik J. Potential human health risks due to environmental exposure to nano- and microplastics and knowledge gaps: a scoping review. Sci Total Environ. 2021;757:143872.
Gimeno-Benito I, Giusti A, Dekkers S, Haase A, Janer G. A review to support the derivation of a worst-case dermal penetration value for nanoparticles. Regul Toxicol Pharmacol. 2021;119:104836.
Biagini Myers JM, Khurana Hershey GK. Eczema in early life: genetics, the skin barrier, and lessons learned from birth cohort studies. J Pediatr. 2010;157(5):704–14.
Gupta V, Mohapatra S, Mishra H, Farooq U, Kumar K, Ansari MJ et al. Nanotechnology in cosmetics and Cosmeceuticals-A review of latest advancements. Gels. 2022;8(3).
World Health Organization. Social determinants of health: WHO Regional Office for South-East Asia; 2008 [Available: https://iris.who.int/bitstream/handle/10665/43943/9789241563703_eng.pdf?sequence=1 [Accessed 26th January 2024].
Yoder BA, Kirsch EA, Barth WH, Gordon MC. Changing obstetric practices associated with decreasing incidence of meconium aspiration syndrome. Obstet Gynecol. 2002;99(5 Pt 1):731–9.
Bhatia BD, Gupta V, Dey PK. Meconium aspiration syndrome: current concepts. Indian J Matern Child Health. 1996;7(1):1–7.
Ragusa A, Notarstefano V, Svelato A, Belloni A, Gioacchini G, Blondeel C et al. Raman Microspectroscopy Detection and Characterisation of Microplastics in Human Breastmilk. Polym (Basel). 2022;14(13).
Kadac-Czapska K, Jutrzenka Trzebiatowska P, Mazurkiewicz M, Kowalczyk P, Knez E, Behrendt M, et al. Isolation and identification of microplastics in infant formulas – A potential health risk for children. Food Chem. 2024;440:138246.
Moya J, Bearer CF, Etzel RA. Children’s behavior and physiology and how it affects exposure to environmental contaminants. Pediatrics. 2004;113(4 Suppl):996–1006.
Amran NH, Zaid SSM, Mokhtar MH, Manaf LA, Othman S. Exposure to Microplastics during early developmental stage: review of current evidence. Toxics. 2022;10(10).
Dusza HM, Manz KE, Pennell KD, Kanda R, Legler J. Identification of known and novel nonpolar endocrine disruptors in human amniotic fluid. Environ Int. 2022;158:106904.
Gray T, Huestis M. Bioanalytical procedures for monitoring in utero drug exposure. Anal Bioanal Chem. 2007;388(7):1455–65.
Dugershaw BB, Aengenheister L, Hansen SSK, Hougaard KS, Buerki-Thurnherr T. Recent insights on indirect mechanisms in developmental toxicity of nanomaterials. Part Fibre Toxicol. 2020;17(1):31.
Leslie HA, van Velzen MJM, Brandsma SH, Vethaak AD, Garcia-Vallejo JJ, Lamoree MH. Discovery and quantification of plastic particle pollution in human blood. Environ Int. 2022;163:107199.
Cox KD, Covernton GA, Davies HL, Dower JF, Juanes F, Dudas SE. Human consumption of Microplastics. Environ Sci Technol. 2019;53(12):7068–74.
Senathirajah K, Attwood S, Bhagwat G, Carbery M, Wilson S, Palanisami T. Estimation of the mass of microplastics ingested - A pivotal first step towards human health risk assessment. J Hazard Mater. 2021;404(Pt B):124004.
Pletz M. Ingested microplastics: do humans eat one credit card per week? J Hazard Mater Lett. 2022;3:100071.
The European Food Safety Authority (EFSA). Presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA J. 2016;14(6):e04501.
Ibrahim YS, Tuan Anuar S, Azmi AA, Wan Mohd Khalik WMA, Lehata S, Hamzah SR, et al. Detection of microplastics in human colectomy specimens. JGH Open. 2021;5(1):116–21.
Di Cristo L, Janer G, Dekkers S, Boyles M, Giusti A, Keller JG, et al. Integrated approaches to testing and assessment for grouping nanomaterials following dermal exposure. Nanotoxicology. 2022;16(3):310–32.
Di Cristo L, Keller JG, Leoncino L, Marassi V, Loosli F, Seleci DA, et al. Critical aspects in dissolution testing of nanomaterials in the oro-gastrointestinal tract: the relevance of juice composition for hazard identification and grouping. Nanoscale Adv. 2024;6(3):798–815.
Innes E, Yiu HHP, McLean P, Brown W, Boyles M. Simulated biological fluids – a systematic review of their biological relevance and use in relation to inhalation toxicology of particles and fibres. Crit Rev Toxicol. 2021;51(3):217–48.
Keller JG, Peijnenburg W, Werle K, Landsiedel R, Wohlleben W. Understanding dissolution rates via Continuous Flow Systems with physiologically relevant Metal Ion Saturation in Lysosome. Nanomaterials. 2020;10(2):311.
Keller JG, Persson M, Müller P, Ma-Hock L, Werle K, Arts J, et al. Variation in dissolution behavior among different nanoforms and its implication for grouping approaches in inhalation toxicity. NanoImpact. 2021;23:100341.
Marques MR, Loebenberg R, Almukainzi M. Simulated biological fluids with possible application in dissolution testing. Dissolution Technol. 2011;18(3):15–28.
Zanoni I, Keller JG, Sauer UG, Müller P, Ma-Hock L, Jensen KA, et al. Dissolution rate of nanomaterials determined by ions and particle size under lysosomal conditions: contributions to standardization of Simulant fluids and Analytical methods. Chem Res Toxicol. 2022;35(6):963–80.
Hirt N, Body-Malapel M. Immunotoxicity and intestinal effects of nano- and microplastics: a review of the literature. Part Fibre Toxicol. 2020;17(1):57.
Fukui H. Increased intestinal permeability and decreased barrier function: does it really influence the risk of inflammation? Inflamm Intest Dis. 2016;1(3):135–45.
Madison A, Kiecolt-Glaser JK. Stress, depression, diet, and the gut microbiota: human-bacteria interactions at the core of psychoneuroimmunology and nutrition. Curr Opin Behav Sci. 2019;28:105–10.
Teixeira TF, Collado MC, Ferreira CL, Bressan J, Peluzio Mdo C. Potential mechanisms for the emerging link between obesity and increased intestinal permeability. Nutr Res. 2012;32(9):637–47.
Viggiano D, Ianiro G, Vanella G, Bibbo S, Bruno G, Simeone G, et al. Gut barrier in health and disease: focus on childhood. Eur Rev Med Pharmacol Sci. 2015;19(6):1077–85.
Bishehsari F, Magno E, Swanson G, Desai V, Voigt RM, Forsyth CB, et al. Alcohol and gut-derived inflammation. Alcohol Res. 2017;38(2):163–71.
Sigthorsson G, Tibble J, Hayllar J, Menzies I, Macpherson A, Moots R, et al. Intestinal permeability and inflammation in patients on NSAIDs. Gut. 1998;43(4):506–11.
Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. 2015;26:26191.
Li B, Ding Y, Cheng X, Sheng D, Xu Z, Rong Q, et al. Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice. Chemosphere. 2020;244:125492.
Martinez JE, Kahana DD, Ghuman S, Wilson HP, Wilson J, Kim SCJ, et al. Unhealthy lifestyle and gut dysbiosis: a better understanding of the effects of Poor Diet and Nicotine on the intestinal microbiome. Front Endocrinol (Lausanne). 2021;12:667066.
Glencross DA, Ho TR, Camina N, Hawrylowicz CM, Pfeffer PE. Air pollution and its effects on the immune system. Free Radic Biol Med. 2020;151:56–68.
Stolfi C, Maresca C, Monteleone G, Laudisi F. Implication of Intestinal Barrier Dysfunction in Gut Dysbiosis and diseases. Biomedicines. 2022;10(2):289.
Wang F, Liu J, Hu X, Zhong Y, Wen F, Tang X, et al. The influence on oxidative stress markers, inflammatory factors and intestinal injury-related molecules in Wahui pigeon induced by lipopolysaccharide. PLoS ONE. 2021;16(5):e0251462.
Schwarzfischer M, Rogler G. The intestinal barrier-shielding the body from Nano- and Microparticles in our Diet. Metabolites. 2022;12(3):223.
Yang S, Cheng Y, Chen Z, Liu T, Yin L, Pu Y, et al. In vitro evaluation of nanoplastics using human lung epithelial cells, microarray analysis and co-culture model. Ecotoxicol Environ Saf. 2021;226:112837.
Delaval M, Boland S, Solhonne B, Nicola MA, Mornet S, Baeza-Squiban A, et al. Acute exposure to silica nanoparticles enhances mortality and increases lung permeability in a mouse model of Pseudomonas aeruginosa pneumonia. Part Fibre Toxicol. 2015;12(1):1.
Inoue K, Takano H, Ohnuki M, Yanagisawa R, Sakurai M, Shimada A, et al. Size effects of nanomaterials on lung inflammation and coagulatory disturbance. Int J Immunopathol Pharmacol. 2008;21(1):197–206.
Thompson LC, Holland NA, Snyder RJ, Luo B, Becak DP, Odom JT, et al. Pulmonary instillation of MWCNT increases lung permeability, decreases gp130 expression in the lungs, and initiates cardiovascular IL-6 transsignaling. Am J Physiol Lung Cell Mol Physiol. 2016;310(2):L142–54.
Hollingsworth JW 2nd, Cook DN, Brass DM, Walker JK, Morgan DL, Foster WM, et al. The role of toll-like receptor 4 in environmental airway injury in mice. Am J Respir Crit Care Med. 2004;170(2):126–32.
Mancini AJ, Skin. Pediatrics. 2004;113(4 Suppl):1114–9.
Walczak AP, Kramer E, Hendriksen PJ, Tromp P, Helsper JP, van der Zande M, et al. Translocation of differently sized and charged polystyrene nanoparticles in in vitro intestinal cell models of increasing complexity. Nanotoxicology. 2015;9(4):453–61.
Lara S, Alnasser F, Polo E, Garry D, Lo Giudice MC, Hristov DR, et al. Identification of receptor binding to the Biomolecular Corona of Nanoparticles. ACS Nano. 2017;11(2):1884–93.
Liu S, Junaid M, Liao H, Liu X, Wu Y, Wang J. Eco-corona formation and associated ecotoxicological impacts of nanoplastics in the environment. Sci Total Environ. 2022;836:155703.
Nasser F, Lynch I. Secreted protein eco-corona mediates uptake and impacts of polystyrene nanoparticles on Daphnia magna. J Proteom. 2016;137:45–51.
Mohamed Nor NH, Kooi M, Diepens NJ, Koelmans AA. Lifetime Accumulation of Microplastic in children and adults. Environ Sci Technol. 2021;55(8):5084–96.
Gaspar L, Bartman S, Coppotelli G, Ross JM. Acute exposure to Microplastics Induced changes in behavior and inflammation in Young and Old mice. Int J Mol Sci. 2023;24(15).
Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368(9553):2167–78.
Ross EJ, Graham DL, Money KM, Stanwood GD. Developmental consequences of fetal exposure to drugs: what we know and what we still must learn. Neuropsychopharmacology. 2015;40(1):61–87.
Qi W, Bi J, Zhang X, Wang J, Wang J, Liu P, et al. Damaging effects of multi-walled carbon nanotubes on pregnant mice with different pregnancy times. Sci Rep. 2014;4(1):4352.
Yang H, Sun C, Fan Z, Tian X, Yan L, Du L, et al. Effects of gestational age and surface modification on materno-fetal transfer of nanoparticles in murine pregnancy. Sci Rep. 2012;2(1):847.
Carter AM. Animal models of human pregnancy and placentation: alternatives to the mouse. Reproduction. 2020;160(6):R129–43.
Bongaerts E, Lecante LL, Bove H, Roeffaers MBJ, Ameloot M, Fowler PA, et al. Maternal exposure to ambient black carbon particles and their presence in maternal and fetal circulation and organs: an analysis of two independent population-based observational studies. Lancet Planet Health. 2022;6(10):e804–11.
Barchiesi M, Kooi M, Koelmans AA. Adding depth to Microplastics. Environ Sci Technol. 2023;57(37):14015–23.
Koelmans AA, Redondo-Hasselerharm PE, Nor NHM, de Ruijter VN, Mintenig SM, Kooi M. Risk assessment of microplastic particles. Nat Reviews Mater. 2022;7(2):138–52.
Kuempel ED, Geraci CL, Schulte PA. Risk assessment and risk management of nanomaterials in the workplace: translating research to practice. Ann Occup Hyg. 2012;56(5):491–505.
European Chemicals Agency (ECHA). Guidance on Information Requirements and Chemical Safety Assessment. Part E: Risk Characterisation. European Chemicals Agency2016 [Available: https://echa.europa.eu/documents/10162/13632/information_requirements_part_e_en.pdf/1da6cadd-895a-46f0-884b-00307c0438fd [Accessed 16th January 2024].
Organisation for Economic Co-operation and Development (OECD). Policies to Reduce Microplastics Pollution in Water 2021 [Available: https://www.oecd.org/environment/waste/policy-highlights-policies-to-reduce-microplastics-pollution-in-water-focus-textiles-and-tyres.pdf [Accessed 16th January 2024].
European Chemicals Agency (ECHA). Microplastics 2023 [Available: https://echa.europa.eu/hot-topics/microplastics [Accessed 7th April 2023].
European Commission. Commission Regulation (EU). 2023/2055 of 25 September 2023 amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as regards synthetic polymer microparticles 2023 [Available: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32023R2055 [Accessed 4th December 2023].
Acknowledgements
We would like to thank the anonymous reviewers for their valuable comments and suggestions, which significantly improved the quality of this manuscript.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under Actionable eUropean ROadmap for early-life health Risk Assessment of micro- and nanoplastics (AURORA) grant agreement No 964827. LDBM, FM and BMW were supported by the Dutch Research Council (NWO) via grant OCENW.GROOT.2019.043.
Author information
Authors and Affiliations
Contributions
MSPB, EAC, AD, LT, and YCdV: conceptualisation and design of study: EAC and MSPB: evaluation of literature, and drafting the manuscript; AD and YCdV: developed and conducted the evidence mapping of the existing RA approaches to MNPs; YCdV: developed the TRL scales for MNP methods, with additional contributions by LDBM, FM, BMW, BMSB, NDS and RZ; KSG, JvB HMC, HMD, JL, JM, LZ, NDS, BMSB, RZ and TSN contributed to review and editing of the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Christopher, E.A., Christopher-de Vries, Y., Devadoss, A. et al. Impacts of micro- and nanoplastics on early-life health: a roadmap towards risk assessment. Micropl.&Nanopl. 4, 13 (2024). https://doi.org/10.1186/s43591-024-00089-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43591-024-00089-3