Abstract
Sustaining high crop yield and profitability on marginal soils, such as degraded ferralsols, calls for the use of adequate farming practices. In this study, we assessed for four cropping seasons the effect of di-ammonium phosphate (DAP) fertilizer application doses (100, 150, and 200 kg ha−1) and plant densities (100,000, 166,666, and 250,000 plants ha−1) on growth, yield, nodulation capacity, and profitability of two pea varieties (Adei and Rwanda) under degraded ferralsols in eastern Democratic Republic of Congo (DRC). Results showed that pea growth, yield, nodulation, and profitability were significantly affected by the DAP dose and the plant density. Application of 150 kg ha−1 of DAP improved pea yields from 730 to 1720 kg ha−1 and nodulation from 17 to 22 nodules per plant. The number of active nodules was positively correlated with total number of nodules, DAP doses, yield parameters, and plant height. High grain yield was recorded during the long rainy season (1400 kg ha−1) compared to the short one (930 kg ha−1). All DAP doses were profitable according to the agronomic efficiency (AE) and value–cost ratio (VCR) analyses. For improved and profitable pea production on degraded South-Kivu ferralsols, applying 150 kg ha−1 of DAP combined with low plant density are to be recommended and preferably in the long rainy season.
Similar content being viewed by others
Introduction
Pea (Pisum sativum L.) is an important grain pulse worldwide, particularly in tropical and subtropical Africa. It is among ten main staple crops worldwide, appreciated for its tolerance to biotic and abiotic constraints and its high nutritional value content (Brkić et al. 2004). For instance, peas are rich in proteins (21–25%), vitamins (A and C), insoluble carbohydrates (5%), fiber, minerals (such as P and Ca), and essential amino acids (such as Tryptophan and Lysine) (Dahl et al. 2012). With 5–20% of Trypsin inhibitors than in soybean, peas are used for livestock feeding without any processing (Bhat et al. 2013; Mishra 2014; Smýkal et al. 2012).
Peas were known in mountainous regions of eastern and central Africa before the arrival of Europeans. It was the main staple food crop in the Rwandan culture, in South-eastern Uganda, eastern Democratic Republic of Congo (DRC), and the entire Great lakes region (DAFF 2016). In DRC and particularly in the South-Kivu province, pea grains (considered as a “rich man’s food”) are consumed as vegetable and are highly appreciated for their flavor and taste (Vwima 2014).
These above-mentioned attributes highlight the crucial role played by peas in smallholder farmers’ food and income security in eastern Africa, thus explaining its high demand at local and regional markets. It is noteworthy that pea production in South-Kivu, eastern DRC does not meet the local demand, such that most pea found at local markets are imported from Rwanda (Kamembe) and/or North-Kivu province (Vwima 2014). In fact, South-Kivu’s agroecological zones (AEZ), and particularly those of Kabare territory, are not conducive to legume production (the average yield is ~ 450 kg ha−1) compared to other African Great lakes sub-regions (~ 800–850 kg ha−1 in Rwanda and Burundi and up ~ 1.7 t ha−1 at the global level) (IFDC 2009). Several factors are responsible of such a low legume yield in eastern DRC: inadequate farming practices, no or low use of fertilizers and other chemicals, soil depletion due to overexploitation, use of degenerated varieties, etc. (Baudouin 2001; Mushagalusa et al. 2016).
Legume production and, particularly peas, allows maintaining high soil fertility by their ability of biological nitrogen fixation (BNF) in association with Rhizobium bacteria (Negi et al. 2006). Though they fix sufficient nitrogen (N) via BNF, their nutrient requirements (for elements, such as P, K, and Mg) are high (DAFF 2016; Batista de Moraisa et al. 2020). For instance, peas and other legumines are demanding in P (Batista de Moraisa et al. 2020). The phosphorous (P) plays crucial role in plant growth and development and is directly linked with root proliferation (in synergy with K), grain formation and maturity (GRDC 2018; Scanlon 2015). According to Bolland et al. (2000) and Karkanis et al. (2018), BNF is an intense and energy-demanding process that requires P for Adenosine Tri-Phosphate (ATP) synthesis. Thus, applying ~ 40 kg ha−1 of P is required for a better BNF. In general, N and P based fertilizers are required for better yields on poor tropical soils (Ferralsols, Acrisols, Cambisols, etc.) (WRB 2015).
As shown previously, peas are not demanding in N fertilizer; hence the application of a high N quantity would reduce the BNF. Instead, P is required for plant growth and proper BNF. Phosphorous (P) uptake by peas varies with varieties and production objectives. On normal soils, applying ~ 30 to 40 kg ha−1 of P2O5 (corresponding to ~ 100 kg ha−1 of DAP) is optimal. In fact, at the establishment growth stage, before nodules are formed (attesting the BNF); peas require N for its growth. Since degraded soils are usually deficient in N and P to sustain sufficient plant vigor and BNF initiation at such establishment stage, it is recommended to supply required N quantity. Gan et al. (2003) and Gregory and Nortcliff (2013) suggested ~ 13 kg ha−1 N, while Blanco-Canqui and Lal (2007) proposed instead ~ 20 to 60 kg ha−1 of N.
On one hand, recommendation for fertilizer dose should meet the plant nutritional needs to promote optimal growth and development but also reduce and minimize environmental harmful effects and make agricultural activities cost-effective (Giller et al. 2013). On the other hand, the efficiency and profitability of fertilizers depend on other farming practices, such as optimal plant density, productive varieties, and adaptation to local conditions (Blanco-Canqui and Lal 2007). Differences in plant growth, development, and yield were recorded with plant densities and pea cultivars (Park et al. 1999; Ayaz et al. 2004; Pageau et al. 2006).
The eastern DRC is characterized by Ferralsols (Chuma et al. 2020). These soils are depleted, poor in nutrients with a very low cations exchange capacity (CEC), low clay activity, acidic and rich in Fe and Al oxides that limit the availability of soil nutrients, such as P and K. These kinds of soils are distributed on ~ 8% of the world surface (WRB 2015). In such tropical soils, N and P are limiting factors. Phosphorous deficiency has been cited as the principal constraint to legume production in these zones; the nutrient requirements becoming difficult to meet in such soils rich in Fe and Al oxides (Bünemann et al. 2011; Ehlers et al. 2010).
Many studies have been conducted on P and N fertilization on legumes in tropical regions (Tittonell et al. 2005; Vandamme et al. 2014; Mushagalusa et al. 2016; Dhital et al. 2017). Amare et al. (2005) and Getachew (2009) found that pea yields increased significantly with P input on tropical soils of Ethiopia, while Jitender (2011) reported their positive effect on nodulation. In eastern DRC, and particularly in the South-Kivu province, IFDC/Catalyst project had conducted experiments to determine the optimal fertilizer doses for main staple crops (IFDC 2009). Unfortunately, it focused only on legumes, such as common bean and soybean. Therefore, no recommendations exist on varietal response, planting density, dose, soil type, and cropping season for peas. As a consequence, pea producers still wonder whether farming practices recommended for common bean and soybean are adequate for pea cultivation. Due to its economic and nutritional values, peas deserve attentions from researchers and other stakeholders in South-Kivu for its full potential as tool for combating hunger and poverty among smallholder farmers.
We hypothesized in the present study that optimal farming practices (plant density, fertilizer dose, varietal response) recommended for common bean could be used by pea farmers to allow high yield and profitability in South-Kivu. This study will, therefore, fill the gap in documentation for pea farming practices in the study area and answer the question whether the combination of optimal plant density and DAP fertilizer dose would improve growth, yield, nodulation capacity, and profitability of pea varieties on poor ferralsols in Kabare territory, eastern DRC.
Materials and methods
Description of the study area
-
a)
Study site
The experiment was conducted in Miti, Kabare territory, in the vicinity of the “Institut National d’Etudes et Recherches Agronomiques” (INERA), Mulungu station, South-Kivu, eastern DRC. It was conducted during long (A) and short (B) rainy seasons of 2017 and 2018. The geographical coordinates of the study site were 02° 18′ 56″ S, 28° 47′ 45.9″ E, and ~ 1700 m above sea level. Figure 1 locates the study area on a DRC soil map.
-
b)
Soil characteristics and vegetation
Soils at Miti (Kabare territory) are of three types: Ferralsols that are predominant across the territory, followed by Nitisols and Cambisols in the surroundings of the Kahuzi Biega National Park (PNKB) and extends to the southern Kabare (Fig. 1). Poor and depleted Ferralsols characterize the study site. Soil laboratory results of the study site are presented in Table 1.
Analyses performed on composite soil samples (collected at depth of 0–50 cm) indicate that the experimental field was acidic (pH = 5.2) with 0.63 charge difference. That soil pH is below legume pH requirements (6.0 to 7.5) (EIAR 2018). It had also low CEC, characteristics of ferralsols (CEC = 20.5). Soil texture analysis according to USDA Soil Texture Triangle classified the study area soil as clayey, rich in Kaolinite. With low CEC and reddish color, these soils are rich in Fe and Al oxides. High concentration in kaolinite, illite, and gibbsite could explain their micro-aggregation as suggested by the World Reference Base for Soil Resources (WRB 2015). Low levels of N (0.28%) and organic matter (OM) (2.16%) were also observed and led us to select binary fertilizer (containing N and P) and organic manure to enhance soil humus and pH during the experiment.
South-Kivu soil map and location of Kabare territory. Classification made based on the WRB classification (extracted from Dewitte et al. 2013)
Climate
Kabare is characterized by a humid tropical climate, temperated by the elevation. According to Köppen–Geiger classification, it is a Aw3 climate type (Chuma et al. 2021; Beck et al. 2018). Such a climate type is favorable for a wide crop diversity and production. During the experimental period (2017–2018), meteorological data indicated an average temperature of ~ 17.5 and ~ 19 °C, for the first and second years, respectively. Across years, the weather data were significantly different. In 2017, November and February were the most humid, followed by January and March. In 2018, the rainfall started early in January but decreased in intensity in February, though it corresponds to the beginning of the short rainy season (B) (Additional file 1). Based on the number of rainy days, November, December, and Avril 2017 had the highest number of rainy days (16 days), followed by March (14 days). June and July were the driest months (with 2–3 rainy days). Nevertheless, rain cumulations during the experimental periods were ~ 1452 mm (2017) and ~ 1386 mm (2018), far above water requirements by peas (600–1000 mm). The year is divided into two main seasons (bimodal): long rainy season (A) which goes from September to January and a short rainy season (B) from mid-February to the end of May. June–August is the dry season, only wetlands and inland valleys are exploited during that period for vegetable production. That extra cropping season is locally referred to as season C.
Methods
Study factors and experimental design
The field experiment was conducted at Miti for four cropping seasons (A and B of 2017 and 2018). Three factors were studied: the main factor was the DAP (46% P2O5 and 16% NH4+) fertilizer dose with four levels (no fertilizer or control, 100, 150, and 200 kg ha−1); the secondary factor was the plant density with three levels (100,000, 166,666, and 250,000 plants ha−1; corresponding to 50 cm × 40 cm, 40 cm × 30 cm, and 40 cm × 20 cm, respectively). The tertiary factor was made of the variety with two levels (a popular local variety called Adei and an introduced variety called Rwanda from the Rusizi district of Rwanda). Seeds of both varieties were sourced from the INERA-Mulungu genebank. The combination of factors provided 24 treatments, replicated in three blocks. The plot size was 3 m × 2.4 m (7.2 m2), plots were spaced by 0.5 m, while 1 m separated two blocks.
Field management
Field activities started with the 2017 long rainy season (A) and ended with the 2018 short rainy season (B). The sowing dates were 15 September and 17 February for seasons A and B, respectively, for both years. Two pea seeds were sown per seeding hole. Weeding was practiced twice a season for weed control. DAP fertilizer was applied at planting at four dose levels: 0, 100, 150, and 200 kg ha−1. As the field was poor in organic matter, an amendment using decomposed cow manure was also supplied (~ 1 t ha−1, corresponding to ~ 52 kg for the whole experiment). Laboratory analyses showed that used cow manure had pH = 8.4, 59 ppm P, 24% C, and 1.7 and 0.21% Ca2+ and Mg2+, respectively. Staking was done 37 days after sowing (DAS). The harvest intervened at physiological maturity when pods were completely dry, 115 DAS.
Assessed parameters
-
a)
Vegetative growth parameters
The seedling emergence rate (%) was recorded at 15 DAS and estimated as the ratio between emerged seedlings and the total number of seeds sown per plot. Leaf area was assessed 50 DAS and calculated using the method by Lecoeur and Sinclair (1996). The collar diameter was evaluated 80 DAS with a calliper, while the plant height was recorded 100 DAS using a rule. Ten plants were randomly selected in each plot to estimate these parameters.
-
b)
Yield parameters
The number of pods per plant and the number of grains per pod were determined by manual counting. The 100-seed weight from each plot lot was determined using a precision weighing balance. Plot grain yields were determined using weighing balance and values were extrapolated to the hectare.
-
c)
Nodulation parameters
Nodulation-related parameters were assessed at flowering. We considered the total number of nodules per plant (on a sample of ten plants per plot); the total number of active nodules from the total number of nodules per plant. “Active nodules” were determined by visual observation of nodule color after sectioning using a blade (Woomer et al. 2011): “red” colored nodules were considered “active”, while “pink” and “white” were considered as “weakly active” and “no active”, respectively. Figure 2 shows nodules (red-colored nodules and white-colored ones) after the blade section. That classification was made according to Corbin et al. (1977) and Woomer et al. (2011).
-
d)
Assessment of agronomic efficiency (AE) and profitability of applied DAP doses
To evaluate the efficiency of each DAP dose, the Agronomic Efficiency (AE) was used, while profitability was evaluated using the value–cost ratio (VCR). AE and VCR were calculated using formulas (1) and (2):
$${\mathbf{EA}}\left( {{\text{kg}}\;{\text{kg}}^{ - 1} } \right) = {{\left( {{\mathbf{Y}}_{{\mathbf{i}}} - {\mathbf{Y}}_{{\mathbf{0}}} } \right)} \mathord{\left/ {\vphantom {{\left( {{\mathbf{Y}}_{{\mathbf{i}}} - {\mathbf{Y}}_{{\mathbf{0}}} } \right)} {{\mathbf{Qi}}}}} \right. \kern-\nulldelimiterspace} {{\mathbf{Qi}}}}$$(1)$${\mathbf{VCR}}\left( {\$ \;\$^{ - 1} } \right) = {{{\mathbf{EA}}*\left( {{\mathbf{Pp}}} \right)} \mathord{\left/ {\vphantom {{{\mathbf{EA}}*\left( {{\mathbf{Pp}}} \right)} {\left( {{\mathbf{P}}_{{{\mathbf{DAP}}}} } \right)}}} \right. \kern-\nulldelimiterspace} {\left( {{\mathbf{P}}_{{{\mathbf{DAP}}}} } \right)}}$$(2)With Yi is the grain yield when applying i quantity of DAP, Y0 is the grain yield in control plot (with no DAP applied) and Qi is the applied DAP dose (in kg ha−1), Pp is the selling price of 1 kg of pea grains at the local market, and PDAP is the price of 1 kg of DAP at the local market. VCR was used as an economic measure of each treatment (CIMMYT 1998; Mushagalusa et al. 2016; Munyahali et al. 2017). At local market, PDAP was 1.5 USD kg−1 and 1 kg of peas was 1.2 USD. Values of VCR were appreciated according to recommendations by Sommer et al. (2013) for eastern and southern African countries. In our case, VCR values were divided into four classes: VCR ≤ 2 was considered as loss or insecure threshold, 3–4 good or secure threshold, and ≥ 5: very good.
Data analysis
Data were encoded using Microsoft Excel 2016 and statistical analyses were performed using R 3.4.3 (de Micheaux et al. 2014). To evaluate the effects of DAP dose and plant density on growth, yield, and nodulation of two pea varieties, the analysis of variance (ANOVA) was run following the experimental design used. When significant differences were observed, treatment means were separated using LSD test at 5% probability threshold. To analyze the link between nodulation parameters and growth, yield, and applied DAP doses, a generalized linear model (GLM) was performed and a correlation matrix was generated. Before running the regression analysis, a two-by-two correlation and collinearity test were performed. When two parameters were highly correlated (r ≥ 70), one was omitted in the regression.
Results
Plant density and DAP dose effects on pea growth under degraded ferralsols across seasons
Results on the effects of plant density and DAP fertilizer doses on growth of two pea varieties grown on ferralsols in Kabare territory are presented in Table 2. Seed emergence rate was not affected by any study factor, while the collar diameter, leaf area, and plant height were significantly affected by DAP doses (p < 0.05). No effects of seasons (for all the growth parameters), variety, and interaction of DAP dose, and season were observed on leaf area. The interaction plant density × DAP dose affected only collar diameter and plant leaf area. No difference (p > 0.05) was observed between the two varieties in terms of seedling emergence rate (%). The collar diameter, plant height, and leaf area significantly and proportionally varied (p < 0.05) with the DAP doses. DAP application enhanced plant growth from ~ 123 (control) to ~ 137 cm when applying 100 kg ha−1 and up to ~ 148 cm with 150 and 200 kg ha−1. Low plant density (100,000 plant per ha) coupled with 150 or 200 kg ha−1 DAP allowed improve the pea growth.
Plant density (p < 0.05) and its interactions with DAP dose had significantly influenced the collar diameter (p < 0.001) and leaf area (p = 0.024). It was found that when peas are sown less densely, plants developed larger collar diameters and leaf areas. Plant height was also influenced by plant density and its interaction with DAP dose. Plants sown at 50 cm × 40 cm (100,000 plants per ha) coupled with 200 kg ha−1 DAP had 145 cm height for variety Rwanda (CR) and 141 cm for Adei. Dense plantation coupled with high amount of DAP led to plants with high leaf area and high height. The local variety Adei presented higher leaf area (24 cm2) and larger collar diameter (0.34 cm) than variety Rwanda (20.5 cm2 and 0.29 cm, respectively). As presented in Table 3, plant densities affected all the yield parameters (p < 0.05). The interaction DAP dose × plant density affected the yield parameters of the two varieties. Application of 150 kg ha−1 DAP to plants sown at 50 cm × 40 cm allowed obtaining 5.7 and 5.0 grains per pod for Adei and Rwanda varieties and 23 and 25 pods per plant, respectively. No effect of the season and all the other factors, and plant density × variety was observed.
Effects of plant density and DAP dose on pea yield and yield-related parameters
Yield-related parameters of the two pea varieties under three DAP doses and three plant densities are presented in Table 3, while variations in grain yields due to study factors are presented in Fig. 3.
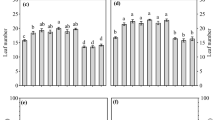
Grain yield variations under effects of DAP dose (a), plant density (b), interaction DAP dose × variety (c), and across seasons and years (d) in Miti, Kabare, eastern DRC. E1, E2, E3: plant densities corresponding to: 100,000, 166,666 and 250,000 plants ha−1, respectively, ADEI and C.R are variety names, A and B refer to the long and short cropping seasons in the study area, respectively
Significant differences were observed among DAP doses (p < 0.001), plant density (p = 0.025), and seasons (p = 0.012). There was a proportional increase in grain yield with increase of the DAP dose; it raised from 730 kg ha−1 (on control plot without fertilizer) to 1200, 1380, and 1720 kg ha−1 with 100, 200, and 150 kg ha−1 DAP, respectively (Fig. 3a). No yield differences were observed between the two varieties regardless of the plant density (Fig. 3b). Densities of 166,666 plants ha−1 (40 cm × 30 cm) and 250,000 plants ha−1 (40 cm × 20 cm) presented statistically the same yields: 1290 and 1310 kg ha−1, respectively, for the variety Rwanda and 1250 and 1260 kg ha−1, respectively, for the variety Adei (Fig. 3c). Significant differences in pea yields were recorded with the interaction DAP dose × variety (p < 0.05) (Fig. 3d). The interaction of the three study factors showed no significant differences, though the interaction season × variety had significant effects on yields (p < 0.05). Highest grain yields were recorded when applying 150 kg ha−1 DAP on variety Adei. Beyond 150 kg ha−1 DAP for both varieties, yields decreased significantly. No differences in pea yields were observed across years (p > 0.05), though there were significant yield differences between cropping seasons. The long rainy season (A) presented higher yields (1450 kg ha−1) than the short rainy season (B) (950 kg ha−1).
Effects of DAP dose and plant density on nodulation capacity
The effects of DAP dose and plant density on nodulation parameters of two pea varieties are presented in Table 4. Applying DAP positively influenced the nodulation capacity of the two pea varieties. Increasing the DAP dose resulted in increase of the total number of nodules per plant and the number of “active” nodules per plant. DAP dose of 150 kg ha−1 yielded 46 nodules per plant, while the control treatment had only 19.9 nodules for variety Adei. The trend was similar for variety Rwanda which had 32 nodules per plant on plots with 150 kg ha−1 and only 18.4 nodules on control plots.
Plant density had slight influence on nodulation. The density of 100,000 plant ha−1 (50 cm × 40 cm) combined with 150 kg ha−1 presented the highest number of nodules per plant. Of the two varieties, local variety Adei presented higher number of nodules (total and active) than variety Rwanda. The two varieties had also responded differently to DAP dose and plant density.
Correlations among growth, yield, and nodulation parameters
Figure 4 shows the relationships among growth and yield-related parameter-based varieties (Fig. 4a) and DAP doses (Fig. 4b). Yield and 100-seed weight, and the number of nodules and the number of active nodules were highly significatively correlated, thus only one of them were integrated in the regression analysis. The number of active nodules was significantly negatively correlated with seed germination rate (%) regardless of DAP doses. The trend was the same for the number of active nodules and plant height [except for 150 kg ha−1 DAP dose, p = 0.031, r = 0.38), leaf area for control plots (r = 0.54) and 150 kg ha−1 (r = 0.3)]. The number of nodules was negatively correlated with 100 kg ha−1 and 200 kg ha−1 DAP. Active nodules were significantly positively correlated with the number of pods per plant, the collar diameter (r = 0.43 with r = 0.45 and 0.62 for Adei and CR, respectively) and crop yield (r = 0.23). A very high correlation was observed between the number of grains per pod and leaf area regardless of DAP doses. Grain yield was in turn positively correlated (p < 0.01) with leaf area (for control and application of 100 kg ha−1), active nodules and number of pods per plant as well as other parameters, such as the number of grains per pod and plant height. Figure 4b shows that grain yield was significantly correlated with DAP doses (r = 0.91 and 0.89 for Adei and Rwanda varieties, respectively) (Additional file 1).
Regression analysis among growth, nodulation and yield-related parameters using doses (a) and varieties as factors (b). Tg germination rate (%), Dc collar diameter, Sfol leaf area, Nodnact total number of active nodules, Ngpp number of grains per plant, Rdt grain yield; values in bold are significant at 5% p value threshold
AE and VCR of applied DAP doses
Results on AE and VCR are presented in Fig. 5. AE significantly varied with the DAP dose, variety, and season (p < 0.05). Applying 100 and 200 kg ha−1 of DAP was efficient regarding AE values. According to plant density, 160,000 and 250,000 plants per ha presented statistically the same AE and VCR. The interaction DAP dose × cropping season also affected AE and VCR. AE was 8.2 and 9.1 when applying 150 kg ha−1 for 100,000 and 250,000 plants per ha, respectively, for variety Adei, while it was 6 and 7.2 for those densities, respectively, for variety Rwanda. Applying 100 kg ha−1 DAP led to AE of 7.2 and 5.8; 6.5 and 3.8; and 6 and 5 for Adei and Rwanda when sown at 100,000, 166,666, and 250,000 plants per ha, respectively. Applying 200 kg ha−1 led to lower AE values (< 3) except for variety Rwanda grown at 250,000 plants per ha.
Agronomic efficiency (EA) (a), and value–cost ratio (VCR) of applied DAP fertilizer under different plant densities (b), and variations in VCR across cropping seasons (c), in Miti, Kabare territory, eastern DRC. E1, E2, E3 are plant densities corresponding to 100,000, 166,666, and 250,000 plants ha−1; A and B refer to the long and short cropping seasons
Profitability assessed through the VCR showed that it varied with varieties, DAP doses, and plant densities. The interaction between the three factors had also significantly influenced the VCR. As for AE, 150 kg ha−1 presented the highest profitability (VCR = 8.5) than 100 and 200 kg ha−1. Variety Rwanda had higher profitability than variety Adei variety under different DAP doses.
Both AE and VCR varied significantly with plant density and cropping season (p < 0.05). Regardless of the DAP dose, the long rainy season (A) presented highest values of both AE and VCR than the short season one (B). In both seasons, 150 kg ha−1 DAP had highest VCR (6.3 and 4 $/$).
Discussion
N and P application improved pea growth and yield under degraded and poor ferralsols
Plant height and leaf area varied with DAP dose and plant density. Applying 200 kg ha−1 DAP led to highest plant leaf area and height but with reduced number of pods per plant and grain yield. High amount of N and P stimulated high plant biomass than grains. High plant density had similar consequences as a result of a high intraspecific competition (for light, water, and nutrients). Densely planted peas were etiolated as a consequence of light searching. In such case, high plant densities can only be adopted for biomass production (to produce fodder or leaves, where the latter are consumed) (Pageau et al. 2006; Ayaz et al. 2004). For grain production, Matthews (2003) and DAFF (2016) suggested that 90,000 plants per ha (9 plants per m2) was optimal, while for biomass production, Johnston et al. (2002a, b) advised increasing density up 50 to 75 plants per m2. Yucel (2013) tested three plant densities (60, 100, and 140 plants m−2) on three pea varieties and reached the conclusion that increasing the number of plants per surface area could significantly reduce leaf area and yield for varieties with erect architecture while increasing pests attacks, especially fungal diseases. In our case, plots with high plant density were more vulnerable to both fungal and nematode pests. High plant density reduced also the grain weight, the number of grains per pod and the number of pods per plant (Ayaz et al. 2004). However, it suppressed weed competition. For weed control, Park et al. (1999) and Agriculture Manitoba (2004) suggested 70–80 plants m−2. In our case, 16 and 25 plants m−2 reached similar results, while 10 plants m−2 allowed highest yields and can be promoted for pea grain production in the study area. Thereby, on one side the plant density affects soil cover and weed inhibition but requires high nutrient concentration to minimize intraspecific competition for soil nutrients (Bakht et al. 2009; Adeux et al. 2019; Colbach and Stéphane, 2019). On the other side, the N applied (as DAP) at the plant establishment helped adequate plant growth, necessary for optimal BNF (in symbiosis with bacteria, such as Rhizobium leguminosarum), an alternative source of N to cover the crop needs. However, pea needs also K+ and mostly P for BNF (Brkić et al. 2004; Getachew 2009; Jitender, 2011; Mishra 2014).
Previous studies on legumes such as common bean (Mushagalusa et al. 2016) and soybean (Zamukulu al. 2018) suggested applying DAP at 100 kg ha−1 in the study area. In this study, high agronomic efficiency, grain yield, and profitability were reached when applying ~ 150 kg ha−1 DAP as top-dressing fertilizer. Thus, on poor and degraded ferralsols of eastern DRC, peas require more nutrient than other legumes. Thus, 100 kg ha−1 currently used by pea farmers (as suggested by many studies on other legumes) needs to be updated to improve pea profitability in South-Kivu. This situation seems reasonable as similar conclusions were made in Kenya by HCDA (2004) that optimal dose on Kenyan ferralsols would be ~ 230 kg ha−1 of DAP that has to be applied as topdressing and ~ 130 kg ha−1 of the urea (46% of N) as booster or topdressing fertilizer. Such result leads to the conclusion that recommendations on fertilizer application are site-specific and depends on the soil degradation status.
The low yield observed on control plots attested the soil poverty of the study site, which is poor and degraded ferralsols, characterized by low pH, associated with very low available P, Mg2+ and Ca2+, and mostly high concentrations in Al, Mn, and Fe oxides. The use of mineral fertilizers, is, therefore, important as it stimulated growth and pea yields on such soils (Karkanis et al. 2018; Chuma et al. 2020).
Nutrient-deficient soils such as that of Kabare territory require an reasonable amount of N and P to improve growth and yield. Such nutrients are known as departure nutrients. Base application of DAP brought not only N (called “departure N”) but also P which is the most limiting nutrient on tropical soils (Mohamed and Babiker 2012; Karkanis et al. 2018). Nevertheless, yield differences were observed when applying different DAP doses. Poor and degraded ferralsols from Kabare responded positively to DAP doses with 150 kg ha−1 being the optimal dose to improve growth, yield and profitability of pea. Pea growth and yield increased when increasing DAP fertilizer doses up to 150 kg ha−1, after which the grain yield and profitability were affected negatively. In fact, Amare et al. (2005) and Getachew (2009) obtained similar results on Ethiopian Nitisols while applying 18 to 20 kg of N and P. Such amount corresponded to ~ 100–130 kg ha−1 of DAP. These authors found that yield increased by ~ 103% independently of the variety (improved or local one). In the same region, Tawaha and Turk (2004) obtained ~ 2600 to 2800 kg ha−1 while applying ~ 120 kg ha−1 DAP (corresponding to 52.5 kg ha−1 of P). Therefore, any increase in P and N up to 150 kg ha−1 DAP is highly correlated with an increase in pea yields according to our findings and thus could be adopted when improving productivity and profitability of peas on poor and degraded lands of eastern DRC. As Kabare farmers are resource-poor and thus unable to afford mineral fertilizers, we would recommend promoting alternative sources such as the organic matter (OM) coupled with BNF and other agricultural practices to reduce required amount of mineral fertilizers. Such an approach was suggested by Hu et al. (2018).
The long rainy season (A) was most productive than the short one (B) and applied DAP dose effect depended on seasons. High productivity of the long rainy season was mentioned by other studies on legumes in Kabare (Mushagalusa et al. 2016). High yields in that season is associated with good rainfall distribution compared to the short rainy season which experienced irregular and erratic rainfall.
Applying DAP fertilizer improves nodulation and BNF on pea under degraded soils
The number of active nodules is an important parameter to evaluate nodulation capacities and BNF. Active nodules contain a protein called leghemoglobin. Its presence in nodule was appreciated with reddish or pink coloration (Gan et al. 2003; Souza 2015). Senescent nodules are generally pink to white and slack. As all legumes, atmospheric-N is fixed by pea thanks to symbiosis relationship with bacteria. These bacteria are of the genus Rhizobium, which naturally occur in the soil. It is thus not necessary to inoculate before sowing unlike other legumes, such as common beans and soybeans. Nitrogen fixation activity increases proportionally with the nodule biomass up to the initiation of the grain filling stage (GFS), where it reaches in the optimum. Applying P improved the N fixation which may have allowed filling up the plant N requirement. Applying P as DAP fertilizer affected nodulation parameters and BNF. This seems evident as P stimulates rhizobia growth (Giri and Joshi 2010). The application of P increases root biomass production (Jemo et al. 2006), BNF, and grain yield of legumes. Wasike et al. (2009a, b) confirmed also that applying P could improve nodulation capacity of different soybean varieties. Differences in nodulation capacity can also be due to varietal effect, soil acidity level, and P concentration (Ndusha et al. 2019). With better nodulation, BNF satisfies up 30–80% of plant N requirement (Erman et al. 2009). Erman et al. (2009) finds also that applying P at 30, 60, and 90 kg ha−1 rates (TSP: Triple Super Phosphate) improved growth, yield, and nodulation of pea. Yield passed from 2265 to 2546 kg ha−1 and growth from 41 to 50 cm height, the number of nodules was enhanced from 11 to 20 per plant. However, effects were more significant when adding inoculation. Nevertheless, applying much DAP brings N as nitrate (NO3−), which inhibits N fixation. Beyond 60 kg ha−1 of N, nodules will not form. A high amount of N affects rhizobia activity in soils by inhibiting Lectin production by host legume which attracts Rhizobia for root infections (Ulzen et al. 2016).
Root pathogen Aphanomyces, water stress, soil compaction, sitones (nodules destroyers), and high weed incidence inhibit BNF (Karkanis et al. 2018). Therefore, required plant density allowed better soil coverage that inhibited weed development, conserved soil humidity, and lowered intraspecific competition. Pea nodulation has been studied by Dixon (1964), while Souza (2015) showed that natural nodulation has an economic importance, legume seeds must be inoculated. Effectiveness is evaluated by counting the number of nodules per plant (Graham et al. 2004). Unkovich et al. (2010) have tested 15 pea varieties and concluded that the crop has atmospheric N-fixation capacities of 128 to 150 kg ha−1 by nodules formation. However, in plant biomass, the fixation capacity goes up to 40 kg ha−1, variations explained by the harvest index which varied from one variety to another and the percentage derivate from fixation. These authors suggested also that inoculation by local strains or exotic Rhizobia must be recommended mostly in regions, where pHKCl is below 6.0 as it is a bit probable that appropriate Rhizobia strains are present in the soil. BNF allowed them, to orient future fertilizer input amounts on the field. Adequate nodulation levels (between 50 and 100 active nodules per plant) have to be then aimed according to Unkovich et al. (2010). In our case, a large amount of active nodules was observed on variety Adei (11 nodules) than Rwanda (9 nodules). The lower number of nodules was due to the fact that observation was done just after flowering, while some nodules were no longer active (discoloration from red to pink and pink to white).
At pH < 5.4, no Rhizobia strain has been detected for pea; however, the number of nodules per plant is highly correlated with the number of Rhizobia for gram pf soil before sowing (Unkovich et al. 2010). The number of nodules is correlated with the dry biomass of plants; it exponentially increased during the vegetative stage when optimal N and P have been furnished to plants (Bildirici and Yilmaz 2005). Applying P at the beginning of the plant stage has allowed root development that increased nutrient absorption. Olivera et al. (2004) highlighted the effect of P on growth and biomass production (not only of plant biomass but also of nodular biomass). One has then to optimize dry matter production of legumes to stimulate N needs and promote BNF. Rhizobia species that prefers symbiosis with pea is Rhizobium legumunosarum, which presents a rapid growth. However, pea nodules are energy-demanding (around 32% of net photosynthetic carbon that has been fixed is used by nodules) during the vegetative stage, i.e., 21–30 DAS (Rawsthorne et al. 1980).
The correlation analysis among assessed parameters showed that pea yield is correlated significantly with seedling emergence rate, leaf area, the number of grains per pod, number of pods per plant, and 100-grain weight. These relationships varied with DAP dose and pea variety. The two nodulation parameters were also correlated with pea yield. Positive correlations between DAP dose and pea yields have been also pointed out by HCDA (2004) et Irambu et al. (2010); the optimal being observed with the application of 90 kg ha−1 of N and 140 kg ha−1 de P which resulted to 3.7 and 3.9 t ha−1 of pea yield. Kandil (2007) found a positive correlation between DAP doses and pea yield, plant height, number of leaves per plant and number of pods per plant, the optimal dose suggested was 120 kg ha−1 applied as topdressing. However, the applied dose varied according to soil type; it was high on soil rich in humus. Application of P as phosphate rocks and TSP is correlated with dry matter of forage legumes grown on Andosols in Chili (Vistoso et al. 2019). Harvest index, dry matter, and root length are correlated with DAP-applied doses; which allow improving P use efficiency in the wheat-pea association as demonstrated by Sandaña and Pinochet (2014).
DAP application and use of improved varieties and agricultural practices improve pea profitability
Application of appropriate DAP dose combined with optimal plant density improved the net income from pea farming. For each US $ spent in buying DAP, the economic gain in terms of VCR was up to 6–8$ when applying 150 kg ha−1. The same trend was observed on Nitisols in Ethiopia (Getachew 2009) and on Nitisols in South-Kivu (Zamukulu et al. 2018). Beyond 150 kg ha−1, the grain yield, AE and VCR decreased significantly. However, Dittoh et al. (2012) suggested that VCR for legumes should be higher than 3–4 for agricultural practices to be attractive and adopted by farmers. Ulzen et al. (2016) fixed the threshold at VCR = 2 for urea but suggested to integrate inoculation, so that it raises up to 4–6. Our VCR was higher than that of Zamukulu et al. (2018) on soybeans and Mushagalusa et al. (2016) on common bean in the same study area.
This is due to the fact that peas are more expensive than common bean and soybean in the area. Agronomic efficiency (AE) and VCR have significantly varied with plant density. These results corroborated with Johnston et al. (2002b) who found that plant densities higher than 50 plants m−2 do not guarantee a surplus of the cost. Nleya and Rickertsen (2011) reported that the economic profitability of fertilizers might vary from 1 year to another but remain very low with plant densities of more than 77 plants m−2. Stepanovic et al. (2018) drew attention to the fact that no difference may be observed with 45, 54, and 60 plants m−2 in terms of profitability. Hu et al. (2018) also suggested applying 90 kg ha−1 of N as topdressing and 45 kg ha−1 at the flowering stage. These results recommend applying DAP at 150 kg ha−1 to improve the yield and profitability of pea in Kabare territory. However, applying 150 kg ha−1 DAP is unaffordable by predominantly resource-poor households in Kabare. For instance, Miti and Kabare farmers live with less than 2$ a day, thus such optimal DAP dose will be unaffordable to them. One of alternative ways as suggested by Brkić et al. (2004); Deslauriers (2014); Ulzen et al. (2016), and Stepanovic et al. (2018) is isolating and making inoculum from natural Rhizobia strains. Inoculating by bacteria for BNF allows to improve legumes yield and minimizes fertilizer costs. Inoculation, for example, using Bradirhizobium has improved 11–38% of yield, 41–68% of nodulation capacity in studies conducted in Ghana (Ulzen et al. 2016), while Karkanis et al. (2018) suggested integrating fungal mycorrhiza to improve legume growth and yield and reduce expenditure on mineral fertilizers. Inoculum application can reduce N and P inputs from 140 to 40–50 kg ha−1 (Johnston et al. 2002a, b; Kandil 2007). However, such integration implies adequate knowledge and materials on soil microbiology diversity. It could be suggested to integrate such research in the study area to facilitate making inoculum from local Rhizobia strains of pea or other legumes, such as soybean, common bean, groundnut, etc. These results indicate that it could be possible to improve pea grain yield in the area by promoting and stimulating BNF by adequate agricultural practices, such as choice of optimal plant density, soil fertility practices, and pest management.
Conclusions
DAP dose and plant density significantly influenced pea yield and nodulation performance regardless of varieties and cropping seasons. Applying 150 kg ha−1 DAP allowed raising yield by two-folds (730 to 1720 kg ha−1), had highest AE and VCR indices, and recorded the highest nodulation capacity compared to other doses. Though differences were not significant across years, cropping seasons were different within years; the long rainy season A (1400 kg ha−1) being more productive than the short rainy season B (930 kg ha−1) regardless of used varieties and DAP doses. This study recommends, therefore, 150 kg ha−1 DAP as optimal fertilizer dose for DAP, encourages lower densities and intensive pea farming during the long rainy season for high yield and profitability in South-Kivu, eastern DRC.
Abbreviations
- AE:
-
Agronomic efficiency
- BNF:
-
Biological nitrogen fixation
- CHAID:
-
Chi-squared Automatic Interaction Detector
- CEC:
-
Cations Exchange Capacity
- CV:
-
Coefficient of variation
- DRC:
-
Democratic Republic of the Congo
- DAP:
-
Di-ammonium phosphate
- DAS:
-
Days after sowing
- GLM:
-
Generalized linear model
- GFS:
-
Grains filling stage
- INERA:
-
Institut National d’Etudes et Recherches Agronomiques
- PNKB:
-
Kahuzi Biega National Park
- NGO:
-
Non-Governmental Organization
- OM:
-
Organic matter
- WRB:
-
World Reference Base for Soil Resources
- ATP:
-
Adenosine Tri-Phosphate
- VCR:
-
Value–cost ratio
References
Adeux G, Munier-jolain N, Meunier D, Farcy P, Carlesi S, Barberi P, Cordeau S. Diversified grain-based cropping systems provide long-term weed control while limiting herbicide use and yield losses. Agron Sustain Dev. 2019. https://doi.org/10.1007/s13593-019-0587-x.
Agriculture Manitoba. Field pea—production and management. [en ligne]. Food and Rural Initiatives. Disponible. 2004. http://www.gov.mb.ca/agriculture/crops/.
Amare G, Du Preez C, Taye B. Effect of phosphorus fertilizer on grain yield and yield components of field pea. Ethiop J Nat Resour. 2005;7:1–21.
Ayaz S, McKenzie BA, Hill GD, McNeil DL. Variability in yield of four grain legume species in a subhumid temperate environment. II. Yields components. J Agric Sci. 2004;142(142):21–8.
Bakht T, Khan IA, Khan IM, Khan I, Khattak MA. Weed control in pea (Pisum sativum L.) through mulching. Pak J Weed Sci Res. 2009;15(1):83–9.
Batista De Moraisa D, Ratke FR, Bortolonc L, Junio De Jesus LJ, Edvana LR, Zuffo MA, Pachecod PL. Maize intercropping systems improve nutrient for the cowpea crop in sandy soils. Commun Soil Sci Plant Anal. 2020. https://doi.org/10.1080/00103624.2020.1718688.
Baudouin J. Contribution des ressources phytogénétiques variétales à la sélection variétale de légumineuses alimentaires tropicales, Gembloux, Belgique. 2001;5(4):221–30.
Beck HE, Zimmermann NE, Mcvicar TR, Vergopolan N, Berg A, Wood EF. Data descriptor: present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci Data. 2018. https://doi.org/10.1038/sdata.2018.214.
Bhat T, Gupta M, Ganai M, Ahanger R, Bhat H. Yield, soil health and nutrient utilization of field pea (Pisum sativum L.) as affected by phosphorus and biofertilizers under subtropical conditions of Jammu. Int J Modern Plant Anim Sci. 2013;1(1):1–8.
Bildirici N, Yilmaz N. The effect of different nitrogen and phosphorus doses and bacteria inoculation (Rhizobium phaseoli) on the yield and yield components of field bean (Phaseolus vulgaris L.). J Agron. 2005;4(3):207–15.
Blanco-Canqui H, Lal R. Soil and crop response to harvesting corn residues for biofuel production. Geoderma. 2007;3–4:355–62.
Bolland MDA, Siddique KHM, Brennan RF. Grain yield responses of faba bean (Vicia faba L.) to applications of fertiliser phosphorus and zinc. Aust J Exp Agric. 2000;40:849–85. https://doi.org/10.1071/EA99164.
Brkić S, Milaković Z, Kristek A, Antunović M. Pea yield and its quality depending on inoculation, nitrogen and molybdenum fertilization. Plant Soil Environ. 2004;50(1):39–45.
Bünemann EK, Prusisz B, Ehlers K. Microorganisms, characterization of phosphorus forms in soil. In: Phosphorus in action. Heidelberg: Springer; 2011. p. 37–57.
Chuma BG, Francine SB, Adrien BN, Wasolu N, Mulalisi B, Espoir MB, Jean MM, Antoine KL, Mushagalusa NG. Optimal fertiliser dose and nutrients allocation in local and biofortified bean varieties grown on ferralsols in eastern Democratic Republic of the Congo. Cogent Food Agric. 2020;6(1):1805226.
Chuma BG, Ndeko BA, Mulalisi B, Safina BF, Ndjadi SS, Mushagalusa NG. Post-harvest constraints of Solanaceae species produced in Kabare Wetlands, Estern Democratic Republic of Congo. Agric Res. 2021. https://doi.org/10.1007/s40003-021-00586-4.
CIMMYT. From agronomic data to farmer recommendations: economics training manual. Completely revised edition, CIMMYT, Mexico; 1998.
Colbach N, Stéphane C. Peut-on réduire les herbicides sans perdre du rendement; 2019.
Corbin EJ, Brockwell J, Gault RR. Nodulation study on chickpea (Cicer arietinum). Aust J Exp Agric Anim Husb. 1977;17:126–34.
DAFF. Field pea production (Department). Rapport Republic of South Africa; 2016.
Dahl WJ, Foster LM, Tyler RT. Review of the health benefits of peas (Pisum sativum L.). Br J Nutr. 2012;108(S1):S3–10.
De Micheaux P, Rémy D, Benoît L. Le logiciel R: Maitriser le langage; effectuer des analyses (bio) statistiques (Statistique et probabilites appliquees). New York: Springer; 2014.
Deslauriers G. Méta-Analyse d’ essais de fertilisation N, P et K sur le haricot et le pois. Thesis, Université Laval, Quebec, Canada; 2014.
Dewitte O, Jones A, Spaargaren O, Breuning-madsen H, Brossard M, Dampha A, Zougmore R. Harmonisation of the soil map of Africa at the continental scale. Geoderma. 2013;211–212:138–53. https://doi.org/10.1016/j.geoderma.2013.07.007.
Dhital B, Sharma G, Khanal A. Effect of nipping at different days in growth and yield of field pea (Pisum sativum) in mid hills of Nepal. Adv Plants Agric Res. 2017;7(1):357–9. https://doi.org/10.15406/apar.2017.07.00266.
Dittoh S, Omotosho O, Belemwire A, Akuriba M, Haider K. Improving the effectiveness, efficiency and sustainability of fertilizer use in sub-Saharan Africa. Briefing Paper Number 3. GDN Agriculture Policy Series. Washington DC: GDN; 2012.
Dixon ROD. The structure of infection threads, bacteria and bacteroids in pea and clover root nodules. Archly Fiir Mikrobiol. 1964;48(2):166–78. https://doi.org/10.1007/bf00408456.
Ehlers K, Bakken LR, Frostegård Å, Frossard E, Bünemann EK. Phosphorus limitation in a ferralsol: impact on microbial activity and cell internal P pools. Soil Biol Biochem. 2010;42(4):558–66.
EIAR. Field pea production guideline: using rhizobial bio-fertilizer technology. Addis Ababa, Ethiopia; 2018.
Erman M, Yildirim B, Togay N, Cig F. Effects of phosphorus application in field peas (Turkey), nodulation and nutrient uptake in field pea. J Anim Vet Adv. 2009;8(2):301–4.
Gaidashova S, De Waele D. Effect of plant parasitic nematodes and arbuscular mycorrhizal fungi on banana (Musa spp.) in the East African Highland cropping systems; 2009. p. 270.
Gan Y, Stulen I, Keulen HV, Kuiper PJC. Effect of N fertilizer top-dressing at various reproductive stages on growth, N2 fixation and yield of three soybean (Glycine max L. Merr.) genotypes. Field Crop Res. 2003;80:147–55.
Getachew A. Phosphate fertilizer and weed control effects on growth and yield of field pea on nitisols of central highlands of Ethiopia. Ethiop J Sci. 2009;32(2):109–16.
Giller KE, Schilt C, Huising J, Franke AC, de Jager I. N2Africa putting nitrogen fixation to work for smallholder farmers in Africa, Podcaster no 22, August, September and October 2013 (No. 22). N2Africa project; 2013.
Giri N, Joshi NC. Growth and yield response of chickpea (Cicer arietinum) to seed inoculation with Rhizobium sp. Nat Sci. 2010;8(9):232–6.
Graham PH, Hungria M, Tlusty B. Breeding for better nitrogen fixation in grain legumes: where do the rhizobia fit in. Crop Manag. 2004. https://doi.org/10.1094/CM-2004-0301-02-RV.
GRDC. Grownotes: field pea, section 6. Nutrition and fertilizer; 2018.
Gregory PJ, Nortcliff S, editors. Soil conditions and plant growth. Chichester: Wiley-Black; 2013.
HCDA. Ministry of agriculture farm management handbook of Kenya, 2nd edition. 2004.
Heath MC, Hebblethwaite PD. Precision drilling combining peas (Pisum sativum L.) of contrasting leaf types at varying densities. J Agric Sci. 1987;108:425–30.
Hu F, Tan Y, Yu A, Zhao C, Coulter JA, Fan Z. Low N fertilizer application and intercropping increases N concentration in pea (Pisum sativum L.) grains. Front Plant Sci. 2018;9:1–14. https://doi.org/10.3389/fpls.2018.01763.
IFDC. Rapport des activités effectuées à l’est de la Re publique Democratique du Congo, Kigali, Rwanda; 2009. p. 24p
Irambu E, Ndegwa AM, Wepukhulu A, Muriuki SB. The effect of nitrogen and phosporus application on the pod yield of snowpeas. KARI.report; 2010.
Jemo M, Abaidoo RC, Nolte C, Horst W. Genotypic variation for phosphorus uptake and dinitrogen fixation in cowpea on low-phosphorus soils of southern Cameroon. J Plant Nutr Soil Sci. 2006;169(6):816–25.
Jitender K. Effect of phosphorus and rhizobium inoculation on the growth, nodulation and yield of garden pea (Pisum sativum L.) Cv. “Mattar Ageta-6.” Legume Res Int J. 2011;34(1):20–5.
Johnston A, Karamanos R, McKenzie R. Field pea responses to phosphorus fertilization. News and Views. 2002a. p. 1–2.
Johnston AM, Clayton GW, Lafond GP, Harke KN, Hogg TJ, Johnson EN, McConnell JT. Field pea seeding management. Can J Plant Sci. 2002b;82:639–44.
Kandil H. Response of pea plants (Pisum sativum L.) to phosphorus levels and humic acid levels. Semantic Scholar. 2007. p. 1–8.
Karkanis A, Ntatsi G, Lepse L, Fernández JA, Vågen IM, Rewald B, Savvas D. Faba bean cultivation—revealing novel managing practices for more sustainable and competitive European cropping systems. Front Plant Sci. 2018;9:1–14. https://doi.org/10.3389/fpls.2018.01115.
Lecoeur J, Sinclair TR. Field pea transpiration and leaf growth in response to soil water deficits. Crop Sci. 1996;36(2):331. https://doi.org/10.2135/cropsci1996.0011183x00360002002.
Matthews C. Field and Pigeon pea variety evaluation in Mpumalanga 1998–2000. In: Summary proceedings of the 2nd pea workshop 2003, 1–11 April 2003. Nelspruit, South Africa: Department of Agriculture and Land administration; 2003.
Mishra N. Growth and yield response of pea (Pisum sativum L.) to integrated nutrient management—a review. J Plant Pest Sci. 2014;1(2):87–95.
Mohamed SSE, Babiker HM. Effects of Rhizobium inoculation and urea fertilization on faba bean (Vicia faba L.) production in a semi-desert zone. Adv Environ Biol. 2012;6:824–30.
Munyahali W, Pypers P, Swennen R, Walangululu J, Vanlauwe B, Merckx R. Responses of cassava growth and yield to leaf harvesting frequency and NPK fertilizer in South Kivu, Democratic Republic of Congo. Field Crop Res. 2017;214:194–201. https://doi.org/10.1016/j.fcr.2017.09.018.
Mushagalusa NG, Kashemwa A, Sinza CB, Bigirinama CL, Karume K, Lubobo AK. Responses of biofortified common bean varieties to di-ammonium phosphate fertilizer under climate variability conditions in South-Kivu, DR Congo. Afr J Agric Res. 2016;11(38):3569–77. https://doi.org/10.5897/AJAR2016.11295.
Ndusha NB, Ndemo OR, Sharwasi NL, Mushagalusa N, Rehema EM, Okoth KS. Indigenous rhizobia strains: the silver bullet for enhanced biological nitrogen fixation and soybean (Glycine max L. Merr.) yield under different soil conditions in South Kivu province, Democratic Republic of Congo. Afr J Agric Res. 2019;14(35):2038–47. https://doi.org/10.5897/AJAR2019.14457.
Negi S, Sing R, Dwivedi O. Effect of biofertilizers, nutrient sources and lime on growth and yield of garden pea. Legum Res. 2006;29(4):282–5.
Nleya T, Rickertsen J. Seeding rate and variety effects on yield, yield components, and economic return of field pea in the northern Great Plains. Crop Manag. 2011. https://doi.org/10.1094/CM-2011-0221-01-RS.
Olivera M, Tejera N, Iribarne C, Ocana A, Lluch C. Growth, nitrogen fixation and ammonium assimilation in common bean (Phaseolus vulgaris): effect of phosphorus. Plant Physiol J. 2004;1(21):498–505.
Pageau D, Lajeunesse J, Lafond J. Influence de la densité de semis sur la productivité du pois sec cultivé sous un climat. Can J Plant Sci. 2006;87:41–8.
Park R, Lopetinsky K, Bjorklund R, Buss T, Eppich S, Laflamme P, Piquette K. Pulse crops in Alberta. Edmonton: Alberta Agriculture, Food and Rural Development; 1999.
Raveneau M, Coste F, Moreau-Valancogne F, Crozat Y, Dürr C. Analyse de la germination-levée de deux légumineuses (pois - haricot): intérêts et complémentarités des approches expérimentales et numériques. Innovations Agronomiques. 2014;35(35):1–11.
Rawsthorne S, Minchin FR, Rodney J, Summerfield J, Cookson C, Coombs J. Review and metabolism in legume. Phytochemistry. 1980;19:341–55.
Sandaña P, Pinochet D. Grain yield and phosphorus use efficiency of wheat and pea in a high yielding environment. J Soil Sci Plant Nutr. 2014;14(4):973–86.
Scanlon C. Diagnosing phosphorus deficiency in field peas. Department of Agriculture and Food, Western Australia; 2015. https://agric.wa.gov.au/n/4478.
Smýkal P, Aubert G, Burstin J, Coyne CJ, Ellis NTH, Flavell AJ, Weller JL. Pisum sativum. Agronomy. 2012;2:74–115. https://doi.org/10.3390/agronomy2020074.
Sommer R, Bossio D, Desta L, Dimes J, Kihara J, Koala S, et al. Profitable and sustainable nutrient management systems for eastern and southern African small holder. Aust J Soil Res. 2013;3:1–91.
Souza D. Legume nodulation and nitrogen fixation studies in Kenya. East Afr Agric for J. 2015;34(3):299–305. https://doi.org/10.1080/00128325.1969.11662308.
Stepanovic SV, Burr C, Peterson JA, Rudnick D, Creech CF, Werle R. Field pea response to seeding rate, depth, and inoculant in West-Central Nebraska. Crop Econ Prod Manag Field. 2018;7:1412–9. https://doi.org/10.2134/agronj2017.10.0600.
Tawaha A, Turk M. Field pea seeding management for semi-arid Mediterranean conditions field pea seeding management for semi-arid Mediterranean conditions. J Agron Crop Sci. 2004;190:86–92. https://doi.org/10.1046/j.1439-037X.2003.00079.x.
Tittonell P, Vanlauwe B, Leffelaar PA, Shepherd KD, Giller KE. Exploring diversity in soil fertility management of smallholder farms in western Kenya: II. Within-farm variability in resource allocation, nutrient flows and soil fertility status. Agric Ecosyst Environ. 2005;110(3–4):166–84. https://doi.org/10.1016/j.agee.2005.04.003.
Ulzen J, Abaidoo RC, Mensah NE, Masso C, Abdelgadir AH. Bradyrhizobium inoculants enhance grain yields of soybean and cowpea in northern Ghana. Front Plant Sci. 2016;7:1–9. https://doi.org/10.3389/fpls.2016.01770.
Unkovich MJ, Baldock J, Peoples MB. Prospects and problems of simple linear models for estimating symbiotic N2 fixation by crop and pasture legumes. Plant Soil. 2010;329(1–2):75–89.
Vandamme E, Pypers P, Vanlauwe B, Merckx R. Severe P deficiency and P adsorption in soils of the highly populated region of South Kivu, Democratic Republic of Congo.Poster. KULeuven, Belgium; 2014.
Vistoso E, Iraira S, Sandaña P. Effects of phosphorus fertilizer solubility on pastures yield and quality in andisols. J Soil Sci Plant Nutr. 2019. https://doi.org/10.1007/s42729-019-00152-6.
Vwima NS. Le rôle du commerce frontalier des produits alimentaires avec le Rwanda dans l’approvisionnement des ménages de la ville de Bukavu (Province du Sud-Kivu). Doctoral thesis, University of Liège; 2014. http://orbi.ulg.ac.be/handle/2268/165882.
Wasike VW, Lesueur D, Wachira FN, Mungai NW, Mumera LM, Sanginga N, Vanlauwe B. Genetic diversity of indigenous Bradyrhizobium nodulating promiscuous soybean (Glycine max (L.) Merr.) varieties in Kenya: impact of phosphorus and lime fertilization in two contrasting sites. Plant Soil. 2009a;22(1–2):151–63.
Wasike VW, Vanlauwe B, Wachira F, Mungai N, Mumera L, Mburu H, Lesueur D. Diversity and phylogeny of indigenous Bradyrhizobium strains nodulating promiscuous soyabean varieties grown in lowland and highland sites in Kenya. In: 9th African crop science, conference proceedings (African Cr). Cape Town, South Africa; 2009b. p. 371–7.
Woomer PL, Karanja N, Kisamuli SM, Murwira M, and Bala A. A revised manual for rhizobium methods and standard protocols. 2011.
WRB. World reference base for soil resources 2014: international soil classification system for naming soils and creating legends for soil maps; 2015. http://www.fao.org.
Yucel DO. Impact of plant density on yield and yield components of pea (Pisum sativum ssp. sativum L.) cultivars. J Agric Biol Sci. 2013;8(2):169–74.
Zamukulu P, Mondo MJ, Ayagirwe RBB, Bagula E, Karume K, Katunga D, Mushagalusa NG. Réponse du soja (Glycine max L.) à des doses croissantes du DAP et Urée au Sud-Kivu, RD Congo. J Appl Biosci. 2018;122:12309–18. https://doi.org/10.4314/jab.v122i1.10.
Acknowledgements
We thank the Université Evangélique en Afrique, Bukavu, for supporting this research. We also thank undergraduate students (Kiriza Eliza, Nathalie Mweze and Mudimbi Mukwasa Serphin) who participated in data collection.
Funding
No funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Meteorological data during the trial period, data were obtained from the INERA/Mulungu weather station for 2017 and 2018 (A), regression between the total number of nodule per plant and operative (active) nodules per plant (B) and, two by two regressions between nodulation parameters, growth and yield from control (C), the first and second doses (100 and 150 kg/ha) and the three doses and the control (E) (D and E).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chuma, G.B., Mulalisi, B., Mondo, J.M. et al. Di-ammonium phosphate (DAP) and plant density improve grain yield, nodulation capacity, and profitability of peas (Pisum sativum L.) on ferralsols in eastern D.R. Congo. CABI Agric Biosci 3, 65 (2022). https://doi.org/10.1186/s43170-022-00130-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43170-022-00130-6