Abstract
Backgrounds
Chronic obstructive pulmonary disease (COPD) is often ascribed to the disparity that exists between ventilatory loading and diaphragmatic function. The principal factor contributing to this imbalance is the mechanical difficulties brought about by pulmonary hyperinflation. This causes the fiber lengths of the respiratory muscles to be suboptimal, thereby decreasing the tension generated. The frailty of the respiratory musculature is further exacerbated by hypercapnia, acidosis, nutritional deficiency, and steroid therapy. As the principal respiratory muscle, the diaphragm is responsible for 75% of quiescent lung ventilation. Ultrasonography has exhibited substantial promise in evaluating the effectiveness of the diaphragm. The goal of the research was to assess the diaphragmatic function in COPD patients using ultrasonographic examination and to study its correlation with pulmonary function tests.
Methods
This work was performed on 60 individuals with COPD and 20 healthy volunteers (control group). All the subjects were sequentially examined by chest X-ray and spirometry in one time and then repeated 15–45 min after the first dose of inhalation therapy (salbutamol); then, 6MWT, ABG, and ultrasonography were done for all patients.
Results
In COPD patients, diaphragmatic thickness and excursion were substantially reduced in comparison to the control group; these parameters were also significantly correlated with pulmonary function tests.
Conclusions
A reduction in diaphragmatic thickness and excursion is observed in patients diagnosed with COPD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Chronic obstructive pulmonary disease (COPD) is a treatable and preventable condition with a high prevalence. Persistent respiratory symptoms and ventilation obstruction resulting from abnormalities in the airway and/or alveoli define this condition [1]. Around 60–80% of the respiratory workload is executed by the diaphragm, the principal muscle responsible for human respiratory movements, during respiration [2]. As a result, the diaphragm’s function in COPD has drawn more attention and is now thought of as an indicator for disease progression. Diaphragmatic dysfunction is caused by the absence of one of the two main factors that affect muscle action: strength and endurance, leading to a weak contraction. Systemic inflammation, hyperinflation, oxidative stress [3, 4], chronic hypoxia, and malnutrition [5, 6] all are some of the potential causes of diaphragm dysfunction in individuals with COPD. One of the most important aspects of evaluating individuals with COPD is the assessment of the diaphragm. Measuring the transdiaphragmatic pressure following magnetic stimulation of the phrenic nerve is the gold standard for determining diaphragmatic dysfunction, but it requires considerable expertise, invasive, and is not available at the bedside [7]. Chest x-ray, fluoroscopy, and computed tomography are used to assess diaphragmatic function, but they are not sensitive beside the hazards of ionizing radiation [8]. Ultrasonography has emerged as a noninvasive and effort-independent tool to assess diaphragmatic function [9, 10]. Diaphragmatic performance was also used to assess weaning from mechanical ventilation [11] as well as evaluation of acute exacerbation of COPD [12].
Methods
Inclusion criteria
This was an observational prospective, case–control study. It was conducted over the interval between April 2020 and April 2021 after approval of the committee for scientific research ethics at Faculty of Medicine, Tanta University (no. 33703) and consent was obtained in writing form from every participant.
Sixty patients > 40 years with clinically stable COPD were recruited throughout their follow-up in outpatient department of Chest University Hospital. They were classified and diagnosed in accordance with GOLD 2021 criteria [1]. Also, 20 age-matched apparently healthy volunteers were included.
Exclusion criteria
Patients with age less than 40 years, COPD exacerbation within the previous 6 weeks, additional pulmonary conditions including pneumothorax, pleural effusion, lung cancer, phrenic nerve palsy, pneumonia, or musculoskeletal diseases, recognized cardiovascular insufficient supply (Ejection fraction < 50%), upper abdominal surgeries, ascites, and being obese [those who have a body mass index (BMI) that is greater than 30] were excluded.
All participants were subjected to the following: detailed history taking, clinical evaluation, BMI evaluation, radiological examination, arterial blood gases assessment, spirometric assessment according to American Thoracic Society guidelines (2019) [13] using computerized spirometry apparatus “Spirosoft spirometry SP-5000, Fukuda Denshi, Japan,” a 6-min walk test in accordance with American Thoracic Society recommendations [14], and ultrasonography of the diaphragm using (ProSound Aloka F37, Hitachi Ltd., Japan) ultrasound machine.
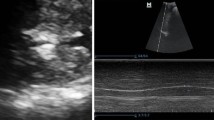
The high-frequency (5–13 MHz) linear probe was used for observing hemidiaphragms in order to determine the diaphragmatic thickness. While the individual was in a semi-sitting position, the probe was positioned above the area of apposition perpendicular to the ninth intercostal space between the midaxillary and anterior axillary lines. Using B-mode, the diaphragm was identified as a three-layer structure, comprising two hyper-echoic outer layers (the peritoneum and pleura) surrounding a hypo-echoic inner muscle layer. As demonstrated in Fig. 1, the thickness of each hemidiaphragm was determined by directly measuring the distance between the center of the pleural membrane and the center of the peritoneal membrane on the frozen B-mode images. The measurements were collected at the ending of maximal inspiration and maximal expiration, specifically at the point of total lung capacity (Tmax) and residual volume (Tmin), respectively. Average measurements from at least three separate breathing cycles were taken. By dividing the average diaphragmatic thickness during maximal inspiration (Tmax) by the average diaphragmatic thickness during maximal expiration (Tmin), the TH ratio was calculated.
Diaphragmatic excursion (DE) shown in Fig. 2 was assessed by visualizing the right hemidiaphragm via the low-frequency (2–6 MHz) convex probe through the hepatic window. In a semi-recumbent posture, the anterior subcostal region between the anterior axillary line and midclavicular line was scanned by B-mode. The right hemidiaphragm, which separates the air-inflated lung and liver parenchyma, appears as a thick hyperechoic line. By positioning calipers at the base and summit of the diaphragmatic slope throughout deep breathing, the diaphragmatic excursion’s amplitude was determined in M-mode. Because of technical issues with the left diaphragmatic excursion caused by the spleen’s limited acoustic window and the interposed stomach gas, only the right diaphragmatic excursion was measured.
Statistical analysis
The statistical analysis was conducted using SPSS (SPSS Inc., Chicago, USA, Version 25.0). In order to compare categorical qualitative parameters expressed as frequency and percentage, the chi-square test was applied. To compare continuous quantitative parameters, the ANOVA test was utilized; the parameters were presented as mean ± SD. In order to ascertain the correlation between the measured ultrasonographic parameters and the clinical characteristics of the patients, Spearman’s correlation analysis was conducted. The receiver operating characteristics curve (ROC) test was employed to evaluate the sensitivity and specificity of all components comprising diaphragmatic ultrasonography.
Results
Of 123 COPD patients were evaluated for eligibility criteria of the study, we selected 20 patients in each COPD GOLD 2, 3, and 4 groups, and 63 patients were excluded as 51 patients with exacerbations over the past 3 months, 7 patients with umbilical hernia, 3 patients with right minimal pleural effusion detected by ultrasound, 1 patient with upper abdominal surgery, and 1 patient with moderate ascites.
Basic demographic and clinical data of all individuals are displayed in Table 1. There were highly significant variations among groups regarding age, smoking prevalence, pH, Pao2, Paco2, and 6MWT.
Regarding diaphragmatic thickness, the mean values of Rt Tmax, Lt Tmax, Rt Tmin, and Lt Tmin were noticeably reduced in COPD groups when contrasted to control group (Table 2).
Significantly higher mean values of Tmax and Tmin were observed in COPD grade 2 compared to grades 3 and 4, and in grade 3 compared to grade 4, although there were nonsignificant differences between patients and controls regarding Rt TH ratio and Lt TH ratio. In contrast to the control group, DE levels were significantly lower in the COPD group. The mean values of diaphragmatic excursion were significantly higher in grade 2 than grades 3 and 4 and were higher in grade 3 than grade 4.
Receiver operating characteristic (ROC) analysis was done for diaphragmatic thickness and excursion (Table 3, Fig. 3). The diaphragmatic excursion showed the highest sensitivity and specificity parameters for COPD with both sensitivity and specificity equal (88% versus 70%) respectively at cutoff value 3.4 cm. Tmax and Tmin showed a lesser sensitivity and specificity percentages (81% versus 60% at cutoff value 4 mm and 77% versus 55% at cutoff value 2.6 mm) for Tmax and Tmin sensitivity and specificity respectively.
As regarding correlations, there was nonsignificant correlation between age, sex, BMI, pO2, pCO2, diaphragmatic thickness and excursion. A positive correlation that was statistically significant was identified between diaphragmatic thickness and excursion as well as 6MWT. A statistically significant positive correlation (FEV1% predicted) was identified between spirometric data and diaphragmatic thickness and excursion (Table 4).
Discussion
COPD is a treatable and preventable condition with a high prevalence. Majority of the respiratory workload is executed by the diaphragm. As a result, the diaphragm’s function in COPD has drawn more attention. Ultrasonography has emerged as a noninvasive and effort-independent tool to assess diaphragmatic function. We used ultrasonography to assess diaphragmatic thickness and excursion and compared it to pulmonary function results.
In the present study, as compared to the control group, Tmax and Tmin were significantly lower in the COPD group.
Our findings were corroborated by Smargiassi A. et al. [15] who assessed diaphragmatic thickness in 23 participants and documented a precipitous decrease in diaphragmatic thickness among COPD patients compared to the control group.
However, in contrast, Baria M. R. et al. [8] discovered that there were no substantial variations observed in the thickness of the diaphragm or the TH ratio between sides when comparing groups (control participants or COPD individuals) or between groups (50 COPD patients and 150 healthy control individuals). The exclusion of the subgroup exhibiting extensive air entrapment (residual volume surpassing 200%) could have contributed to this outcome.
The current study found that the COPD group exhibited a significantly shorter diaphragmatic excursion than the control group.
This was in agreement with Scheibe N. et al. [16] who studied diaphragmatic thickness and mobility in 20 healthy persons and 60 COPD patients and stated that COPD patients had less diaphragm mobility than did healthy individuals.
In the current study, the mean values of diaphragmatic excursion were significantly higher in group 2 than groups 3 and 4 and were higher in group 3 than group 4 with a statistically high significant difference among the three groups.
Scheibe N. et al. [16] also reported decreasing diaphragmatic mobility with enhanced COPD severity.
As determined by the receiver operating characteristics (ROC) analysis, a number of the ultrasonographic parameters utilized in the diagnosis of COPD exhibited inadequate sensitivity and specificity. At a cutoff value of 3.4 cm, the diaphragmatic excursion exhibited the highest sensitivity and specificity (88% versus 70%). These findings increase the diagnostic efficacy of diaphragmatic ultrasonographic excursion measurement in COPD. These results are in agreement with several study groups including Kareem J. et al. [17] and Essawy T. et al. [18].
In the current study, there was a moderate positive association among Tmax and Tmin of both hemidiaphragms and BMI.
Comparable findings were observed in this investigation and the one documented by Smargiassi A. et al. [15] and Boon A. J. et al. [20], who established a correlation between diaphragmatic thicknesses and physical attributes including body mass index (BMI) and height.
But Cimsit C. et al. [19] found out that BMI dropped as the illness stage advanced. The correlation between BMI and diaphragm thickness, however, was not found.
In the current research, there was also a highly substantial positive correlation between diaphragmatic thicknesses and the spirometric data (FEV1% predicted) with a nonsignificant correlation between ratios of diaphragmatic thickness and FEV1% predicted.
According to Smargiassi A. et al. [15] who assessed diaphragmatic thickness in 23 participants, Tmax was most strongly correlated with fat-free mass (FFM), BMI, and lung hyperinflation.
Yalçin B. et al. [21], who assessed diaphragmatic thickness and mobility in 67 COPD patients and 53 healthy volunteers, discovered a significant correlation between pulmonary function tests and diaphragmatic thickness in patients with COPD.
On the contrary, Cimsit C. et al. [19] assert that there is no correlation between diaphragmatic thickness and pulmonary function tests in patients with chronic obstructive pulmonary disease (COPD), with the exception of those with mild COPD. This lack of correlation is likely attributable to the fact that the measurements were exclusively performed during the end-expiratory phase. In addition, the limited sample size of the study group might have an impact on the correlation between ultrasound measurements and the severity of COPD.
In the present study, the diaphragmatic excursion was found to be positively correlated with BMI.
Smargiassi A. et al. [15] who enrolled 23 clinically stable COPD patients found that diaphragmatic excursion was closely related to BMI.
There was a statistically high significant positive correlation between diaphragmatic excursion and 6-min walk test (6MWT).
Scheibe N. et al. [16] who studied diaphragmatic thickness and mobility in 20 healthy persons and 60 COPD patients stated that there is a moderate correlation between diaphragmatic excursion and 6MWT.
A significant correlation among diaphragmatic excursion and the spirometric data (predicted FEV1%) was found in the current study. It has been demonstrated that ultrasonographic measurements of the diaphragmatic excursion in patients with COPD are negatively correlated with the progression of COPD, with grade IV COPD patients exhibiting the lowest ultrasonographic measurements.
In agreement with our results, Scheibe N. et al. [16] who studied diaphragmatic thickness and mobility in 20 healthy persons and 60 COPD patients found out that diaphragmatic movement was related to lung function parameters,
while Smargiassi A. et al. [15] who studied diaphragmatic thickness and mobility in 23 COPD patients found no relation between diaphragmatic excursion and the spirometric data (FEV1) and stated that it is only related to BMI.
Limitations
A limitation of this research is the relatively small sample size of enrolled patients. Lack of comparative analysis with gold standard techniques used to evaluate diaphragm function constitutes an additional limitation of our research.
Conclusion
Diaphragmatic thickness and excursion significantly positively correlated to the spirometric data (FEV1% predicted). Diaphragmatic assessment could be a prognostic factor for COPD severity and could guide the treatment plan.
Future directions
Assessment of diaphragmatic performance could be a worthy substitute for spirometry in the future. Diaphragmatic performance could also be used to assess weaning from mechanical ventilation and estimation of acute exacerbation of COPD.
Availability of data and materials
Materials and data are accessible upon the author’s reasonable request.
Abbreviations
- 6MWT:
-
Six-minute walk test
- BMI:
-
Body mass index
- COPD:
-
Chronic obstructive pulmonary disease
- DE:
-
Diaphragmatic excursion
- FFM:
-
Fat-free mass
- ROC:
-
Receiver operating characteristic
- SD:
-
Standard deviation
- TH ratio:
-
Thickening ratio
- FEV1%:
-
Forced expiratory volume during the first second
- Rt Tmax:
-
Thickness of right hemidiaphragm total lung capacity
- Rt Tmin:
-
Thickness of right hemidiaphragm at residual volume
- Rt TH ratio:
-
Thickness ratio resulting from the equation of Rt Tmax/Rt Tmin
- Lt Tmax:
-
Thickness of left hemidiaphragm at total lung capacity
- Lt Tmin:
-
Thickness of left hemidiaphragm at residual volume
- Lt TH ratio:
-
Thickness ratio resulting from the equation of Lt Tmax/Lt Tmin
- Excursion:
-
Diaphragm movement between total lung capacity and residual volume
References
Agusti A, Criner G, Beasley R, et al. (2021) Global strategy for prevention, diagnosis and management of COPD: 2021 report. Global Initiative for Chronic Obstructive Lung Disease
Pickering M, Jones JF (2002) The diaphragm: two physiological muscles in one. J Anat 201:305–312
Ogan N, Aydemir Y, T EV, Ataç GK, Baha A, Katipoğlu B, et al (2019) Diaphragmatic thickness in chronic obstructive lung disease and relationship with clinical severity parameters. Turk J Med Sci 49:1073–1078
Rittayamai N, Chuaychoo B, Tscheikuna J, Dres M, Goligher EC, Brochard L (2020) Ultrasound evaluation of diaphragm force reserve in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc 17:1222–1230
Agustí A (2007) Systemic effects of chronic obstructive pulmonary disease: what we know and what we don’t know (but should). Proc Am Thorac Soc 4:522–525
Cao Y, Li P, Wang Y, Liu X, Wu W (2022) Diaphragm dysfunction and rehabilitation strategy in patients with chronic obstructive pulmonary disease. Front Physiol 13:872277
Ricoy J, Rodríguez-Núñez N, Álvarez-Dobaño JM, Toubes ME, Riveiro V, Valdés L (2019) Diaphragmatic dysfunction Pulmonology 25:223–235
Laghi FA Jr, Saad M, Shaikh H (2021) Ultrasound and non-ultrasound imaging techniques in the assessment of diaphragmatic dysfunction. BMC Pulm Med 21:85
Baria MR, Shahgholi L, Sorenson EJ, Harper CJ, Lim KG, Strommen JA et al (2014) B-mode ultrasound assessment of diaphragm structure and function in patients with COPD. Chest 146:680–685
Boussuges A, Rives S, Finance Jand Brégeon F (2020) Assessment of diaphragmatic function by ultrasonography: current approach and perspectives. World J Clin Cases 8:2408–2424
Saeed AM, El Assal GI, Ali TM, Hendawy MM (2016) Role of ultrasound in assessment of diaphragmatic function in chronic obstructive pulmonary disease patients during weaning from mechanical ventilation. Egypt J Bronchol 10:167–172
Lim SY, Lim G, Lee YJ, Cho YJ, Park JS, Yoon HI et al (2019) Ultrasound assessment of diaphragmatic function during acute exacerbation of chronic obstructive pulmonary disease: a pilot study. Int J Chron Obstruct Pulmon Dis 14:2479–2484
Graham BL, Steenbruggen I, Barjaktarevic IZ et al (2019) Standardization of spirometry 2019 update an official American Thoracic Society and European Respiratory Society technical statement. Am J Respir Crit Care Med 200(8):E70-88
Enright PL (2003) The six-minute walk test. Respir Care 48(8):783–785
Smargiassi A, Inchingolo R, Tagliaboschi L, Di Marco BA, Valente S, Corbo GM (2014) Ultrasonographic assessment of the diaphragm in chronic obstructive pulmonary disease patients: relationships with pulmonary function and the influence of body composition - a pilot study. Respiration 87:364–371
Scheibe N, Sosnowski N, Pinkhasik A, Vonderbank S, Bastian A (2015) Sonographic evaluation of diaphragmatic dysfunction in COPD patients. Int J Chron Obstruct Pulmon Dis 10:1925–1930
Kareem J, Al-Waely N, Al-Hashimi A (2022) Diaphragmatic thickness in chronic obstructive pulmonary disease. Med J Babylon 19:427–433
Essawy TS, Al-Arag AH, mousa h, abdelsattar Me and elsawy R, (2021) Ultrasound assessment of diaphragmatic thickness in chronic obstructive pulmonary disease patients as a predictor for disease severity. Benha med j 38:353–367
Cimsit C, Bekir M, Karakurt S, Eryuksel E (2016) Ultrasound assessment of diaphragm thickness in COPD. Marmara Med J 29:8–13
Boon AJ, Harper CJ, Ghahfarokhi LS, Strommen JA, Watson JC, Sorenson EJ (2013) Two-dimensional ultrasound imaging of the diaphragm: quantitative values in normal subjects. Muscle Nerve 47:884–889
Yalçın B, Sekmenli N, Baktık B, Bekçi TT (2022) Evaluation of diaphragm thickness and function with ultrasound technique and comparison with spirometry in stable chronic obstructive pulmonary disease. Tuberk Toraks 70:76–84
Acknowledgements
Nil.
Funding
Nil.
Author information
Authors and Affiliations
Contributions
MMD and EMD were tasked with data acquisition, while WME and DEE conceived and oversaw the research. The data were analyzed and interpreted by WSE. The manuscript was subject to feedback from all authors at different phases of its development. The final manuscript was reviewed and endorsed by all authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
It was approved by the Tanta University Faculty of Medicine’s ethics committee and commenced in April 2020 and concluded in April 2021. Written informed assent was acquired from each participant.
Consent for publication
Permission to publish in the journal is obtained from all authors.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dahma, E.M., El-lawaty, W.M., Shaarawy, D.E. et al. Ultrasonographic evaluation of diaphragmatic thickness and movement for assessment of diaphragmatic dysfunction in COPD patients. Egypt J Bronchol 18, 69 (2024). https://doi.org/10.1186/s43168-024-00313-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43168-024-00313-x