Abstract
Background
The survival benefit of venovenous extracorporeal membrane oxygenation (VV-ECMO) in adult patients with severe acute respiratory distress syndrome (ARDS) remains controversial. This study aimed to investigate the efficiency and potential prognostic factors of VV-ECMO for severe ARDS in adults by evaluating our institutional experience and results.
Materials and methods
This research studied ARDS patients receiving VV-ECMO between June 2011 and May 2023. The inclusion criteria were PaO2/FiO2 < 100 mmHg at FiO2 of 1.0. Retrospective data was analyzed to identify factors associated with successful ECMO weaning and hospital discharge survival.
Results
A total of 18 patients were included in this study, with 7 cases (38.9%) successfully weaned from ECMO and 5 cases (27.8%) surviving hospital discharge. The overall complication rate was 77.8%. After treatment with VV ECMO, there were statistically significant improvements in both PaO2 and PaCO2 (P < 0.05). Patients in the successful weaning group had a lower pTB value, less accumulative volume of sodium bicarbonate during ECMO, and lower accumulative volume of intravenous immunoglobulin in the hospital compared to the unsuccessful weaning group (all P < 0.05). Furthermore, compared to the non-survivors, the survivors had less severe acidosis, higher mean arterial pressure before ECMO, a lower level of pCr, and a lower pTB value during ECMO (all P < 0.05).
Conclusion
ECMO can effectively promote oxygenation and carbon dioxide (CO2) removal in patients with severe ARDS. Early initiation of ECMO with appropriate management could benefit in reducing comorbidities and mortality.
Similar content being viewed by others
Introduction
A majority of patients with ARDS require mechanical ventilation for gas exchange to prolong survival time to receive supportive care or lung recovery. However, in some critically ill patients with profound hypoxemia, despite using “lung-protective ventilation strategy” to reduce ventilator-induced lung injury (VILI) [1], an associated complication which mortality rate is up to 46% [2, 3], gas exchange targets are difficult to achieve.
Venovenous extracorporeal membrane oxygenation (VV-ECMO) improves oxygenation and promotes the removal of carbon dioxide (CO2). In the most severe form of ARDS, ECMO rapidly facilitates improvement in gas exchange [4]. Advances in ECMO technology have improved safety and facility, thus expanding its application scope [5,6,7]; however, its benefit for severe ARDS remains controversial [8,9,10]. The results of the first two randomized trials of ECMO for ARDS were less favorable, yet these trials were performed decades ago. Nevertheless, the results of the most recent trial [9], an international multicenter, randomized, open trial that evaluated the impact of ECMO on the morbidity and mortality associated with severe ARDS, were discouraging. Compared with traditional mechanical ventilation, the primary endpoint (mortality rate at 60 days) of patients with the most severe ARDS showed no significant difference in early ECMO, the 28% crossover rate made it difficult to draw clear conclusions on the usefulness of ECMO for severe forms of ARDS.
Despite the existing inconsistent study results, ECMO is currently considered an established rescue therapy for treating refractory ARDS. Based on the recognition, we analyzed a series of patients receiving VV-ECMO treatment for severe ARDS and described our experience and results in this retrospective study, aiming at evaluating the effect of VV-ECMO on severe ARDS patients and exploring appropriate management strategies. Meanwhile, we also sought possible explanations for treatment failure.
Materials and methods
Study patients and design
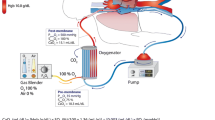
This study included patients with VV-ECMO who visited Zhongshan Hospital of Sun Yat-sen University, a tertiary care hospital and supra-regional ECMO center in southern China, between June 2011 and May 2023 (Fig. 1a). Patients were identified on the basis of the Berlin definition of severe ARDS. The criteria for VV-ECMO included PaO2/FiO2 < 100 at a FiO2 of 1.0, with > 5 cm H2O of positive end-expiratory pressure (PEEP), after the treatment of mechanical ventilation and optimal conventional therapy. In order to avoid selection bias, patients were excluded with (i) intubation and mechanical ventilation for ≥ 7 days; (ii) age < 18 years; (iii) BMI > 45 kg/m2; (iv) pregnancy; (v) advanced malignancy; (vi) severe immunocompromise; (vii) VA-ECMO; (viii) previous history of heparin-induced thrombopenia. The included patients were divided into two groups according to in-hospital mortality and ECMO weaning, respectively. Successful weaning was defined as Survival > 48 h after weaning off ECMO. Survival was defined as weaning off ECMO and improvement of clinical conditions after hospital discharge.
Some pre-ECMO data (demographic data, causes of ARDS for ECMO initiation, Acute Physiology and Chronic Health Evaluation II score (APACHE II), IMV duration), laboratory parameters (iCr, pCr, iTB, pTB, iLactate, and pLactate), complications during ECMO and causes of hospital death were obtained. The following variables were analyzed at the time of presentation (before ECMO) and 2, 6, 12, 24, and 48 h after ECMO initiation: ABGA results (PaO2, PCO2, SaO2, PH, HCO3-) and ventilator settings (FiO2, PEEP, PS, VT, PIP, respiratory rate, dynamic pulmonary compliance). In addition, total fluid balance was determined daily from the time of presentation (day 1) to the seventh day after ECMO initiation (day 7). Transfusion during ECMO and in the hospital was also presented. To assess outcomes, the following parameters were evaluated: successful weaning and in-hospital mortality rate, duration of ECMO and IMV, length of stay in the ICU (intensive care unit) and hospital, and complications.
Data collection was approved by the Institutional Review Board of Zhongshan Hospital of Sun Yat-sen University. Informed consent was not required due to the retrospective study design.
Statistical analysis
SPSS 24 software (IBM Corp, Armonk, NY, USA) was used to perform statistical analyses. Continuous variables were tested for normality using the Shapiro–Wilk test. Continuous variables with normal distribution were analyzed using Student’s t-test and expressed as mean ± standard deviation, while continuous variables with non-normal distribution were analyzed using the Mann–Whitney U test and expressed as median (25–75% interquartile range). Categorical variables were displayed as frequency distribution and evaluated with Pearson's chi-square test or Fisher’s exact test, as appropriate. P values < 0.05 were considered statistically significant. Overall survival was calculated following the Kaplan–Meier method. Figures were created using the software Prism 7 (GraphPad Software, San Diego, CA, USA).
Results
Baseline characteristics
During the defined study period, 242 adult patients underwent ECMO and only 18 VV-ECMO patients were eventually included in the retrospective study. Yet, we observed an increase in patients meeting the inclusion criteria in recent 3 years (Fig. 1a, b). According to the ECMO weaning and in-hospital mortality, the baseline characteristics of the included patients are summarized in Table 1.
The mean age of patients was 39 ± 13 years with a BMI of 21.1 ± 2.2 kg/m2, and 10/18 patients were male. The overall mean APACHE II score was 20 [18,19,20,21,22,23,24,25,26]. A total of 11 cases developed ARDS by bacterial pneumonia; and 5 by avian influenza A (H7N9) virus; the remaining 2 were due to idiopathic lung fibrosis and severe acute pancreatitis, respectively. Moreover, 2 patients were transferred from other hospitals with ECMO, established by our ECMO team. Vascular access was established via femoral veins (drainage cannula) and internal jugular veins (return cannula), of which, 72.2% of patients used percutaneous catheterization. The most prevalent mode was VV-ECMO (83.3%), while 3 (16.7%) patients converted VV-ECMO into VA-ECMO because of hemodynamic instability during the catheter-establishment process. Patients received ECMO with a mean blood flow of 2.7 ± 0.6 L/min, of which the PaO2/FiO2 and MAP were 57 ± 18 mmHg and 74 ± 16 mmHg, respectively. Compared to the non-survivors, the survivors had less severe acidosis (pH 7.42 ± 0.16 vs. 7.24 ± 0.14, P = 0.029) before ECMO. Additionally, there was a significant difference in arterial pressure between the successful and unsuccessful weaning group (85 ± 12 vs. 67 ± 14, P = 0.009), so as the survivors and non-survivors (86 ± 15 vs. 69 ± 14, P = 0.041). However, the other variables of the demographic and characteristics showed no significant differences between the successful and unsuccessful weaning group, as well as the survivors and the non-survivors (Table 1).
Clinical data
All patients underwent IMV, with an average IMV duration of 29 (12,80) h, VT of 413 (323–531) ml, PEEP of 12 (10–18) cmH2O, and PIP of 28 (25–32) cmH2O before ECMO. After 48 h on ECMO, PaO2 increased from 60 (47–74) mmHg to 90 (71–120) mmHg (P = 0.003), PaCO2 declined from 50 (39–64) mmHg to 42 (34–45) mmHg (P = 0.033), and SaO2 increased from 84 (68–94) to 97 (96–100) % (P = 0.001). As shown in Table 2 and Fig. 2 summarizes time-varying changes in arterial gas blood analysis and ventilator settings, showing a trend of gradual improvement in several circulatory and respiratory physiological indicators. Considering the laboratory results, the successful weaning group had a lower level of pTB (31.3 ± 8.8 vs. 132.7 ± 109, P = 0.012) compared to the unsuccessful weaning group. Furthermore, compared to the non-survivors, the levels of pCr (110 ± 46 vs. 203 ± 89, P = 0.043) and pTB (29.1(24.1–32.3) vs. 81.0 (34.4–207.4), P = 0.046) in survivors were lower.
Figure 3 describes the usage of red blood cells (RBC), sodium bicarbonate (SB), albumin (ALB), and intravenous immunoglobulin (IVGB) during ECMO and in the hospital. We adopted a restricted transfusion strategy. The results showed that there was no significant difference in the total number of concentrated red blood cells transfused in hospitals between survivors and death groups. For patients with severe infection, “Intravenous immunoglobulin (IVIG) 5%:50 ml” is given to enhance immunity and anti-infection ability. Through comparison, we found that the successful weaning group had a lower accumulation of sodium bicarbonate during ECMO (0 (0–125) vs. 225(30–1275), P = 0.035), and a higher accumulation of intravenous immunoglobulin in hospital (3500 (1000–4000) versus 0 (600–1600), P = 0.035). Yet by the same analysis, there was no significant difference in total immunoglobulin infusion during hospitalization between the survivors and non-survivors.
As shown in Fig. 4, we did not observe a significant difference between survivors and non-survivors in terms of fluid balance prior to ECMO support from day 1 to day 7. Patients in the successful weaning group were more likely in a negative daily fluid balance state, while the unsuccessful weaning group was the opposite.
Outcomes
Out of 18 patients, 7 were successfully weaned off ECMO, 2 of whom died of multiple organ dysfunction syndrome later on. Of these 13 death cases, 4 died of septic shock, 3 of multiple organ dysfunction syndrome (MODS), 2 of respiratory failure, and 4 of treatment abandonment made by relatives due to unsatisfied therapeutic effect, respectively (Table 3). Figure 5 shows Kaplan–Meier plots of a total survival. The total durations of ECMO, IMV, ICU and hospitalization were approximately 5 (3,9) d, 10 (5,17) d, 13 (6,19) d and 18(6,28) d, respectively Table 3. Compared to the unsuccessful weaning group, the successful weaning group had a longer duration on IMV (13 (12-28) vs. 7 (4-9), P =0.008), a longer stay in ICU (18 (15-28) vs. 6 (3-10), P =0.004), a longer stay in hospital (28 (25-101) vs. 6 (3-17), P =0.001), and a lower mortality (28.6% vs. 100%, P =0.002). (Fig. 5).
Compared to the unsuccessful weaning group, the successful weaning group had a longer duration of IMV (13 (12–28) vs. 7 (4–9), P = 0.008), ICU stay (18 (15–28) vs. 6 (3–10), P = 0.004), and hospital stay (28 (25–101) vs. 6 (3–17), P = 0.001), whereas with lower mortality (28.6% vs. 100%, P = 0.002). The complications during ECMO included ventilator-associated pneumonia in 12 patients (66.7%), hemorrhage in 12 patients (66.7%), of which were pulmonary hemorrhage (n = 7), gastrointestinal hemorrhage (n = 6), cannula or surgical site bleeding (n = 5), and cerebral hemorrhage (n = 1), acute renal failure in 12 patients (66.7%), hyperbilirubinemia in 8 patients (44.4%), mechanical complications in 6 patients (33.3%), and hemolysis in 5 patients (27.8%). Almost all patients had hyperglycemia (n = 17, 94%), which has been described in Table 4.
Discussion
The main findings of the present retrospective investigation are as follows: (a) the rate of successfully weaning from ECMO was 38.9%, and the overall mortality and the complication rate were 72.2% and 77.8%, respectively; (b) overall, patients experienced a gradual ascending tendency of PaO2 and the declining tendency of PaCO2 at the early stage of ECMO, particularly within the first 12 h; (c) patients in the successful weaning group had a lower pTB value and a less accumulation of sodium bicarbonate during ECMO, a less accumulation of intravenous immunoglobulin in hospital and lower mortality when compared to the unsuccessful weaning group; (d) the increased mortality was associated with more severe acidosis and lower mean arterial pressure before ECMO, as well as increased serum creatinine and total bilirubin values during ECMO.
This investigation focused on refractory ARDS patients supported by VV-ECMO, aiming at evaluating the therapeutic effect and exploring appropriate management of the rescue option. None of the studied patients had severe cardiac dysfunction before ECMO.
It is now well established that bilirubin is a marker of hepatic dysfunction, which is not only caused by hepatic diseases but also by hemolysis and hypoxia, simultaneously or/and at different times. We identified a lower level of total bilirubin in survivors during ECMO (29.1 (24.1,32.3) vs. 81.0 (34.4,207.4), P < 0.05), which accords with a previous report that bilirubin was a risk factor of mortality in patients with VV-ECMO for ARDS [11]. Serum creatinine might have various values during ECMO, yet it needs further investigation by larger cohort studies [12, 13].
Another key finding is that the cumulative volume of sodium bicarbonate during ECMO and intravenous immunoglobulin (IVIG) in hospitals has distinct different values between the successful and unsuccessful weaning groups. Sodium bicarbonate compensates for an acidotic cellular environment to a certain extent; however, its impact on metabolic acidemia remains controversial [14, 15]. A recent multicenter, randomized, controlled trial revealed that in patients with severe metabolic acidemia (pH ≤ 7.20), sodium bicarbonate treatment had no effect on mortality by day 28 or the presence of at least one organ failure at day 7, but reduced the need for renal-replacement therapy [16]. Additionally, the effect of IVIG treatment remains controversial in patients with sepsis [17, 18]. Recent evidence has reported that immunoglobulins did not benefit patients with severe ARDS requiring ECMO support [20]. Hence, additional research is needed to better understand the impact of sodium bicarbonate and IVIG therapy on this kind of population.
A large randomized trial has discovered that a conservative fluid-management strategy contributes to shortening ventilation duration and improving treatment outcomes of ICU patients [19, 20]. Recent research revealed a positive fluid balance on ECMO day 3 was associated with higher hospital mortality [19]. However, no statistically significant difference was detected in our research, suggesting more future studies would be needed to better understand the discrepancy.
The overall complication rate in our study was 77.8%, of which bleeding complication was the most common (66.7%). Hemostasis is a complex process with many cellular interactions that might affect patients with ECMO. Continuous use of heparin and intensive consumption of coagulation factors might relate to the complication. The abnormal level of bilirubin, associated with hepatic dysfunction, might also contribute to a decrease in clotting factors. Hemorrhage is highly related to ECMO mortality in some large series and registry data [21, 22]. One case with a fatal intracranial hemorrhage survived because of the timely response and appropriate treatment of our intensivist and surgeons. Therefore, identifying and treating complications in the early stage is critical. Several studies reported that hemorrhage and hemolysis increased blood product usage, indicating an independent association with outcomes of patients with ECMO [23, 24]. Although a restrictive transfusion policy for critically ill patients has been reported to better prognosis [25, 26], the benefit for patients on ECMO remains uncertain. There is no statistically significant difference in the volume of PRBCs (Packed Red Blood Cells) received between survivors and non-survivors during ECMO or in-hospital from this finding, which is in line with a previous report [27].
The rate of mortality and complication in the present analysis was 72.2 and 77.8%, respectively. The high incidence might be attributed to the low utility of suggested managements such as prone positioning [28, 29] and neuromuscular blocking agents [30] before ECMO. These strategies are recommended by ELSO when patients have received optimal care for at least 6 h, their PaO2/FiO2 is still lower than 100 with the FiO2 > 90% [31]. The best outcome for adults with respiratory failure happens when the ECMO initiation is on the early onset of the disease (1–2 days), while in our case, it was [11 (7, 20)] h. Nevertheless, the patients in our study had more critical conditions, with mean APACHE II scores of 20 (18–26). The learning curve of VV-ECMO by all ECMO members, especially intensivists in ICU, and a difference in patient selection, might also affect the outcomes. Hence, we emphasized early implementation according to the recognition and patient characteristics.
Despite the undesirable results, patients had a gradual ascending tendency of PaO2 and a declining tendency of PaCO2 at the early stage of ECMO. Therefore, VV-ECMO is an ultimate rescue measure for refractory ARDS because of the rapid correction of severe gas exchange disorders. Critical illness is a complex situation in which development is influenced by various factors, better outcomes could be expected through larger randomized controlled trials, experience accumulation, and ECMO support.
There are several limitations in this study. First, it was conducted in a single institution, which might limit the generalizability. Second, the retrospective analysis of the data could have been biased by residual confounders so it might not be suitable for the multivariable analysis. Finally, the sample size was relatively small to accurately assess the efficacy of VV-ECMO. Despite the above limitations, our medical center took the lead in developing ECMO technology and conducting the first investigations on whether VV-ECMO improves the prognosis of severe ARDS patients in China. Our treatment experience reflects the clinical situation in China to some extent. In the future, we plan to collaborate with multiple ECMO centers in China to conduct multicenter clinical trials, providing reliable clinical evidence for the application of VV-ECMO in treating severe ARDS.
In conclusion, severe ARDS is a disease with complex pathogenesis and time-varying progression. Therefore, a single therapeutic strategy often fails to achieve satisfactory outcomes. However, our findings enhance the existing knowledge that VV-ECMO can effectively improve the oxygenation and ventilation of patients with severe ARDS. Besides, we highlight the early initiation of ECMO, with appropriate management strategies, which could contribute to reducing the risk of comorbidities and mortality.
Availability of data and materials
Raw data for all the figures and tables presented in this study are not publicly available due to patients’ privacy.
Abbreviations
- ECMO:
-
Extracorporeal membrane oxygenation
- ARDS:
-
Acute respiratory distress syndrome
- IPF:
-
Idiopathic pulmonary fibrosis
- ABGA:
-
Arterial blood gas analysis
- PaO2:
-
Arterial oxygen tension
- PaCO2:
-
Arterial carbon dioxide tension
- SaO2:
-
Arterial oxygen saturation
- FiO2:
-
Fraction of inspiration O2
- PEEP:
-
Positive end-expiratory pressure
- PS:
-
Pressure support
- PIP:
-
Peak inspiratory pressure
- Compliance:
-
Dynamic pulmonary compliance
- IMV:
-
Invasive mechanical ventilation
- VV:
-
Venovenous
- VA:
-
Venoarterial
- Cr:
-
Creatinine
- TB:
-
Total bilirubin
- ICU:
-
Intensive care unit
- PRBCs:
-
Packed red blood cells
References
Goligher EC, Dres M, Patel BK et al (2020) Lung- and diaphragm-protective ventilation. Am J Respir Crit Care Med 202(7):950–961
Bellani G, Laffey JG, Pham T et al (2016) Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 315(8):788–800
Chiu LC, Chuang LP, Lin SW et al (2021) Cumulative fluid balance during extracorporeal membrane oxygenation and mortality in patients with acute respiratory distress syndrome. Membranes (Basel) 11(8):567
Gehron J, Bandorski D, Mayer K et al (2023) The impact of recirculation on extracorporeal gas exchange and patient oxygenation during veno-venous extracorporeal membrane oxygenation-results of an observational clinical trial. J Clin Med 12(2):416
Wang H, Deng S, Fan X et al (2021) Research trends and hotspots of extracorporeal membrane oxygenation: a 10-year bibliometric study and visualization analysis. Front Med (Lausanne) 8:752956
Valencia E, Nasr VG (2020) Updates in pediatric extracorporeal membrane oxygenation. J Cardiothorac Vasc Anesth 34(5):1309–1323
Brodie D, Abrams D, MacLaren G et al (2022) Extracorporeal membrane oxygenation during respiratory pandemics:past, present, and future. Am J Respir Crit Care Med. 205(12):1382–1390
Morris AH, Wallace CJ, Menlove RL et al (1994) (1994) Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med 149(2 Pt 1):295–305
Combes A, Hajage D, Capellier G et al (2018) Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med 378(21):1965–1975
Fan E, Gattinoni L, Combes A, Schmidt M et al (2016) Venovenous extracorporeal membrane oxygenation for acute respiratory failure: a clinical review from an international group of experts. Intensive Care Med. 42(5):712–724
Lazzeri C, Bonizzoli M, Cianchi G et al (2018) (2018) Bilirubin in the early course of venovenousextracorporeal membrane oxygenation support for refractory ARDS. J Artif Organs. 21(1):61–67
Pilarczyk K, Huenges K, Bewig B et al (2022) Acute kidney injury in patients with severe ARDS requiring extracorporeal membrane oxygenation: incidence, prognostic impact and risk factors. J Clin Med 11(4):1079
Roedl K, De Rosa S, Fischer M et al (2023) Early acute kidney injury and transition to renal replacement therapy in critically ill patients with SARS-CoV-2 requiring veno-venous extracorporeal membrane oxygenation. Ann Intensive Care. 13(1):115
Weisbord SD, Gallagher M, Jneid H et al (2018) Outcomes after angiography with sodium bicarbonate and acetylcysteine. N Engl J Med 378(7):603–614
Kim S, Hwang J, Kim JH (2022) Sodium bicarbonate buffer for weaning from venovenous extracorporeal membrane oxygenation in patients with hypercapnic respiratory failure and acute renal failure. Ann Thorac Med. 17(4):237–240
Jaber S, Paugam C, Futier E et al (2018) Sodium bicarbonate therapy for patients with severe metabolic acidaemia in the intensive care unit (BICAR-ICU): a multicentre, open-label, randomised controlled, phase 3 trial. Lancet 392(10141):31–40
Pan B, Sun P, Pei R et al (2023) Efficacy of IVIG therapy for patients with sepsis: a systematic review and meta-analysis. J Transl Med. 21(1):765
Jarczak D, Kluge S, Nierhaus A (2020) Use of intravenous immunoglobulins in sepsis therapy-a clinical view Int J Mol Sci 21(15):5543
Seitz KP, Caldwell ES, Hough CL (2020) Fluid management in ARDS: an evaluation of current practice and the association between early diuretic use and hospital mortality. J Intensive Care 8:78
Silversides JA, Major E, Ferguson AJ et al (2017) Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: a systematic review and meta-analysis. Intensive Care Med 43(2):155–170
Nunez JI, Gosling AF, O’Gara B et al (2022) Bleeding and thrombotic events in adults supported with venovenous extracorporeal membrane oxygenation: an ELSO registry analysis. Intensive Care Med. 48(2):213–224
Martucci G, Giani M, Schmidt M et al (2024) Anticoagulation and bleeding during veno-venous extracorporeal membrane oxygenation: insights from the PROTECMO study. Am J Respir Crit Care Med 209(4):417–426
Martucci G, Panarello G, Occhipinti G et al (2019) Anticoagulation and transfusions management in veno-venous extracorporeal membrane oxygenation for acute respiratory distress syndrome: assessment of factors associated with transfusion requirements and mortality. J Intensive Care Med 34(8):630–639
O’Halloran CP, Alexander PMA, Andren KG et al (2018) (2018) RBC exposure in pediatric extracorporeal membrane oxygenation: epidemiology and factors associated with large blood transfusion volume. Pediatr Crit Care Med 19(8):767–774
Abbasciano RG, Yusuff H, Vlaar APJ (2021) Blood transfusion threshold in patients receiving extracorporeal membrane oxygenation support for cardiac and respiratory failure-a systematic review and meta-analysis. J Cardiothorac Vasc Anesth 35(4):1192–1202
Singh G, Nahirniak S, Arora R et al (2020) Transfusion thresholds for adult respiratory extracorporeal life support: an expert consensus document. Can J Cardiol. 36(9):1550–1553
Worku ET, Win AM, Parmar D et al (2023) Haematological trends and transfusion during adult extracorporeal membrane oxygenation: a single centre study. J Clin Med. 12(7):2629
Ong SL, Tantawy H, Assi R et al (2022) Combined use of ECMO, prone positioning, and APRV in the management of severe COVID-19 patients. Clin Med Insights Circ Respir Pulm Med. 16:11795484221134452
Poon WH, Ramanathan K, Ling RR et al (2021) Prone positioning during venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care. 25(1):292
Qadir N, Sahetya S, Munshi L et al (2024) An update on management of adult patients with acute respiratory distress syndrome: an official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 209(1):24–36
Tonna JE, Abrams D, Brodie D et al (2021) Management of adult patients supported with venovenous extracorporeal membrane oxygenation (VV ECMO): guideline from the extracorporeal life support organization (ELSO). ASAIO J 67(6):601–610
Acknowledgements
We appreciate all medical staff in our institution for the tender care of the patients entrusted to our care.
Funding
Funding for this study was provided by the National Natural Science Foundation of China (No.82374216) and the Medical Scientific Research Foundation of Guangdong Province of China (no. A2023198 and no. A2024135), Zhongshan Science and Technology Bureau (no. 2020B1081).
Author information
Authors and Affiliations
Contributions
Su Ying-ying analyzed and interpreted statistics analysis. Fan Wen-ding was a major contributor to writing the manuscript. Wu Zhi-xin supervised the entire process and made an equal contribution to Jiang Chong-hui in the study. Su Yi graphed the data. Chen Qiao was responsible for the language editing. Huang Shao-juan and Chen Ping offered the equipment and place needed. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This research has been approved by Zhongshan City People’s Hospital Clinical Research and Animal Experimental Ethic Committee, project no.: 2024–014.
Competing interest
Dr. Wu Zhi-xin received support from the National Natural Science Foundation of China (no. 82374216) and the Medical Scientific Research Foundation of Guangdong Province of China (no. A2023198 and no. A2024135). All other authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chong-hui, J., Ying-ying, S., Wen-ding, F. et al. Venovenous extracorporeal membrane oxygenation (VV-ECMO) for severe acute respiratory distress syndrome (ARDS) in adults—a single-center experience. Egypt J Bronchol 18, 58 (2024). https://doi.org/10.1186/s43168-024-00310-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43168-024-00310-0