Abstract
Background
Knowledge of the sequelae of new coronavirus disease 2019 (COVID-19) is still limited owing to the relative recent onset of the disease. However, the study of other different types of coronavirus infections prior to COVID-19 infection reports that the patients may experience persistent symptoms following the infection.
The aim of this study
Assessment and follow-up of persistent respiratory symptoms in patients recovered from acute COVID-19 infection.
Methods
In this prospective cohort study, COVID-19 patients diagnosed at Beni-Suef University hospital and followed up prospectively at 3, 6, and 12 months after discontinuation of quarantine. Patients were interviewed for persistent respiratory symptoms then underwent assessment by physical examination and routine labs.
Results
Seventy-one patients were evaluated and participated in this study. The mean age of the patients was 47 years and 46 (64%) of them were females. After 3 months, 77.5% of the patients had persistent dyspnea, 57.7% persistent fatigue, 15.5% persistent cough, and 8.5% persistent chest pain. At the 6th month, dyspnea and fatigue persisted in 33.8% and 22.5% of cases respectively while at the 12th month dyspnea persisted in 22.5% of cases. Old age, smoking, diabetes mellitus, severity of the disease, and hypoxemia on admission were associated factors with persistent symptoms.
Conclusion
Our result added to the growing evidence that there are pulmonary sequelae in COVID-19 survivors, which may become a significant chronic global pulmonary health problem.
Similar content being viewed by others
Introduction
On 31 December 2019, the China Health government alerted the World Health Organization (WHO) to severe cases of pneumonia of unknown cause in Wuhan City [1]. On 7 January, a novel infection by coronavirus was detected, originally abbreviated as 2019-nCoV by WHO and identified from a throat swab sample of infected patient [2]. This virus then renamed as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [3]. On 11 March 2020, the WHO documented COVID-19 infection as pandemic [4]. Despite the majority of patients completely recovered, a significant proportion of the patients—including mild cases—still complaining of persistent symptoms as fatigue and exertional dyspnea up to 6 and even 12 months [5]. Post-viral syndromes are reported following other coronavirus infection outbreaks as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) [6]. Persons who have recovered from COVID-19 but still have long-lasting consequences or who have experienced the usual symptoms but for longer duration than expected have acquired importance in recent scientific literature [7]. The long-term symptoms and how they influence the patient’s quality of life are not yet understood. Several authors have documented lung injury, including fibrosis and pulmonary function impairment, as well as the persistence of respiratory symptoms up to months following release from COVID-19 infection [8]. Patient-reported outcomes, such as fatigue or dyspnea, are critical for enhancing healthcare delivery, increasing patient involvement, and ensuring that the treatment and research are oriented to this patient [9]. “Long COVID-19” patients, those with symptoms that persist after the acute viral illness has been subsided, are becoming well recognized [7]. There is a significant incidence of respiratory symptoms, including dyspnea, cough, and chest discomfort among the persistent symptoms [10]. One in three patients—those with or without comorbidities—has a worse quality of life as a result of the duration of these symptoms. Additionally, it might postpone a person’s return to work [11].
The aim of the study
Assessment and follow-up of persistent pulmonary symptoms in long COVID-19 patients aiming to identify the clinical needs of COVID-19 survivors.
Methods
Study design and participants
A prospective observational (cohort) study was performed on adult patients infected with COVID-19 at Beni-Suef University hospital according to the illustrated flowchart (Fig. 1). Patients met the World Health Organization criteria for quarantine discontinuation (10 days after symptoms onset, not feverish for 3 additional days, and improvement in symptoms like cough, dyspnea, or fatigue) were followed up. This study was performed in the period between November 2020 and February 2022. The study was approved by the local ethical committee at Faculty of Medicine, Beni-Suef University (FMBSUREC/04102020/Ibrahim).
A written informed consent obtained from all study participants.
Procedures
Assessment was done including full history taking, physical examination, routine labs, measurement of peripheral oxygen saturation, and follow-up of persistent respiratory symptoms also identifying risk factors for persistent respiratory symptoms as age of the patients, DM, severity of hypoxemia, and smoking status. Symptoms were assessed at 3, 6, and 12 months after recovery.
Dyspnea was assessed by a modified Medical Research Council dyspnea scale (mMRC scale) for measuring the degree of disability due to breathlessness that poses day-to-day activity on a scale from 0 to 4 [12].
Severity of the disease was classified according to the Egyptian management protocol of COVID-19 into:
-
1)
Mild: mild symptoms, normal imaging
-
2)
Moderate: pneumonia without hypoxia
-
3)
Severe: pneumonia with hypoxia responding to oxygen therapy
-
4)
Critical: pneumonia with hypoxia not responding to oxygen therapy and/or by organ dysfunction [13]
Statistical analysis
All data were collected then entered and coded into SPSS version 25 for Windows. Numeric variables expressed as mean, standard deviation, median, minimum, and maximum, while categorical variables expressed as number and percent.
McNemar test was used for follow-up of the symptoms between 3, 6, and 12 months. Pearson correlation was used to detect the correlation between length of hospital stay and different scale parameters.
Multivariable binary logistic regression analysis was conducted for assessment of the risk factors for residual symptoms at 3, 6, and 12 months.
*P value less than or equal 0.05 was considered as significant.
Results
The mean value for the age of the studied patients was 47 ± 15.6 years, most of them were females. Half of the participants were managed at home, 35.2% were managed in a hospital ward, and only 14.1% were managed in ICU (Table 1). Most of the studied patients had moderate to severe COVID-19 symptoms. The commonest management regimen based on diagnosis was steroids, parenteral antiviral, and parenteral anticoagulant regimen (Table 2).
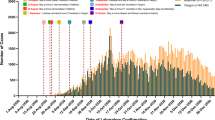
Figure 2 shows that the most common persistent pulmonary symptom in the 3rd month of follow-up was dyspnea followed by fatigue then cough. In the 6th month, dyspnea and fatigue persisted in 33.8% and 22.5% of cases respectively while at the 12th month dyspnea and fatigue persisted in 22.5% of cases and 11.3% of cases, respectively (Table 3). There was a marked significant change in the proportion of patients who had dyspnea at 12-month follow-up after 6 months (Table 4). There was no significant change in patients who had cough at 12-month follow-up after 6 months (Tables 5 and 6). All cases of expectoration totally improved at 6 months (Table 7). Regarding fatigue showed that there was a statistically significant change in the patients who had fatigue at 6 months after 3 months (Table 8). There was no significant change in the patients who had chest pain at follow-up 6 months after 3 months; 4 of 6 patients became free at 6 months (Tables 9 and 10). All cases of other constitutional manifestations totally improved at 6 months (Tables 11 and 12). After adjustment for age, sex, presence of DM, HTN, smoking, chest problems, and oxygen saturation on admission to detect the associated risk factors for prediction of the probability of having symptoms persistent after 3 months, it was detected that every increase in age 1 year increased the probability of having symptoms persistent after 3 months about one time and half OR (95%CI for OR) was 1.4 (1.05 to 1.9) (Table 13). The probability of having symptoms persistent after 6 months, it was detected that smoking increased the probability of having symptoms persistent after 6 months OR (95%CI for OR) was 8.5 (1.3 to 58.2) and presence of diabetes also. In addition, it was found that every increase in oxygen saturation on admission one unit decreased the probability of having symptoms persistent after 6 months with about 13% OR (95%CI for OR) was 0.917 (0.863 to 0.974) (Table 14). Prediction of the probability of having symptoms persistent after 12 months, it was detected that smoking increased the probability of having symptoms persistent after 12 months OR (95%CI for OR) was 6.9 (1.04 to 45.9). In addition, increase in oxygen saturation one unit decreased the probability of having symptoms persistent after 12 months with about 13% OR (95%CI for OR) was 0.920 (0.868 to 0.976) (Table 15). Regarding Tables 13, 14, and 15, severity of the disease and site of care were excluded from the model as they were correlated with oxygen saturation on admission. There was a moderate linear positive correlation between the length of hospital stay and ferritin level on admission (Table 16, Figs. 3 and 4).
Discussion
The term “post-COVID 19 syndrome” includes persistent symptoms that may be caused by residual inflammation (convalescent phase), end organ damage, non-specific effects from hospital admission or prolonged mechanical ventilation, social isolation, or impact of pre-existing health problem [14].
Owing to the high number of patients infected by COVID-19 infection, and because it is important to detect the risk of persistent respiratory symptoms to plan management modalities for this the long COVID syndrome, this study was performed on 71 adult patients more than 18 years diagnosed with COVID-19 infection at Beni-Suef University hospital in the period between November 2020 and February 2022. Patients died or not attending the follow-up visits were excluded from the study. The most common persistent symptom in 3rd month of following-up was dyspnea followed by fatigue then cough. In the 6th month, dyspnea and fatigue persisted in 33.8% and 22.5% of cases respectively while at the 12th month dyspnea and fatigue persisted in 22.5% of cases and 11.3% of cases, respectively. There was a significant change in the percentage of the patients who had dyspnea at 6 months after 3 months and also at 12 months after 6 months. 15.5% of patients had cough at follow-up 3 months and 2.8% at follow-up 6 months with significant change. There was no significant change in the percentage of the patients who had cough at 12 months after 6 months. All cases of expectoration totally improved at 6-month follow-up. There was a significant change in the percentage of the patients who had fatigue at 6 months after 3 months. Follow-up of fatigue from 6 months till 12 months showed significant improvement. Smoking, diabetes mellitus, hypoxemia on admission, and severity of the disease were risk factors for persistent symptoms on follow-up. There was a moderate linear positive correlation between the length of hospital stay and ferritin level on admission. This study agrees with a study by Wu et al. who followed up patients at 3, 6, 9, and 12 months after hospital discharge. Dyspnea was very frequent in patients at 3 months. The number of patients significantly reduced at 6 months, 9 months, and 12 months [15]. Another study showed that the percentage of patients with at least one residual symptom decreased from 68% at follow-up 6 months to 49% at follow-up 12 months [16]. Lorent et al. [17] showed that about half of the studied patients detected fatigue, dyspnea, and/or cognitive impairment at follow-up 3 and 12 months, respectively.
Martino et al. [18] studied persistent symptoms after infection by COVID-19 at 12-month follow-up; the most common persistent symptoms were dyspnea (18.7%), then cough (6.2%), and finally fatigue (12.5%). Female sex and having underlying comorbidities were associated with fatigue [19]. Cirulli et al. [20] showed that severity of the illness has a higher risk of long-term symptoms. Being current or ex-smoker, having diabetes mellitus, and having a longer length of hospital admission were risk factors with persistent pulmonary symptoms [21]. Martino et al. [18] reported that there is no influence of biological sex on persistent respiratory symptoms at all time points (6-month, 12-month follow-up). Huang et al. [16] showed that increasing age and severity of acute illness were positively associated with fatigue at follow-up. Pre-existing pulmonary comorbidity, type 2 diabetes mellitus, and malignancy were associated with persistent symptoms [22]. Elevated ferritin level was an associated factor with prolonged hospital stay [23]. The exact mechanisms that explain these chronic pulmonary symptoms after COVID-19 infection still not yet fully known. Added to the direct effects of SARS-CoV-2 infection, the host immune response to the virus may be responsible for the presence of these long-lasting symptoms, through facilitating an ongoing hyperinflammatory state [24]. Compared to the previous studies, the strength and novelty of our study consisted of a long-term follow-up, including a broad spectrum of different patient severity, as we included unselected COVID-19 patients with various comorbidities then analysis of the characteristics of acute viral infection associated with persistent pulmonary symptoms.
Limitations of the study
The small sample size of the studied patients then including patients with severe cases may over estimate post-acute infectious sequelae or other comorbidity in patients with mild COVID-19. Also, we did not have data about the functional status of the studied patients before infection with COVID-19 also follow-up laboratory parameters as CRP, ferritin, and d-dimer not included in the study due to increasing number of variables of the study, number of vaccinated patient not included in the study as plan for this study was introduced for ethical committee approval in 2020 before obligation of COVID-19 vaccine in Egypt, and lastly, the lack of control group.
Conclusion
There is evidence that persistent symptoms are common after 1 year in patients recovered from COVID-19. Dyspnea and fatigue were the most common. Dyspnea and fatigue continued in 22.5% and 11.3% of cases respectively in the twelfth month of follow-up. It was detected that every increase in age 1 year increased the probability of having symptoms persistent after 3 months while increase in oxygen saturation on admission one unit decreased the probability of having symptoms persistent after 6 and 12 months but smoking increased the probability of having symptoms persistent after 6 and 12 months. Timely follow-up of survivors is recommended.
Availability of data and materials
Not applicable.
References
Lu H, Stratton CW, Tang YW (2020) Outbreak of pneumonia of unknown etiology in Wuhan China: the mystery and the miracle. J Med Virol 92(4):401–402
Hui D, Azhar I, E, Madani T, Ntoumi F, Kock R, Dar O, et al (2020) The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis 91:264–266
Gorbalenya A, Baker S, Baric R, de Groot R, Drosten C, Gulyaeva A, et al (2020) severe acute respiratory syndrome-related coronavirus: the species and its viruses – a statement of the Coronavirus Study Group. BioRxiv 2020. 02.07.937862.
Sharma A, Farouk I, Lal S (2021) COVID-19: a review on the novel coronavirus disease evolution, transmission, detection, control and prevention. Viruses 13(2):202
Wynberg E, van Willigen H, Dijkstra M, Boyd A, Kootstra N, van den Aardweg J, et al (2022) RECoVERED Study Group. Evolution of COVID-19 symptoms during the first 12 months after illness onset. Clin Infect Dis 75(1):e482-e490
Moldofsky H, Patcai J (2011) Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurol 11:37
Mahase E (2020) COVID-19: what do we know about ‘long COVID’? BMJ 370:m2815
Shaw B, Daskareh M, Gholamrezanezhad A (2021) The lingering manifestations of COVID-19 during and after convalescence: update on long-term pulmonary consequences of coronavirus disease 2019 (COVID-19). Radiol Med 126(1):40–46
Wong A, Shah A, Johnston J, Carlsten C, Ryerson C et al (2020) Patient-reported outcome measures after COVID-19: a prospective cohort study. Eur Respir J 26(5):2003276
Rosales-Castillo A, de Los RC, Garc´ıa J, (2021) Persistent symptoms after acute COVID-19 infection: importance of follow-up. Med Clin (Barc) 156(1):35–36
Halpin S, McIvor C, Whyatt G, Adams A, Harvey O, McLean L et al (2021) Post discharge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol 93(2):1013–1022
Rajala K, Lehto J, Sutinen E, Kautiainen H, Myllärniemi M, Saarto T (2017) mMRC dyspnoea scale indicates impaired quality of life and increased pain in patients with idiopathic pulmonary fibrosis. ERJ Open Res 3(4):00084
Masoud HH, Elassal G, Zaky S, Kamal EK (2019) Management protocol for COVID-19 patients version 1.4/30th May 2020 Ministry of health and population (MOHP), Egypt. Coronavirus Disease.
Garg P, Arora U, Kumar A, Wig N (2021) The “post-COVID” syndrome: how deep is the damage? J Med Virol 93(2):673–674
Wu X, Liu X, Zhou Y, Yu H, Li R, Zhan Q et al (2021) 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med 9(7):747–754
Huang L, Yao Q, Gu X, Wang Q, Ren L, Wang Y et al (2021) 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet 398:747–758
Lorent N, Weygaerde Y, Claeys E, Fajardo I, De Vos N, De Wever W et al (2022) Prospective longitudinal evaluation of hospitalised COVID-19 survivors 3 and 12 months after discharge. ERJ Open Res 8:00004–02022
Martino G, Benfaremo D, Bitti G, Valeri G, Postacchini L, Marchett A et al (2022) 6- and 12-month outcomes in patients following COVID-19-related hospitalization: a prospective monocentric study. Intern Emerg Med 9:1–9
Amin-Chowdhury Z, Harris R, Aiano F, Zavala M, Bertran M, Borrow R et al (2021) Characterising post-COVID syndrome more than 6 months after acute infection in adults; prospective longitudinal cohort study. England Med Rxiv 03(18):21253633
Cirulli E, Schiabor Barrett K, Riffle S, Bolze A, Neveux I, Dabe S et al (2020) Long-term COVID-19 symptoms in a large unselected population. Med Rxiv 10(07):20208702
Robey R, Kemp K, Hayton P, Mudawi D, Wang GM et al (2021) Pulmonary sequelae at 4 months after COVID-19 infection: a single-centre experience of a COVID follow-up service. Adv Ther 38:4505–4519
Sonnweber T, Tymoszuk P, Sahanic S, Boehm A, Pizzini A, Luger A, et al (2022) Investigating phenotypes of pulmonary COVID-19 recovery: a longitudinal observational prospective multicenter trial. eLife 11:e72500
Caoa P, Wuc Y, Wu S, Wu T, Zhang Q, Zhang R et al (2021) Elevated serum ferritin level effectively discriminates severity illness and liver injury of coronavirus disease 2019 pneumonia. Biomarkers 26(3):207–212
Korompoki E, Gavriatopoulou M, Hicklen R, Ntanasis- Stathopoulos I, Kastritis E, Fotiou D et al (2021) Epidemiology and organ specific sequelae of post-acute COVID19: a narrative review. J Infect 83(1):1–16
Acknowledgements
NA.
Funding
Nil.
Author information
Authors and Affiliations
Contributions
EME collected the patients’ data and performed the statistical component, ME conceived the publication design and prepared the manuscript, RS and LA revised the methods and results, and all authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the hospital research ethics board of Beni-Suef University, and a written informed consent was obtained from either patients themselves or their relatives. The study was approved by the local ethical committee at the Faculty of Medicine, Beni-Suef University (FMBSUREC/04102020/Ibrahim).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Emad Eldin, E.M., Mohammed, R.S., Batanony, M.M.E.L. et al. 12-month risk factor evaluation for persistent pulmonary symptoms in long COVID-19 patients. Egypt J Bronchol 18, 16 (2024). https://doi.org/10.1186/s43168-024-00265-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43168-024-00265-2