Abstract
Acute lung injury (ALI) is a life-threatening clinical syndrome with high morbidity and mortality. The main pathological features of ALI are increased alveolar-capillary membrane permeability, edema, uncontrolled migration of neutrophils to the lungs, and diffuse alveolar damage, resulting in acute hypoxemic respiratory failure. We performed a systematic review and meta-analysis to elucidate the antioxidant activities of flavanols in a rat model of acute lung injury (ALI). PubMed, EMBASE, Scopus, ProQuest, Web of Science, and Google Scholar databases were searched to obtain the relevant papers. Nine studies with 343 rat models of ALI were included in this study. We investigated oxidative stress with the corresponding 95% CI. Estimating the correlation and 95% CIs for the inflammatory agents and oxidative stress in the intervention group, compared with that in the control group (ALI), respectively (correlation: 0.635; 95% CI, 0.560–0.699, P value = 0.000, Z value= 12.648) and (correlation: 0.317; 95% CI, 0.189–0.434, P value = 0.00, Z value= 4.7). In conclusion, investigating the effects of different flavanols on oxidative stress in lung injury may provide a useful therapeutic strategy in ALI mouse models. However, the final conclusion on treatment efficacy should be sufficient for prospective controlled randomized trials.
Similar content being viewed by others
Introduction
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are life-threatening lung diseases, and severe sepsis is one of the most important factors in causing the above diseases. However, it is not the only trigger that leads to ALI and ARDS. Factors such as severe bacterial pneumonia, trauma, exposition to injurious mechanical ventilation, and capillary endothelial cell damage are caused. ALI is associated with acute and severe inflammation that disrupts the endothelial and epithelial barriers of the lung. Alveolar-capillary membrane damage, pulmonary edema, neutrophil-induced inflammation, and perfusion-ventilation mismatch ultimately reduce lung compliance and cause profound hypoxemia and may lead to loss of lung function [1, 2]. However, drug treatments are not available to improve patients’ conditions. ALI has a high prevalence and the mortality rate is alarmingly high in the critically ill patient population. Treatment of acute lung injury is based on both ventilatory and nonventilatory strategies [3]. The results of several clinical trials have shown that pharmaceutical strategies are not effective in reducing mortality. But the use of ventilators for management in supportive care of lung injury patients has been very effective [4]. A ventilator is a lifesaving feature in the treatment of critically ill patients. The purpose of ventilators is to ensure sufficient gas exchange while taking over the work of breathing during the resolution of the underlying disease that caused respiratory failure. Unfortunately, MV can lead to excessive mechanical strain on lung tissues causing damage to them, especially in patients already suffering from lung diseases [5]. To date, the most significant advances in supportive care for lung injury patients have been related to improved ventilator management. Recent advances in understanding the pathophysiology of ALI have led to research into several potential drug therapies. Despite previous encouraging clinical evidence, pre-clinical have not confirmed the use of statins, beta-agonists, non-steroidal anti-inflammatory drugs, antioxidants, exogenous surfactants, neutrophil elastase inhibitors, anticoagulants, anti-TNF biologics, etc., as a treatment for ALI [6]. Therefore, an urgent need remains for the development of novel therapeutic strategies with minimal side effects.
Alveolar epithelial cell death, inflammation, and oxidative stress are typical features of ALI. These cytokines are thought to play an important role in ALI. In ALI, neutrophils and macrophages infiltrate the lung tissue and secrete cytokines that stimulate regional pro-inflammatory cascades. Inflammatory factors lead to irreversible damage to the lung epithelium [6]. Nuclear factor-kappa B (NF-κB) is a nuclear transcription factor that regulates inflammatory processes. NF-κB is required for maximal transcription of multiple cytokines, including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) [7]. Therefore, it has been suggested that NF-κB function inhibitors may be useful as anti-inflammatory agents [8]. The results of a study showed that a neutrophil membrane-coated liposome-loaded acidic fibroblast growth factor has pro-inflammatory cytokine binding capabilities and can promote cellular uptake, substantially attenuate inflammatory responses, and enhance cellular antioxidant capacity [6]. The damage induced by free radicals is an important etiological factor related to many diseases. Since oxidative stress is the main cause of ALI-induced cytotoxicity in the lung, it seems that substances with their antioxidant properties can be a suitable approach for the treatment of ALI-induced toxicity [9]. Some studies have shown that antioxidant enzymes (AOEs) including superoxide dismutase (SOD), catalase, and glutathione peroxidase (GPx) can protect tissue against oxidative damage [10, 11].
Flavonoids are secondary metabolites that have extensive metabolic functions in plants. They are widely distributed in fruits, flowers, seeds, vegetables, and in both legumes and non-legumes. They have a variety of medicinal activities, acting as anti-cancer, antioxidant, and anti-inflammatory agents [12,13,14]. In recent years, quercetin has attracted increasing attention due to its anti-inflammatory and anti-oxidation effects in cell models. It can suppress the ongoing oxidative stress, acute inflammation, and cytokine storm, which can increase animal survival rate and improve lung edema and pathological symptoms [15]. To elucidate the anti-inflammatory and antioxidant activities of quercetin, we conducted a systematic review and meta-analysis in ALI-induced rats.
Main text
Literature search
This study was conducted based on the Preferred Reporting Method for Systematic Review and Meta-Analysis (PRISMA) [16]. We searched PubMed, EMBASE, Scopus, Web of Science, and Google Scholar databases from June 30, 2017, to August 30, 2022. The systematic search has used the following keywords: “Acute lung injury” OR “lung injury” OR “acute respiratory distress syndrome “OR “ALI” OR “ARDS” OR “lung disease” OR “respiratory failure” OR “Chronic obstructive pulmonary disease” OR “COPD” OR “Idiopathic pulmonary fibrosis” OR “IPF”) AND (“Quercetin” OR “4 3,3′,4′,5,7-pentahydroxy-2-phenylchromen-4-one”) AND (“pro-inflammatory” OR “anti-inflammatory“ OR “oxidative stress”). We also looked at the references of some related articles, especially the referenced articles. In addition, the references of all related articles and the articles that were referred to were checked manually to find relevant studies. The full text of the selected articles was evaluated for an additional and independent analysis. This is a systematic review and meta-analysis of previous studies, so there was no need for ethical approval or patient consent.
Selection criteria
Studies with the following appropriate standards were included in this analysis: All research articles in English, studies that focused on ALI models in rats, studies that examined the effects of quercetin administration on oxidative stress in ALI models, studies that examined the effects of quercetin administration on inhibition of inflammatory responses in ALI models, and (D) studies that looked at specific concentrations of quercetin. Studies were excluded with the following criteria: human experiments, cell culture experiments, animal studies other than rats, duplicate studies, review articles, case report studies, and conference abstracts. We excluded studies that did not provide us with enough information and articles that were not in English.
Data extraction
We used EndNote software (version X8, Thomson Reuters, USA) to review the literature and removed duplicate articles. Then, two authors separately checked the title and abstract of the searched articles. Disputes between the two evaluators were referred to a third party. Articles that did not meet the eligibility criteria were excluded. The following data were extracted from each eligible article: the first author, year of publication, number of cases and controls, how the ALI animal model was created, age/weight, quercetin drug prescription type, changes in oxidative stress levels in the ALI model rat (cases and controls), changes in levels of inflammatory factors in the ALI model rat (cases and controls), effects of quercetin on levels of inflammatory factors, and oxidative stress in the ALI model.
Quality assessment
The quality of included studies was assessed based on Hayden et al. guidelines for evaluating quality in prognostic studies [17].
Statistical analysis
Statistical analyses were performed using Comprehensive Meta-Analysis V3 (Biostat, Inc., USA and the UK). We used levels of inflammatory factors and oxidative stress with the appropriate 95% CI to measure the effect size of the studies, and two-sided (bilateral) p values of 0.05 were considered significant. Mixed effects analysis A random effects model is used to combine studies within each subgroup. A fixed effect model is used to combine subgroups and yield the overall effect. The study-to-study variance (tau-squared) is assumed to be the same for all subgroups—this value is computed within subgroups and then pooled across subgroups. The Egger’s and Begg’s tests were performed to evaluate the probability of the publication bias [18].
Results
Study identification and selection
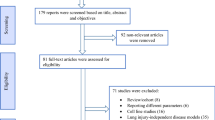
The steps for identifying and selecting literature are given in (Fig. 1). We identified 221 potentially related records in the initial systematic search. After initial screening, 154 duplicated studies were excluded. Titles and abstracts of studies were conducted to examine eligibility criteria and 47 studies were excluded. The full text of the remaining 22 records was evaluated and 15 articles were deleted. The remaining 9 eligible articles were briefly evaluated and included in the study [15, 19,20,21,22,23,24,25,26]. The total sample size was 343 rats. Table 1 aims to summarize the main features of the included studies.
Study characteristics
All nine of the studies included were case-control studies. These articles were published between 2017 to 2022. The studies were published in Europe (4 articles; Şengül [27], Boots [19], Tiboc-Schnell [20], Ileriturk [21]), African (1 article; Oka [22]), East Asia (1 article; Chen [15]), North America (1 article; Farazuddin [23]) and South American (2 articles; Maturu [24], da Silva Araújo [25]) populations. Sample sizes ranged from 6 (Boots [19]) to 50 (Şengül [27], Tiboc-Schnell [20]). Cyclophosphamide [27], rhinovirus [23], oxygen exposure [24], amiodarone [22], bleomycin [19], LPS [20], cigarette smoke [25], cypermethrin [21], and paraquat [15] were used to create models of the rat model of ALI. In all studies, changes in levels of inflammatory agents and oxidative stress in ALI rats were measured and changes were examined after apigenin administration [15, 19,20,21,22,23,24,25, 27]. Inflammatory factors TNF-α [15, 19,20,21, 23], IFN-α and IFN-β [23], IL-1β [15, 19,20,21], IL-6 [20, 21], and IL-17a [19, 23] were measured by ELISA. The activities of superoxide dismutase (SOD) [19, 25, 27], glutathione peroxidase (GSH) [25, 27], MDA [15, 19,20,21, 24, 27], and catalase (CAT) [15, 19, 22, 25] in the lung tissue were measured by ELISA.
Quality assessment
Based on the quality assessment form, we entered them all because the overall quality score of the studies was acceptable.
Quercetin and inflammation
Figure 2 shows the results of the correlation coefficient, lower and upper bound statistics, and Z value and P value significance statistics for each of the inflammatory factors after treatment with quercetin. Quercetin reduces inflammatory factors in the rat model of ALI, which was seen as a direct correlation between quercetin and a decrease in inflammatory factors (correlation: 0.635; 95% CI, 0.560–0.699, P value = 0.000, Z value= 12.648). Quercetin reduces inflammatory factors, including TNF-α, IFN-α, NF-κB, IL-1β, and IL-17a in rat ALI model, which are statistically significant (P< 0.001, Z> 2). But it was not statistically significant for IL-6 (P= 0.995, Z= 0.057).
Funnel plot of the correlation of quercetin and inflammation. Forest plot of the meta-analysis for estimating the correlation and 95% CIs for the inflammatory agents in the intervention group, compared with that in the control group (correlation: 0.635; 95% CI, 0.560–0.699, P value = 0.000, Z value= 12.648).
Quercetin and oxidative stress
Figure 3 shows the results of the correlation coefficient, lower and upper bound statistics, and Z value and P value significance statistics for each of the oxidative stress after treatment with quercetin. Quercetin reduces oxidative stress factors in the rat model of ALI, which was seen as a direct correlation between quercetin and a decrease in oxidative stress factors (correlation: 0.317; 95% CI, 0.189–0.434, P value = 0.00, Z value= 4.7). Six studies (4 sub-studies) were included in the fixed-effect model (I2=0.0%). The pooled correlation coefficient was estimated to be 0.317, which was statistically significant (P<0.0001), as shown in Fig. 3. Quercetin reduces oxidative stress factors, including CAT, SOD, MDA, GSH, and GSTs in rat ALI model, which are statistically significant (P< 0.001, Z> 2).
Funnel plot of the correlation of quercetin and oxidative stress. Forest plot of the meta-analysis for estimating the correlation and 95% CIs for the oxidative stress s in the intervention group, compared with that in the control group (correlation: 0.317; 95% CI, 0.189–0.434, P value = 0.00, Z value= 4.7)
Discussion
ALI/ARDS is characterized by severe alveolar inflammation, alveolar-capillary membrane damage, and pulmonary edema. Cytokines, as well as inflammatory factors, initiate, strengthen, and perpetuate the inflammatory response in acute lung injury. Inflammatory reactions in acute lung injury are caused by the release of pro-inflammatory cytokines from neutrophils that accumulate at the site of injury. Proinflammatory cytokines such as TNF-α, IL-6, and IL-1β are known as early indicators of inflammation and lead to lung damage by inducing acute phase reactions [26, 28]. TNF-α is one of the first inflammatory factors for the inflammatory reaction, which is mainly produced by monocytes/macrophages and induces the production of other cellular factors such as IL-6 by damaging vascular endothelial cells [29]. Quercetin has been noted for its anti-inflammatory and antioxidant properties [30]. The results of a study showed that quercetin pretreatments reduced tissue damage in mice with LPS-induced lung damage and reduced lactate production, which was associated with reduced release of pro-inflammatory cytokines [31]. Idiopathic pulmonary fibrosis (IPF) patients have significantly reduced endogenous antioxidant defenses, as shown by reduced total antioxidant capacity and reduced levels of glutathione and uric acid compared to healthy subjects. This confirms that the redox balance is disturbed in IPF. Quercetin reduces the production of pro-inflammatory cytokines IL-8 and TNFα in IPF patients and healthy subjects; however, the anti-inflammatory effect was more pronounced in IPF patients. In bleomycin-stimulated BEAS-2B cells, quercetin was found to enhance the antioxidant response by increasing Nrf2 activity and reducing the production of pro-inflammatory cytokines in a concentration-dependent manner [32]. Cyclophosphamide [27], rhinovirus [23], oxygen exposure [24], amiodarone [22], bleomycin [19], LPS [20], cigarette smoke [25], cypermethrin [21], and paraquat [15] were used to create models of the rat model of ALI. The results of our analysis showed quercetin reduces inflammatory factors, including TNF-α, IFN-α, NF-κB, IL-1β, and IL-17a in the rat ALI model, which are statistically significant (P< 0.001, Z> 2). But it was not statistically significant for IL-6 (P= 0.995, Z= 0.057). In the study of Oka and et al., they stated that the effect of quercetin on inflammatory factors was not statistically significant; however, they refused to highlight the inflammatory factors studied [22]. It seems that further studies to target pro-inflammatory factors in lung diseases with quercetin are more evident to achieve more accurate results. But what was obtained from the studies was the positive effect of this drug in animal models.
Reactive oxygen species (ROS) are involved in the activation of many proinflammatory cytokines produced in the acute inflammatory response of lung injury. It is known that the balance between oxidant and antioxidant enzymes causes oxidative damage in essential organ systems [33]. MDA is one of the most effective and comprehensive indicators of oxidative stress. MDA levels fluctuate in many diseases, including cardiovascular disease and neurological diseases [34, 35]. Oxidative damage plays an important role in ALI. Some enzymes, such as SOD and GSH-Px, protect cells from damage by oxygen-derived free radicals. SOD is an enzyme that is widely used as a biochemical indicator of pathological conditions associated with oxidative stress [36]. Quercetin reduces oxidative stress factors, including CAT, SOD, MDA, GSH, and GSTs in rat ALI model, which are statistically significant (P< 0.001, Z> 2). Our results support the reduction of oxidative stress factors under the influence of quercetin.
Conclusions
The aim of this study was to highlight the anti-inflammatory and antioxidant activities of quercetin in acute lung injury. We examined changes in cytokines, inflammatory mediators, and antioxidants after administration of quercetin in ALI models. The results of our study showed that quercetin can act as a protective agent by inhibiting inflammatory factors and reducing oxidative stress factors in ALI mouse models. It seems that quercetin alone cannot be a useful therapeutic strategy in mouse models of ALI. However, final conclusions about treatment efficacy should be investigated in prospective randomized controlled trials.
Availability of data and materials
Data will be made available on request.
References
Tian C, Zhang P, Yang J, Zhang Z, Wang H, Guo Y, Liu M (2019) The protective effect of the flavonoid fraction of Abutilon theophrasti Medic. leaves on LPS-induced acute lung injury in mice via the NF-κB and MAPK signalling pathways. Biomed Pharmacother 109:1024–1031
Matthay MA, Zemans RL (2011) The acute respiratory distress syndrome: pathogenesis and treatment. Annual Review of Pathology: Mechanisms of Disease 6:147–163
Suhail MN, Kadhim ZH, Al-Mowali A (2023) Bismuth oxyiodide nanocomposites supported on strontium hydroxyapatite enhance UV-Vis light-driven photocatalytic activity. Al-Kitab J Pure Sci 6(1):14–29. https://doi.org/10.32441/kjps.06.01.p2
Johnson ER, Matthay MA (2010) Acute lung injury: epidemiology, pathogenesis, and treatment. Journal of aerosol medicine and pulmonary drug delivery 23(4):243–252
Joelsson JP, Asbjarnarson A, Sigurdsson S, Kricker J, Valdimarsdottir B, Thorarinsdottir H, Starradottir E, Gudjonsson T, Ingthorsson S, Karason S (2022) Ventilator-induced lung injury results in oxidative stress response and mitochondrial swelling in a mouse model. Laboratory animal research 38(1):1–5
Huang Z, Wang H, Long J, Lu Z, Chun C, Li X (2022) Neutrophil membrane-coated therapeutic liposomes for targeted treatment in acute lung injury. Int J Pharm 624:121971
Yang R, Yang L, Shen X, Cheng W, Zhao B, Ali KH, Qian Z, Ji H (2012) Suppression of NF-κB pathway by crocetin contributes to attenuation of lipopolysaccharide-induced acute lung injury in mice. Eur J Pharmacol 674(2-3):391–396
Di R, Huang MT, Ho CT (2011) Anti-inflammatory activities of mogrosides from Momordica grosvenori in murine macrophages and a murine ear edema model. J Agric Food Chem 59(13):7474–7481
Mahlooji MA, Heshmati A, Kheiripour N, Ghasemi H, Asl SS, Solgi G, Ranjbar A, Hosseini A (2022) Evaluation of protective effects of curcumin and nanocurcumin on aluminium phosphide-induced subacute lung injury in rats: modulation of oxidative stress through SIRT1/FOXO3 signalling pathway. Drug Research 72(02):100–108
Aldoori QA, Albyti AM, Mohammed Ameen AH (2023) Futile care in Kirkuk teaching hospital burn unit. Al-Kitab J Pure Sci 3(2):131–138. https://doi.org/10.32441/kjps.03.02.p11
Kachungwa Lugata J, Ortega AD, Szabó C (2022) The role of methionine supplementation on oxidative stress and antioxidant status of poultry-a review. Agriculture 12(10):1701
Shen N, Wang T, Gan Q, Liu S, Wang L, Jin B (2022) Plant flavonoids: classification, distribution, biosynthesis, and antioxidant activity. Food Chem:132531
Rakha A, Umar N, Rabail R, Butt MS, Kieliszek M, Hassoun A, Aadil RM (2022) Anti-inflammatory and anti-allergic potential of dietary flavonoids: a review. Biomed Pharmacother 156:113945
de Menezes Filho AC, Ventura MV, Alves I, Taques AS, Batista-Ventura HR, de Souza Castro CF, Teixeira MB, Soares FA (2022) Phytochemical prospection, total flavonoids and total phenolic and antioxidant activity of the mushroom extract Scleroderma verrucosum (Bull.) Pers. Brazilian Journal of. Science 1(1):1–4
Chen YB, Zhang YB, Wang YL, Kaur P, Yang BG, Zhu Y, Ye L, Cui YL (2022) A novel inhalable quercetin-alginate nanogel as a promising therapy for acute lung injury. Journal of nanobiotechnology 20(1):1–7
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group* (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151(4):264–269
Hayden JA, Côté P, Bombardier C (2006) Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med 144:427–437
Higgins JP. Cochrane handbook for systematic reviews of interventions. Version 5.1. 0 [updated March 2011]. The Cochrane Collaboration. www. cochrane-handbook. Org, 2011.
Boots AW, Veith C, Albrecht C, Bartholome R, Drittij MJ, Claessen SM, Bast A, Rosenbruch M, Jonkers L, van Schooten FJ, Schins RP (2020) The dietary antioxidant quercetin reduces hallmarks of bleomycin-induced lung fibrogenesis in mice. BMC Pulmonary Medicine 20(1):1–6
Tiboc-Schnell CN, Filip GA, Man SC, Decea N, Moldovan R, Opris R, Sas V, Tabaran F (2020) Quercetin attenuates naso-sinusal inflammation and inflammatory response in lungs and brain on an experimental model of acute rhinosinusitis in rats. J Physiol Pharmacol 71(4)
Ileriturk M, Kandemir O, Kandemir FM (2022) Evaluation of protective effects of quercetin against cypermethrin-induced lung toxicity in rats via oxidative stress, inflammation, apoptosis, autophagy, and endoplasmic reticulum stress pathway. Environ Toxicol 37(11):2639–2650
Maturu P, Wei-Liang Y, Androutsopoulos VP, Jiang W, Wang L, Tsatsakis AM, Couroucli XI (2018) Quercetin attenuates the hyperoxic lung injury in neonatal mice: implications for Bronchopulmonary dysplasia (BPD). Food Chem Toxicol 114:23–33
Farazuddin M, Mishra R, Jing Y, Srivastava V, Comstock AT, Sajjan US Quercetin prevents rhinovirus-induced progression of lung disease in mice with COPD phenotype. PLoS One 13(7):e01996122018
Oka VO, Okon UE, Osim EE (2019) Pulmonary responses following quercetin administration in rats after intratracheal instillation of amiodarone. Nigerian Journal of Physiological Sciences 34(1):63–68
da Silva Araújo NP, de Matos NA, Leticia Antunes Mota S, Farias de Souza AB, Dantas Cangussú S, Alvim C, de Menezes R, Silva BF (2020) Quercetin attenuates acute lung injury caused by cigarette smoke both in vitro and in vivo. COPD: J Chron Obstruct Pulmon Dis 17(2):205–214
Park HK, Kim SJ, Kwon DY, Park JH, Kim YC (2010) Protective effect of quercetin against paraquat-induced lung injury in rats. Life Sci 87:181–186
Şengül E, Gelen V, Gedikli S, Özkanlar S, Gür C, Çelebi F, Çınar A (2017) The protective effect of quercetin on cyclophosphamide-induced lung toxicity in rats. Biomed Pharmacother 92:303–307
Matute-Bello G, Frevert CW, Martin TR (2008) Animal models of acute lung injury. Am J Phys Lung Cell Mol Phys 295(3):L379–L399
Giebelen IA, van Westerloo DJ, LaRosa GJ, de Vos AF, van der Poll T (2007) Local stimulation of α7 cholinergic receptors inhibits LPS-induced TNF-α release in the mouse lung. Shock 28(6):700–703
Li Y, Yao J, Han C, Yang J, Chaudhry MT, Wang S, Liu H, Yin Y (2016) Quercetin, inflammation and immunity. Nutrients 8(3):167
Chen LL, Song C, Zhang Y, Li Y, Zhao YH, Lin FY, Han DD, Dai MH, Li W, Pan PH (2022) Quercetin protects against LPS-induced lung injury in mice via SIRT1-mediated suppression of PKM2 nuclear accumulation. Eur J Pharmacol 936:175352
Al-Hamadiny SQ, salman, R. I., & Al-Rawe, A. M. (2023) Nanocarriers and Beyond: Innovations in Overcoming Barriers for Effective CNS Drug Delivery. Trends Pharm Biotechnol 1(1):34–47. https://doi.org/10.57238/tpb.2023.144275.1003
Cicek M, Unsal V, Doganer A, Demir M (2021) Investigation of oxidant/antioxidant and anti-inflammatory effects of apigenin on apoptosis in sepsis-induced rat lung. J Biochem Mol Toxicol 35(5):e22743
Mehri H, Aslanabadi N, Nourazarian A, Shademan B, Khaki-khatibi F (2021) Evaluation of the serum levels of Mannose binding lectin-2, tenascin-C, and total antioxidant capacity in patients with coronary artery disease. J Clin Lab Anal 35(10):e23967
Ajoolabady A, Sogutlu F, Nikanfar M, Nourazarian A, Laghousi D (2021) Investigation of the potential serum levels of autophagy 5–protein, Apo-lipoprotein B-48, and oxidative stress markers in the early diagnosis of patients with ischemic stroke
Abd Alameer N, Alammar H (2023) Some Trace Elements and Oxidative Stress Status in Patients with Chronic Rheumatoid Arthritis. J Biomed Biochem 2(1):21–27. https://doi.org/10.57238/jbb.2023.6412.1026
Acknowledgements
Not applicable.
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
Sally Salih Jumaa: writing—original draft, and methodology. Sally Salih Jumaa, Abduladheem Turki Jalil: writing—original draft, and data curation. Abbas F. Almulla: data analysis. Abduladheem Turki Jalil: conceptualization and writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ismaeel, G.L., Abdulhadi, M.A., Al-Ameer, L.R. et al. Quercetin for inhibition of inflammatory responses and oxidative stress in lung injury model: a systematic review and meta-analysis. Egypt J Bronchol 17, 71 (2023). https://doi.org/10.1186/s43168-023-00245-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43168-023-00245-y