Abstract
Peripheral muscle dysfunction is a well-recognized complication of COPD associated with exercise (in)tolerance and worse prognosis. The evaluation of peripheral muscle integrity is an attractive marker in COPD patients.
The aim of the study was to identify the correlation between the rectus femoris muscle cross-sectional area (RFCSA) and the severity of airway obstruction in COPD patients using ultrasound.
The study enrolled 24 outpatients diagnosed with stable COPD (post-bronchodilator FEV1/FVC < 0.001*. There was a significant negative correlation between RFCSA and age only in cases with very severe airflow limitation. Among COPD patients, there was a significant negative correlation between average RFCSA and mMRC score (r = − 0.864*, p < 0.001*), but not with FEV1 (r = 0.165, p = 0.442). No significant correlation between average RFCSA and neither CRP nor heart rate in COPD patients (r = − 0.169, p = 0.431, r = − 0.285, p = 0.178, respectively. Among COPD, there was a significant positive correlation between RFCSA and QMVC (r = 0.881*, p < 0.001*). COPD cases showed reduced RFCSA, reduced QMVC, shorter 6MWD distance, and higher mMRC score in comparison to the control group.
Ultrasound measurement of RFCSA is an effort-independent, radiation-free method that relates to strength and should be applied as an assessment tool for COPD patients.
Similar content being viewed by others
Introduction
Chronic obstructive pulmonary disease (COPD) is defined as a common treatable complex condition, characterized by persistent respiratory symptoms due to airflow limitation that is caused by high exposure to noxious particles or gases and impacted by host factors such as abnormal lung development [1]. COPD prevalence and burden are expected to rise in the next decades because of continued exposure to COPD risk factors and global population aging; as people live longer, more individuals will experience the long-term impacts of COPD risk factors [2]. According to World Health Organization (WHO) estimates, COPD will be the third greatest cause of mortality by 2030. The most prevalent symptom of COPD is chronic and persistent dyspnea. In up to 30% of patients, coughing with sputum production is reported. These symptoms can change from day to day and can occur years before airflow limitation develops [3].
Cardiovascular disease, depression, lung cancer, osteoporosis, skeletal muscle dysfunction, anxiety, and metabolic syndrome are common comorbid disorders. COPD also has extra-pulmonary systemic consequences, such as skeletal muscular dysfunction [4]. Patients with COPD, particularly when the disease is severe and during exacerbations, have evidence of systemic inflammation, which is measured either as increased circulating cytokine, chemokine, and acute-phase protein levels or as abnormalities in circulating cells [5]. It is still uncertain whether these systemic markers of inflammation are a “spillover” from inflammation in the peripheral lung or are a parallel abnormality. The systemic inflammation seen in patients with COPD might contribute to its systemic manifestations and could worsen comorbid diseases [6]. In a large population study, systemic inflammation (increased C-reactive protein, fibrinogen, and leukocyte levels) was associated with a 2- to fourfold increased risk of cardiovascular disease, diabetes, lung cancer, and pneumonia, although not with depression [5].
Muscle dysfunction is defined as the inability of a muscle to perform its task, as a result of loss of strength (muscle ability to create maximum force) or endurance (the required ability to sustain submaximal force over time), or both. Dysfunction of the respiratory muscles or the muscles of the limbs (also known as peripheral muscles) is common in respiratory diseases [7]. Peripheral muscle dysfunction/weakness and sarcopenia are well-recognized complications of COPD and are associated with disease severity, exercise (in)tolerance, and worse prognosis [8]. As such, the evaluation of peripheral muscle integrity is an attractive marker of disease severity in patients with COPD. However, routine direct evaluation of this marker is difficult because of the need to rely on imaging techniques such as computed tomography or dual-energy X-ray absorptiometry which are expensive, ionizing, time-consuming, and not readily available at the bedside [9]. In recent years, ultrasonography has emerged as a point-of-care tool that allows access to a rapid, simple, widely available and non-ionizing evaluation of many physiological variables, including peripheral muscle and diaphragm function and integrity. Ultrasound-derived measurement of the rectus femoris cross-sectional area has been shown to be a reliable estimator of muscle strength in patients with COPD [9]. Rectus femoris is predominantly made up of type II fibers [10]. Type I fiber number is lower in COPD patients than in healthy people, and an increase in type IIb fiber occurs consequently and might be a factor in increasing limb muscle endurance and fatigability [11]. In COPD, muscle fiber atrophy is a severe systemic complication resulting in diminished muscular function, limited exercise capacity, and poor health conditions. Muscle fiber cross-sectional area (CSA) decrease is a well-acknowledged sign of muscle wasting and predicts COPD mortality [9]. The complex etiology of limb and respiratory muscle dysfunction in COPD involves many variables and biological processes. Tobacco smoke, hypoxia, hypercapnia, and acidosis, metabolic changes of various types, systemic inflammation, malnutrition, genetics, aging, comorbidities, concomitant therapies, and exacerbations are important etiologic contributing factors to muscle dysfunction in COPD patients through a variety of biological pathways [12]. Age, malnutrition, and systemic corticosteroid therapy may exacerbate COPD-related fast-twitch fiber loss. Another contributing biological process is a decrease in capillary numbers and connections, which might potentially compromise oxygen transport to myofibers [12]. The limb muscles show a higher proportion of type II fibers (fast-twitch contraction, predominantly anaerobic metabolism), while the diaphragm and intercostal muscles seem to show changes in the opposite direction since patients increase the proportion of type I fibers (slow-twitch contraction, aerobic metabolism, and fatigue-resistant) [13].
Ultrasound allows for a quick, easy, widely accessible, and non-ionizing assessment of peripheral muscle and diaphragm function. Ultrasound-derived measurements of the rectus femoris cross-sectional area have been considered a reliable predictor of muscle strength in patients with COPD [11]. Ultrasound measurement of rectus femoris muscle cross-sectional area (RFCSA) is an effort-independent and radiation-free method that relates to strength [9]. The RFCSA (in mm2) is related to voluntary quadriceps strength, and this method has been utilized to characterize peripheral muscle wasting in COPD patients [9].
Aim of the work
The aim of the study was to identify the correlation between rectus femoris muscle cross-sectional area and severity of airway obstruction in COPD patients using ultrasound as an effort independent and radiation-free method.
Subjects
This is a case–control study performed at Alexandria Main University Hospital. The study enrolled 24 male outpatients diagnosed with stable COPD of the 4 degrees of airway obstruction, 6 patients for each category: mild (FEV1 ≥ 80% predicted), moderate (50% ≤ FEV1 < 80% predicted), severe (30% ≤ FEV1 < 50% predicted), and very severe (FEV1 < 30% predicted) [14]. And the control group of 24 healthy male individuals of the same age group was recruited from the general population.
Inclusion criteria were a smoking history ≥ 20 pack years, COPD patients confirmed by the presence of post-bronchodilator FEV1/FVC < 0.7 in patients complaining of chronic cough, sputum production or dyspnea, and/or a history of exposure to risk factors for the disease, CT scan done (to exclude other respiratory diseases), NO COPD exacerbation or hospital admission within the past month, and a BMI of 18.5–40.
Exclusion criteria were age < 40, patients with organ dysfunction, malignancies or comorbidities affecting lower limbs, other respiratory diseases (as interstitial pulmonary fibrosis, bronchiectasis, etc.), abnormal CT findings other than findings related to COPD, and prior history of lobectomy or pneumonectomy.
Methods
The study was approved by the institutional ethics committee, and an informed consent was taken from all patients before participation in the study according to the guidelines of the ethics committee, Alexandria Faculty of Medicine, IRB NO.00012098 (available from www.med.alexu.edu.eg/wp-content/uploads /2012/ 04/ ethics-guide.pdf).
All included individuals were subjected to thorough history taking, smoking history (pack year index), past medical history, comorbidities and past surgical history, and history of previous exacerbation (date, severity, hospital, or ICU admission and use of systemic steroids). A full clinical examination was done on both the general and local chest (inspection, palpation, percussion, and auscultation), and routine laboratory investigations and radiological studies including plain chest X-ray and CT chest without IV contrast.
Assessment of dyspnea
According to the Modified British Medical Research Council (mMRC dyspnea) questionnaire [14], arterial blood gas analysis (ABG), 6-min walk distance: subjects walked as far as they can along a minimally trafficked 30-m corridor for 6 min, then the distance, rest times during the test, oxygen saturation, heart rate and borg scale of dyspnea, and fatigue were written at the end of the test. Spirometry: spirometry was done to confirm the diagnosis of COPD as the presence of post-bronchodilator FEV1/FVC < 0.7 confirms the presence of persistent airflow limitation. A monitor LTD spirometer device was used to perform spirometry. Then, the selected cases were classified into 4 groups (mild, moderate, severe, very severe) according to the severity of airflow limitation. Severity of airflow limitation was classified according to post-bronchodilator FEV1 into mild (FEV1 ≥ 80% predicted), moderate (50% ≤ FEV1 < 80% predicted), severe (30% ≤ FEV1 < 50% predicted), and very severe (FEV1 < 30% predicted) [14].
Measurement of isometric quadriceps femoris muscle maximum voluntary contraction (QMVC) using a strain gauge device to quantify quadriceps muscle strength in each subject [15]. Each subject was asked to sit down on a high chair with his feet not touching the floor, with hip joint flexed at 90°, forearms crossing over anterior chest and knee joint flexed at 60° to maximize quadriceps muscle contraction. The device was attached posteriorly to a fixed object and anteriorly to the ankle joint of the dominant lower limb, and the subject tried to extend his leg straight forward without lateral angulation and without using other muscles of the body, just using the quadriceps femoris muscle of the dominant limb (Figs. 1a and b).
Calculation of QMVC/height2 and correlation of the result to the sex-specific cutoff point (which is 8.3 for males) for QMVC strength normalized to height squared (QMVC/height2) were done [16].
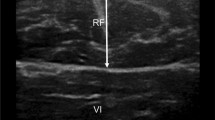
The cross-sectional area of the rectus femoris (RFCSA) was measured using B-mode ultrasonography with an 8 MHz linear transducer array. A sonoscape device with a digital ultrasound system was used, and the transducer was placed perpendicular to the long axis of the thigh (of the dominant lower limb) on its superior aspect, three-fifths of the distance from the anterior superior iliac spine to the superior patellar border. This is the highest point in the thigh where the entire rectus femoris crosssection could be visualized in a single field in all subjects. Imaging was conducted supine with the rested leg supported in passive extension. Excess contact gel was applied to minimize underlying soft tissue distortion. Oblique imaging is minimized by the operator, using visual feedback to obtain the smallest cross-sectional image. Scanning depth was set to where the femur could be discerned for orientation (Fig. 2) [17].
Gentle contraction-relaxation maneuvers are employed to delineate muscle septa prior to image acquisition. RFCSA was calculated by a planimetric technique after the inner echogenic line of the rectus femoris was outlined by a movable cursor on a frozen image. RFCSA was taken as an average of three consecutive measurements within 10% (Fig. 2) [17].
Statistical analysis of the datacm
Data were fed to the computer and analyzed using IBM SPSS software package version 20.0. (Armonk, NY: IBM Corp). Qualitative data were described using numbers and percentages. The Shapiro–Wilk test was used to verify the normality of distribution. Quantitative data were described using range (minimum and maximum), mean, standard deviation, and median. The significance of the obtained results was judged at the 5% level.
The used tests were as follows:
Student t test: For normally distributed quantitative variables, to compare between two studied groups. F test (ANOVA): For normally distributed quantitative variables, to compare between more than two groups, and post hoc test (Tukey) for pairwise comparisons. Pearson coefficient: To correlate between two normally distributed quantitative variables. Mann–Whitney test: For abnormally distributed quantitative variables, to compare between two studied groups. Kruskal–Wallis test: For abnormally distributed quantitative variables, to compare between more than two studied groups, and post hoc (Dunn’s multiple comparisons test) for pairwise comparisons. Spearman coefficient: To correlate between two abnormally distributed quantitative variables.
Results
A total of 48 participants were included in the study, 24 COPD cases and 24 controls. Their mean age was 55 ± 7.93. Both cases and control groups were matched regarding age, sex, and employment. The mean BMI among COPD cases was 24.69 ± 4.48 kg/m2 while in the control group, it was 28.57 ± 5.16 kg/m2 and the difference between the two groups was statistically significant (p = 0.008*). No statistically significant difference was found among the 4 categories of the studied COPD cases (p > 0.05).
The CRP in COPD cases ranged between (1.30–21.0) mg/dl with a mean of 7.87 ± 4.15 mg/dl and a median of 8.0 mg/dl while in the control group, it ranged between (1.0–5.0) mg/dl with mean 2.06 ± 1.11mg/dl and median 2.0 mg/dl and the difference between the two groups was statistically significant (p < 0.001**). The mean FEV1 in cases was 51.92 ± 24.15 versus 88.67 ± 5.54 in controls and that difference between the two groups was statistically significant (p < 0.001**).
Table 1 shows that there was a statistically significant difference between the control group and each of the 4 groups of airflow limitation in COPD patients (p < 0.001*) regarding average RFcsa. Also regarding QMVC, there was a statistically significant difference between the control group and each of the 4° groups of airflow limitation in COPD patients (p < 0.001*).
The 6-min walking distance walked by cases ranged between 140.0 and 330.0 m with a mean of 239.4 ± 55.37 m, while it ranged between 350.0 and 510.0 m and a mean of 441.3 ± 44.07 m in controls and this difference was statistically significant as indicated by t test result (t = 13.98, p < 0.001**). There was no statistically significant difference among cases with different airflow limitations.
The correlation between average RFCSA and different variables in COPD cases is shown in Table 2. There was only a statistically significant negative correlation between average RFCSA and age in cases with very severe airflow limitation (r = − 0.821, p = 0.045*). There was a statistically significant negative correlation between average RFCSA and mMRC score (r = − 0.864*, p < 0.001*), Fig. 3, but there was no significant correlation between average RFCSA and FEV1 (r = 0.165, p = 0.442) among neither total COPD patients nor in each category of airflow limitation. There was no significant correlation between average RFCSA and PYI (r = − 0.353, p = 0.091). Similarly, there was no significant correlation between average RFCSA and CRP (r = − 0.169, p = 0.431). There was no significant correlation in each category of airflow limitation either. There was no significant correlation between average RFCSA and heart rate as well (r = − 0.285, p = 0.178).
Figure 4 showed that among all COPD cases, there was a statistically significant positive correlation between average RFCSA and QMVC (r = 0.881*, p < 0.001*).
Table 2 shows a correlation between the average RFCSA and different parameters among the studied cases.
Table 3 presents the correlation between QMVC and different variables: among COPD cases, there was a statistically significant negative correlation between QMVC and age (r = − 0.520, p = 0.009*), and there was a statistically significant negative correlation between QMVC and mMRC score as well (r = − 0.896*, p < 0.001*). There was no significant correlation between QMVC and FEV1 (r = 0.158, p = 0.460). There was no significant correlation between QMVC and FEV1 in each category of airflow limitation either.
Table 4 shows a correlation between FEV1 and different variables. Among all COPD cases, there was a significant correlation only between FEV1 and PaO2 (p < 0.001).
Regarding total COPD cases, there was a statistically significant negative correlation between heart rate and both FEV1 (r = − 0.689*, p < 0.001*) and PaO2 (r = − 0.600*, p = 0.002*). The heart rate measured after 6MWT ranged between 90.0 and 120.0 beat/minute in cases with a mean of 103.0 ± 9.27 b/m and a median of 100.0 b/m while it ranged between 70.0 and 90.0 b/m with a mean of 81.08 ± 5.41 b/m and a median of 80.0 b/m in controls and that the difference between the two groups was statistically significant (p < 0.001**). In this study, there was a statistically significant difference between the control group and each of the 4° of airflow limitation in COPD patients (p < 0.001**) regarding heart rate following a 6-min walking test.
Discussion
Sarcopenia and peripheral muscular dysfunction are well-known COPD complications [18] and are linked to disease severity, exercise intolerance, and critical prognosis. As a result, the assessment of peripheral muscles in COPD patients is a promising indicator of disease severity [19]. Ultrasonography has evolved as a point-of-care tool in recent years, allowing for a quick, easy, and non-ionizing assessment of peripheral muscle performance and size [20].
This study was conducted to identify the correlation between rectus femoris muscle cross-sectional area and severity of airway obstruction in COPD patients using ultrasound as an effort independent and radiation-free method.
Both cases and control groups were matched regarding age, sex, and employment to avoid confounding factors that might determine quadriceps strength [21]. The mean body mass index (BMI) of the cases was 24.69 ± 4.48 vs 28.57 ± 5.16 in controls according to the findings of this study, and this could be explained by COPD itself as shown in a previous study that followed patients for 16 months found that 15% of COPD patients lost more than 5% of their body weight [22]. In a group of COPD patients admitted to a pulmonary rehabilitation center, the prevalence of weight loss was 49% [23]. Patients with COPD are more likely to develop additional diseases such as heart failure and cancer [24], which also can lead to weight loss. Our studied COPD patients have a significantly higher baseline CRP level (7.87 ± 4.15) than the control group (2.06 ± 1.11) (p< 0.001). Previous studies found that greater levels of CRP, fibrinogen, and leukocytes in COPD patients were linked to an increased risk of exacerbation, but that there was no significant difference between COPD patients and controls [25]. Decreased lung function was linked to higher levels of systemic inflammatory markers in a preceding meta-analysis [25], which could have substantial pathophysiological and therapeutic impacts on people with stable COPD. CRP is an excellent predictor of acute exacerbations and hospitalization [26]. Individuals with greater levels of CRP assessed at baseline had a higher risk of early mortality, according to a previous meta-analysis [27].
Our study also showed that there was neither significant correlation between CRP and QMVC, nor between CRP and RFCSA. Also, there was no significant correlation between CRP and FEV1. In contrast to our results, a previous work found that quadriceps muscle thickness (the distance between two fascias of a muscle determined by M mode-US and significantly correlated to the cross-sectional area) [28] was reduced more in the CRP-positive patients than in the CRP-negative patients and a positive CRP on admission were both significantly correlated with decreased quadriceps muscle thickness after acute stroke [29]. Another study reported a positive correlation between systemic inflammation biomarkers and quadriceps muscle atrophy in COPD patients with reduced RFMCSAand increased systemic inflammation biomarkers such as erythrocyte sedimentation rate (ESR), CRP, and procalcitonin (PCT) [30]. Waterer et al. as well have reported a negative association between systemic inflammation markers and quadriceps strength in stable COPD patients [31]. The results of the abovementioned studies [31] do not match our results. This may be due to the small sample size enrolled in our study or may be attributed to the fact that we only tested one inflammatory marker.
On comparing COPD patients to the control group regarding RFMCSA, COPD patients had significantly lower values of the mean RFMCSA (802.54 ± 178.07 mm2) than the control group (1163.17 ± 41.89 mm2) (p< 0.001) which was consistent with the difference reported in prior studies that found that the mean RF(CSA) was reduced in patients with COPD by 25% of the mean value in healthy subjects [17, 32]. Also, quadriceps maximum voluntary contraction (QMVC) was lower in COPD cases (14.03 ± 3.15kg) than in the control group (23.38 ± 3.64kg) (p < 0.001). Similarly, Shrikrishna et al. reported that both RFCSA and the quadriceps strength QMVC were lowered in all GOLD stages [33].
Regarding 6MWT COPD, patients walked a shorter distance (239.38 ± 55.37m) than healthy subjects (441.25 ± 44.07m) and this difference was statistically significant (p < 0.001). This matches the findings of a previous study that compared the distance walked by COPD patients to the distance walked by the non-COPD group [34]. The decline of 6MWT distance in COPD patients could be attributed to the sedentary lifestyle because it is a strong characteristic in this group, as suggested by Hernandes et al. [35], who found that COPD patients spend the majority of their time sitting or lying down to avoid the symptom of dyspnea, as well as walking with less intensity of movement. Other authors claim that inactivity is a precursor to systemic alterations rather than a result of illness severity [36].
Regarding RFCSA, in our study, RFCSA was correlated with QMVC and 6MWT distance showing a strong positive significant correlation in both cases and control groups. In contrast, a previous study found no significant relationships between rectus femoris thickness (which positively correlates with RFCSA) and the 6MWT [37]. On the other hand we found no significant correlation between RFMCSAand FEV1. In contrast, a previous work found that forced vital capacity (FVC) and percent-predicted forced expiratory volume in 1 s (FEV1%pred) were positively correlated with RF thickness [38]. In our study, there was a statistically significant negative correlation between the average RFCSA and mMRC score (p < 0.001*). These results are consistent with the results of a previous study that showed that mMRC scores were negatively correlated with RFCSA thickness [38].
Regarding QMVC, in the present study, there was a strong significant negative correlation between QMVC and mMRC and between QMVC and age. A positive correlation was found between QMVC with both RFCSA and 6MWT distance. There was no significant correlation between FEV1 and QMVC. These results are inconsistent with the results of previous studies that found a significant positive link between FEV1 values and bilateral quadriceps strength (P < 0.05) in a research by Shah et al. [39] So more studies with a larger number of patients are needed in this field to correlate FEV1 with the previous parameters.
There is a lot of debate in the literature about the link between muscle function and COPD severity. Some studies show that muscle dysfunction can occur even in the early stages of COPD. A large number of patients with COPD in GOLD stages 1 and 2 reported quadriceps weakness, according to research by Seymour et al. These percentages increased to 38% in GOLD stage 4 patients and 43% in those with an MRC score of 4 or 5 [21]. This demonstrates that quadriceps weakness worsens as the disease progresses.
In our study, we found no significant correlation between FEV1 and mMRC dyspnea scale. But FEV1 correlated positively with PaO2. This agrees with the findings of a previous study that showed that PaO2positively correlated with FEV1 at rest and during exercise [40].
There were several studies that have linked 6MWD to spirometry in COPD [41, 42]. In our study, neither FEV1 nor FEV1/FVC correlated with 6MWD distance in cases in the current study. These findings contradicted research conducted by Agrawal et al. [43], Ameri [44], and Asmita et al. [45], who reported a positive correlation between 6MWT and spirometry parameters (FEV1 and FVC). Our findings may be due to the small sample size enrolled as well as some cases in our study did not adopt the sedentary lifestyle like others. One of them was a farmer with severe airflow obstruction but adopted heavy daily duties, the 2nd was a marathon runner with severe airflow obstruction, and the 3rd was a cook with moderate airflow obstruction but undergoing pulmonary rehabilitation. The three patients walked a 6MWD distance greater than other patients with similar degree of airflow limitation. Also, the three patients showed greater RFMCSA and greater QMVC in comparison to other patients with similar airflow limitation severity.
In our study, a negative correlation was observed between heart rate and PaO2 and between heart rate and FEV1. Similar results were reported by Bhattacherjee [46] who found that arterial oxygen saturation decreased with increased heart rate in COPD. Our study showed that the heart rate measured after 6MWT in COPD cases was higher than that in the control group and that the difference between the two groups was statistically significant (p< 0.001**). These results were consistent with a previous study, showing that the mean pulse rate was significantly higher during rest and exercise in COPD patients compared with the controls [47]. That could be explained by several hemodynamic factors, such as hypoxia and decreased stroke volume in COPD. Pulmonary dysfunction in COPD is associated with a decrease in left ventricular size and stroke volume. When stroke volume is decreased, cardiac output can be maintained by an increase in heart rate. Moreover, COPD is additionally known to be associated with autonomic dysfunction resulting in decreased parasympathetic and increased sympathetic activity [48].
Finally, it is necessary to point out that the current study had some limitations. For example, a larger sample size should be included to confirm our results and to correlate FEV1 and RFMcsa, whether ultrasound assessment of the rectus femoris could predict exercise tolerance in healthy subjects is unclear and whether the findings can be applied to patients with acute exacerbations of COPD, and those undergoing pulmonary rehabilitation remain to be determined.
In conclusion, our study indicated that all COPD patients have lower limb sarcopenia denoted by reduced RFCSA and QMVC regardless of the degree of airflow limitation. COPD patients have a higher level of C-reactive protein than the control group which may be associated with the systemic effects of COPD. Sarcopenia in COPD patients correlates positively with reduced 6MWT distance and higher mMRC dyspnea score. COPD patients maintaining a degree of daily physical activities have a greater RFCSA with better muscle strength, longer 6MWT distance, and lower m MRC dyspnea score. Ultrasound measurement of rectus femoris muscle cross-sectional area (RFCSA) is an effort-independent and radiation-free method of measuring quadriceps muscle cross-sectional area in patients with COPD that relates to strength and should be applied as an assessment tool of COPD patients.
We recommend that further research with a larger sample size is needed to confirm the correlation between the degree of airflow limitation and sarcopenia. Ultrasound measurement of the rectus femoris muscle cross-sectional area (RFCSA) should be applied as an assessment tool for COPD patients. All COPD patients should be involved in a training system to maintain a good degree of physical daily activities including a pulmonary rehabilitation program. Serial ultrasound assessment of (RFCSA) before and after the rehabilitation program should be applied as a follow-up tool in COPD patients.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 6MWT:
-
6-Minute walk test
- ABG:
-
Arterial blood gas analysis
- COPD:
-
Chronic obstructive pulmonary disease
- CRP:
-
C-reactive protein
- CSA:
-
Cross-sectional area
- FEV1:
-
Forced expiratory volume 1
- FVC:
-
Forced vital capacity
- HF:
-
Heart failure
- mMRC:
-
Modified Medical Research Council
- MT:
-
Muscle thickness
- PH:
-
Pulmonary hypertension
- QMVC:
-
Quadriceps femoris muscle maximum voluntary contraction
- RF:
-
Rectus femoris
- RFCSA:
-
Rectus femoris muscle cross-sectional area
- US:
-
Ultrasound
References
Singh D, Agusti A, Anzueto A, Barnes PJ, Bourbeau J, Celli BR et al (2019) Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Int J Chron Obstruct Pulmon Dis 53(5):1900164
Soriano JB, Polverino F, Cosio BG (2018) What is early COPD and why is it important? Eur Respir J 52(6):1801448
Mattos WL, Signori LG, Borges FK, Bergamin JA, Machado V (2009) Accuracy of clinical examination findings in the diagnosis of COPD. J Bras Pneumol 35(5):404–408
Oliveira AS, Munhá J, Bugalho A, Guimarães M, Reis G, Marques A (2018) Identification and assessment of COPD exacerbations. Pulmonology 24(1):42–47
Kalhan R, Tran B, Colangelo L, Rosenberg S, Liu K, Thyagarajan B et al (2010) Correction: systemic inflammation in young adults is associated with abnormal lung function in middle age. PloS one 5:11431
Baines KJ, Backer V, Gibson PG, Powel H, Porsbjerg CM (2015) Impaired lung function is associated with systemic inflammation and macrophage activation. Eur Respir J 45(2):557–559
Rossi A, Aisanov Z, Avdeev S, Di Maria G, Donner CF, Izquierdo JL et al (2015) Mechanisms, assessment and therapeutic implications of lung hyperinflation in COPD. Respirat Med 109(7):785–802
Gredic M, Blanco I, Kovacs G, Helyes Z, Ferdinandy P, Olschewski H et al (2021) Pulmonary hypertension in chronic obstructive pulmonary disease. Br J Pharmacol 178(1):132–151
Maynard-Paquette A-C, Poirier C, Chartrand-Lefebvre C, Dubé B-P (2020) Ultrasound evaluation of the quadriceps muscle contractile index in patients with stable chronic obstructive pulmonary disease: relationships with clinical symptoms, disease severity and diaphragm contractility. Int J Chron Obstruct Pulmon Dis 15:79–88
Parry SM, El-Ansary D, Cartwright MS, Sarwal A, Berney S, Koopman R et al (2015) Ultrasonography in the intensive care setting can be used to detect changes in the quality and quantity of muscle and is related to muscle strength and function. J Crit Care 30(5):1151
Ramachandran P, Devaraj U, Patrick B, Saxena D, Venkatnarayan K, Louis V et al (2020) Ultrasonographic assessment of skeletal muscle mass and diaphragm function in patients with chronic obstructive pulmonary disease: a case-control study. Lung India 37(3):220–226
Gea J, Pascual S, Casadevall C, Orozco-Levi M, Barreiro E (2015) Muscle dysfunction in chronic obstructive pulmonary disease: update on causes and biological findings. J Thorac Dis 7(10):E418–E438
Gosker HR, Zeegers MP, Wouters EF, Schols AM (2007) Muscle fibre type shifting in the vastus lateralis of patients with COPD is associated with disease severity: a systematic review and meta-analysis. Thorax 62(11):944–949
Global Initiative for Chronic Obstructive Lung Disease (GICOLD) (2021) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. GICOLD, USA
Kharbanda S, Ramakrishna A, Krishnan S (2015) Prevalence of quadriceps muscle weakness in patients with COPD and its association with disease severity. Int J Chron Obstruct Pulmon Dis 10:1727–1735
Canavan JL, Maddocks M, Nolan CM, Jones SE, Kon SS, Clark AL et al (2015) Functionally relevant cut point for isometric quadriceps muscle strength in chronic respiratory disease. Am J Respir Crit Care Med 192(3):395–397
Seymour JM, Ward K, Sidhu PS, Puthucheary Z, Steier J, Jolley CJ et al (2009) Ultrasound measurement of rectus femoris cross-sectional area and the relationship with quadriceps strength in COPD. Thorax 64(5):418–423
Remels AH, Gosker HR, Langen RC, Schols AM (2013) The mechanisms of cachexia underlying muscle dysfunction in COPD. J Appl Physiol 1985 114(9):1253–62
Yin HL, Yin SQ, Lin QY, Xu Y, Xu HW, Liu T (2017) Prevalence of comorbidities in chronic obstructive pulmonary disease patients: a meta-analysis. Medicine (Baltimore) 96(19):e6836
Dubé BP, Dres M, Mayaux J, Demiri S, Similowski T, Demoule A (2017) Ultrasound evaluation of diaphragm function in mechanically ventilated patients: comparison to phrenic stimulation and prognostic implications. Thorax 72(9):811–818
Seymour JM, Spruit MA, Hopkinson NS, Natanek SA, Man WD, Jackson A et al (2010) The prevalence of quadriceps weakness in COPD and the relationship with disease severity. Eur Respir J 36(1):81–88
Wu DD, Song J, Bartel S, Krauss-Etschmann S, Rots MG, Hylkema MN (2018) The potential for targeted rewriting of epigenetic marks in COPD as a new therapeutic approach. Pharmacol Ther 182:1–14
Schols AM, Soeters PB, Mostert R, Saris WH, Wouters EF (1991) Energy balance in chronic obstructive pulmonary disease. Am Rev Respir Dis 143(6):1248–1252
Negewo NA, McDonald VM, Gibson PG (2015) Comorbidity in chronic obstructive pulmonary disease. Respir Investig 53(6):249–258
Gan WQ, Man SF, Senthilselvan A, Sin DD (2004) Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 59(7):574–580
Moy ML, Teylan M, Danilack VA, Gagnon DR, Garshick E (2014) An index of daily step count and systemic inflammation predicts clinical outcomes in chronic obstructive pulmonary disease. Ann Am Thorac Soc 11(2):149–157
Fermont JM, Masconi KL, Jensen MT, Ferrari R, Di Lorenzo VAP, Marott JM et al (2019) Biomarkers and clinical outcomes in COPD: a systematic review and meta-analysis. Thorax 74(5):439
Zhang YB, Zuo HY, Tian DH, Ouyang XH, Wang XA (2018) Correlation between peripheral skeletal muscle functions and the stable phase of COPD in older patients. Eur Rev Med Pharmacol Sci 22(16):5317–5326
Nozoe M, Kanai M, Kubo H, Kitamura Y, Yamamoto M, Furuichi A et al (2016) Changes in quadriceps muscle thickness, disease severity, nutritional status, and C-reactive protein after acute stroke. J Stroke Cerebrovasc Dis 25(10):2470–2474
Ahmadi M, Nazemiyeh M, Ghaebi M, Mikaeili H, Tarzamani M, Valizadeh H et al (2019) Quadriceps muscle atrophy and inflammatory markers in COPD patients. Res Square 16:1–14
Yende S, Waterer GW, Tolley EA, Newman AB, Bauer DC, Taaffe DR et al (2006) Inflammatory markers are associated with ventilatory limitation and muscle dysfunction in obstructive lung disease in well functioning elderly subjects. Thorax 61(1):10–16
Ramírez-Fuentes C, Mínguez-Blasco P, Ostiz F, Sánchez-Rodríguez D, Messaggi-Sartor M, Macías R et al (2019) Ultrasound assessment of rectus femoris muscle in rehabilitation patients with chronic obstructive pulmonary disease screened for sarcopenia: correlation of muscle size with quadriceps strength and fat-free mass. Eur Geriatr Med 10(1):89–97
Shrikrishna D, Patel M, Tanner RJ, Seymour JM, Connolly BA, Puthucheary ZA et al (2012) Quadriceps wasting and physical inactivity in patients with COPD. Eur Respir J 40(5):1115–1122
Gomes L, Santos D, Santos D, Souza G, Cruz V, Lima Y (2020) Muscle strength, functionality and distance traveled in patients with chronic obstructive pulmonary disease. J Physiother Res 10(2):195–202
Hernandes NA, Teixeira Dde C, Probst VS, Brunetto AF, Ramos EM, Pitta F (2009) Profile of the level of physical activity in the daily lives of patients with COPD in Brazil. J Bras Pneumol 35(10):949–956
Evanirso da Silva A, Thais Miranda P, Lopes IBDV, Castro FDMR, Coelho CC, Cunha Filho IT (2010) Correlation between body composition and respiratory muscle strength, endurance, and exercise capacity in patients with chronic obstrutive pulmonary disease. Fisioter 17(1):58–62
Cruz-Montecinos C, Guajardo-Rojas C, Montt E, Contreras-Briceño F, Torres-Castro R, Díaz O et al (2016) Sonographic measurement of the quadriceps muscle in patients with chronic obstructive pulmonary disease: functional and clinical implications. J Ultrasound Med 35(11):2405–2412
Deng M, Liang C, Yin Y, Shu J, Zhou X, Wang Q et al (2021) Ultrasound assessment of the rectus femoris in patients with chronic obstructive pulmonary disease predicts poor exercise tolerance: an exploratory study. BMC Pulm Med 21(1):304
Shah S, Darekar B, Salvi S, Kowale A (2019) Quadriceps strength in patients with chronic obstructive pulmonary disease. Lung India 36(5):417–421
RahimiFard M, Zarezadeh N (2004) Relationship between FEV1 and PaO2, PaCO2 in patients with chronic bronchitis. TANAFFOS (Respiration) 3(2(spring))):41–6
Gayan-Ramirez G, Decramer M (2013) Mechanisms of striated muscle dysfunction during acute exacerbations of COPD (1985). J Appl Physiol 114(9):1291–9
Mantilla CB, Sieck GC (2013) Neuromotor control in chronic obstructive pulmonary disease (1985). J Appl Physiol 114(9):1246–52
Agrawal MB, Awad NT (2015) Correlation between six minute walk test and spirometry in chronic pulmonary disease. J Clin Diagn Res 9(8):0Oc1–4
Al Ameri HF (2006) Six minute walk test in respiratory diseases: a university hospital experience. Ann Thorac Med 1(1):16–19
Asmita M, Indira K (2011) Correlation of six minute walk test with spirometry and DLCO in chronic respiratory diseases: a tertiary care hospital experience. Pulmon 13(2):55–58
Bhattacharyya P, Bhattacherjee PD, Saha D, Das SK, Paul M, Samanta S (2015) The change in pulse rate and saturation pattern in normal COPD and DPLD patients. PulmoFace 15:9–13
Roca M, Mitu F, Roca IC, Mihăescu T (2013) Heart rate variations in chronic obstructive pulmonary disease. Rev Med Chir Soc Med Nat Iasi 117(3):616–622
Jensen MT, Marott JL, Lange P, Vestbo J, Schnohr P, Nielsen OW et al (2013) Resting heart rate is a predictor of mortality in COPD. Eur Respir J 42(2):341–349
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Contributions
Corresponding author: Dr. Rania Ahmed Sweed directing the practical part of the research, presenting the results, and writing the manuscript. Prof. Dr. Yehya Mohamed Khalil decided the main idea of the research and the methodology and revised the whole manuscript. Mahmoud Abdelrahman Abdelgawad Ali performed the practical part, statistics, and data collection. Prof. Dr. Anwar Ahmed Elganady guided the practical part and revised the manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All subjects enrolled in the study signed an informed consent before participation. The study was accepted by the local ethical committee of Alexandria Faculty of Medicine (available from www.med.alexu.edu.eg/wp-content/uploads/2012/ 04/ ethics-guide.pdf).
Consent for publication
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sweed, R.A., Khalil, Y.M., Elganady, A.A. et al. Correlation between rectus femoris muscle cross-sectional area and the severity of airflow limitation in COPD patients. Egypt J Bronchol 17, 53 (2023). https://doi.org/10.1186/s43168-023-00229-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43168-023-00229-y