Abstract
Background
Due to limited capacity, health care systems worldwide have been put in challenging situations since the emergence of COVID-19. To prioritize patients who need hospital admission, a better understanding of the clinical predictors of disease severity is required. In the current study, we investigated the predictors of mortality and severity of illness in COVID-19 from a single center in Cairo, Egypt.
Methods
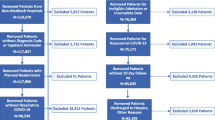
This retrospective cohort study included 175 patients hospitalized with COVID-19 pneumonia and had positive real-time polymerase chain reaction (RT-PCR) results for SARS-CoV-2 from 1 May 2020 to 1 December 2020. Severe COVID-19 was defined as requiring high-flow oxygen (flow rate of more than 8 L/min or use of high flow oxygen cannula), noninvasive ventilation, or invasive mechanical ventilation at any time point during the hospitalization. We used univariate and multivariate regression analyses to examine the differences in patient demographics and clinical and laboratory data collected during the first 24 h of hospitalization related to severe disease or death in all 175 patients.
Results
Sixty-seven (38.3%) of the study subjects had a severe or critical disease. Elevated d-dimer, leukocytosis, and elevated CRP were found to be independent predictors of severe disease. In-hospital mortality occurred in 34 (19.4%) of the cases. Elevated TLC, urea, the use of invasive mechanical ventilation, and the presence of respiratory bacterial co-infection were found to be independently associated with mortality.
Conclusion
Clinical and laboratory data of COVID-19 patients at their hospital admission may aid clinicians in the early identification and triage of high-risk patients.
Similar content being viewed by others
Introduction
The SARS-CoV-2 pandemic began in Wuhan, China, in December 2019, and continues to pose a challenge to the healthcare system worldwide [1]. According to the WHO’s most recent update, there have been over 255 million cases worldwide, with over 5 million deaths reported.
Since the emergence of the SARS-CoV-2 in late 2019 and the WHO’s official declaration of a worldwide pandemic in March 2020, there have been tremendous efforts to identify prognosticators that clinicians utilize to assess the risk at the early stage of the disease, thus aiding in tailoring management strategies as well as facilitating decision-making and improving outcomes for COVID-19 patients via increasing the cure rate and decreasing the case fatality rate.
Despite ongoing research, the clinical characteristics and outcomes of COVID-19 patients as a population have yet to be thoroughly studied in Egypt.
We conducted a retrospective cohort study of COVID-19 patients hospitalized in an Egyptian tertiary hospital to determine the risk factors associated with a severe course and worse outcomes, including mortality, to assist healthcare systems in triaging patients who present to the hospital.
Methods
Study subjects and settings
We performed a retrospective cohort study of 175 patients hospitalized with COVID-19 from 1 May 2020 to 1 December 2020 in the quarantine section of Misr International Hospital, Cairo, Egypt.
Patients who were hospitalized with radiological evidence of COVID-19 pneumonia and had positive real-time polymerase chain reaction (RT-PCR) results for SARS-CoV-2 were included in the study. Patients with missing data and negative (RT-PCR) results for SARS-CoV-2 were excluded from the study.
Data collection
Demographic and laboratory data were extracted from the medical records. The following clinical data were collected: baseline comorbidities, presenting symptoms, vital signs, microbiology results, imaging results, medical treatments, supplemental oxygen (O2), noninvasive and invasive forms of ventilation, respiratory co-infections, complications, and hospitalization outcome (death or discharge).
Ethics approval
The institutional review board of the Ministry of Health, Cairo, Egypt (No: 3- 2021/19) approved the study. The data was collected from the hospital records, and informed consent was not required as the data was anonymized and no personal identifiers were collected.
Patient categorization
We defined severe COVID-19 as requiring high-flow O2 (flow rate of more than 8 L/min or use of high-flow oxygen cannula), noninvasive ventilation, or invasive mechanical ventilation at any time point during the hospitalization. Among all 175 patients, we investigated the differences in the demographic, clinical, and laboratory data collected during the first 24 h of hospitalization regarding severe disease or death at any time during hospitalization.
Statistical methods
The data was collected and tabulated for statistical analysis using Minitab 17.1.0.0 for Windows (Minitab Inc., 2013, PA, USA). Continuous data were presented as mean and standard deviation (SD), whereas categorical data were presented as number and percentage (%). The normality of data was examined using the Shapiro-Wilk test. The association between severity and mortality was performed using the chi-square test, independent t-test, or Mann-Whitney test. Moreover, the prognostic utility of TLC, d-dimmer, urea, and CRP was done using the receiver operating characteristic curve (ROC curve); the area under the curve AUC above 0.6 is considered acceptable for test capability. Multiple logistic regression analysis models with the step forward selection model technique were used for finding the predictors for COVID-19 severity and mortality. All tests were two-sided; P-value was considered significant if < 0.05.
Results
A total of 175 patients were included in the study, with an average age of 59 years, and the majority were males (77.7%). 54.9% of the patients were comorbid; DM and HTN were the most frequently encountered comorbidities, as reported in one-third of the cases. Among cases with COVID-19 pneumonia, 38.3% had a severe and critical disease. In-hospital mortality occurred in 19.4% of the cases. More than half of the cases (62.9%) needed supplemental oxygen. Standard oxygen supply (low-flow nasal cannula, standard oxygen mask) was the most frequently used (52%). High-flow nasal cannula (HFNC) and noninvasive and invasive mechanical ventilation were used in 18.3%, 8.6%, and 20.6% of patients, respectively (Table 1).
Factors associated with the severity of COVID-19 pneumonia
Sixty-seven subjects (38.2%) were categorized as severe COVID pneumonia among the studied group. Comorbidities (diabetes, hypertension, and renal impairment) were significantly associated with the severe COVID pneumonia group versus the non-severe group (Table 2).
Patients with severe COVID pneumonia had significantly higher levels of leukocytosis, lymphopenia, elevated liver enzymes, urea, d-dimer, ferritin, and CRP compared to the non-severe group (Table 2).
In multivariate regression, only leukocytosis (adjusted odds ratio [aOR] 1.14, 95% confidence interval [CI] 1.1, 1.2), elevated d-dimer (adjusted odds ratio [aOR] 1, 95% confidence interval [CI] 1.0001, 1.0004), and elevated CRP (adjusted odds ratio [aOR] 1.01, 95% confidence interval [CI] 1.0015, 1.0103) were found to be independent predictors of severe disease (Fig. 1; Table 3).
Factors associated with in-hospital mortality
The mortality rate in our cohort was 19.4%, which was significantly associated with old age and comorbidity, especially renal disease, P = 0.002, 0.02, and < 0.001, respectively. Moreover, patients with severe disease and those who needed NIV and IMV for oxygen supply had a significantly higher mortality rate, P < 0.01 for all. Elevated levels of TLC, AST, ALT, urea, creatine, ferritin, d-dimer, and CRP, as well as lower levels of lymphocyte, platelet, and hemoglobin, were found to be significantly associated with mortality (Table 2).
Multivariate analysis was performed to identify the independent predictors for mortality. Elevated TLC (adjusted odds ratio [aOR] 1.17, 95% confidence interval [CI] 1.005, 1.363), urea (adjusted odds ratio [aOR] 0.99, 95% confidence interval [CI] 0.974, 0.997), and the use of invasive mechanical ventilation (adjusted odds ratio [aOR] 1597.5, 95% confidence interval [CI] 60.112, 42,458.58), and presence of respiratory bacterial respiratory co-infection adjusted (odds ratio [aOR] 71.2, 95% confidence interval [CI] 1.5, 3381.9) were found to be independently associated with mortality (Fig. 2; Tables 4 and 5).
Discussion
In this study, we provided a relatively comprehensive estimate for the early predicting factors affecting the COVID-19 disease state. We report on 175 patients with confirmed SARS-CoV-2 infection; 67 patients (38.29%) had severe and critical COVID-19 pneumonia, with the mortality rate in our cohort being 19.4%.
Multivariate regression analysis revealed that elevated CRP, d-dimer, and TLC were independent predictors of COVID-19 disease severity, whereas elevated TLC, urea, presence of respiratory bacterial co-infection, and the need for invasive mechanical ventilation were independent predictors of COVID-19 mortality.
CRP is a non-specific acute phase reactant induced by IL-6 in the liver. Elevated CRP levels are directly correlated with the level of inflammation and disease severity. A meta-analysis conducted by Malik et al. revealed that higher CRP levels are associated with disease severity and the formation of lung lesions in the early stages of COVID-19 [2]. Elshazli et al. found CRP to be a valid biomarker of death from COVID-19 when examining a range of hematological and immunological markers [3]. Elevated CRP may not be attributable to COVID-19 alone and may represent concomitant pathology such as secondary bacterial pneumonia [4].
Regarding the d-dimer levels, they were significantly higher in patients with severe COVID-19, whereas mortality was significantly associated with elevated d-dimer levels. Since the emergence of COVID-19, several data have reported that elevated d-dimer is more prevalent in deceased patients, and increasing odds of in-hospital death were associated with elevated d-dimer levels [5,6,7]. This finding is attributed to severe virus infection that developed into sepsis and induced coagulation dysfunction. Also, the increase of d-dimer may be an indirect manifestation of inflammatory reaction, as inflammatory cytokines could cause the imbalance of coagulation and fibrinolysis in the alveoli, which may activate the fibrinolysis system and then increase the level of d-dimer [8].
However, evidence regarding the causal mechanisms and whether the associations are specific effects of SARS-CoV-2 infection or are consequences of systemic inflammatory response is still lacking.
With respect to TLC, the present study revealed that higher TLC was associated with severe COVID-19 infection and higher mortality. Yuan et al. reported similar findings in severe COVID-19 cases [9]. Zhao et al. evaluated 52 COVID-19 patients with increased leukocyte at admission and compared them with COVID-19 patients with non-increased leukocyte count, and it was found that the patients with increased leukocyte count were more likely to develop critical illness (P < 0.01) and had a higher rate of death (P < 0.01) [10].
This finding could be explained due to high levels of serum IL-6, which induce an inflammatory response leading to neutrophil migration, recruitment, and activation. Phagocytosis, the release of granular contents, and the production of cytokines are significant functions of activated neutrophils, suggesting a protective immune response against the virus. However, excessive neutrophils can cause cytokine storm and tissue damage, leading to severe COVID-19 pneumonia and death [10].
Overall, data obtained from the multivariate analysis revealed that a higher mortality rate is expected in patients suffering from renal impairment, which could be attributed to lowered immunity and the underlying immune responses in patients with comorbid conditions [11, 12]. As evidenced by the reduction in nitric oxide in diseases such as hypertension, diabetes, and kidney dysfunction, endothelial dysfunction is also thought to be a key factor [13,14,15].
Henry and Lippi found over 3-fold higher risk of developing severe COVID-19 in patients suffering from chronic renal disease [16].
Tian et al. and Cheng et al. reported that the presence of chronic renal disease is a significant predictor of mortality in COVID-19 patients [6, 17]. Therefore, paying more attention to the presence of renal impairment at the time of admission and implementing an effective intervening strategy handling it as early as possible might help reduce mortality in COVID-19 patients suffering from underlying kidney disease.
It is well known that viral respiratory infections predispose patients to bacterial infections and that co-infections have a worse outcome than that of either infection on its own [18]. Different studies have addressed the presence of documented respiratory co-infection in COVID-19 pneumonia patients with varying degrees of prevalence, which may be due to different diagnostic methods applied to diagnose respiratory co-infections [19,20,21].
In the current study, 10.9% of our patients have documented respiratory co-infection in whom mortality was significantly increased, which is consistent with a recent meta-analysis in which investigators have shown a positive association between co-infection and increased risk of death among patients with the SARS-CoV-2 infection [22].
The use of invasive mechanical ventilation was also found to be an independent predictor of mortality in our study, which is compatible with Manal et al., who found that the need to ventilate a COVID-19 patient mechanically is likely associated with higher mortality [23].
There was a significant range of mortality rates reported for COVID-19 patients receiving mechanical ventilation ranging from 9.4 to 97% [24]. Studies from China reported mortality in COVID-19 patients receiving IMV reaching as high as 97% [7].
The wide variation in mortality rates among patients receiving invasive mechanical ventilation (IMV) could be multifactorial in different countries. Studies from different areas with varying degrees of expertise and hospital resources may explain the variable reported outcome. In addition, during the surge of the pandemic, different institutional protocols were applied according to the available resources, like in some Italian regions, giving priority to patients who are more likely to survive, as was recommended by the Italian Society of Anesthesia, Analgesia, Resuscitation and Intensive Care [25, 26].
Studies from China early in the pandemic had reported higher mortality rates than US, UK, and Spanish studies that recruited patients later in the pandemic [27,28,29,30,31].
In other words, decreasing mortality rates as the pandemic progresses have been observed in studies including ICU COVID-19 patients and in which the proportion of patients receiving IMV ranged from 0 to 100%. This finding could explain why clinicians gained more knowledge and expertise as time passed, and medical treatments became more available compared to early in the pandemic [24].
Our study has some limitations. First, due to the retrospective study design, not all laboratory tests were done in all patients, including lactate dehydrogenase and IL-6. Therefore, we could not investigate their role in predicting the outcome in COVID-19 patients. Second, the study was performed in a limited hospital setting and included a relatively small sample size with disproportion in the different study groups. In order to validate our findings, a large multicenter prospective observational study would be preferable.
In conclusion, according to this study, COVID-19 infection was more aggressive in patients presenting with elevated TLC, d-dimer, and CRP levels. Mortality was found to be higher in patients with renal impairment and documented respiratory co-infection as well as in patients with elevated TLC and mechanically ventilated patients.
Hence, pretreatment clinical and laboratory data from COVID-19 patients at hospital admission can assist clinicians in identifying high-risk patients early and providing special and prompt care for those in need.
Availability of data and materials
Data are available.
Abbreviations
- COVID-19:
-
Coronavirus disease of 2019
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- CRP:
-
C-reactive protein
- TLC:
-
Total leukocytic count
- WHO:
-
World Health Organization
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- ROC:
-
Receiver operating characteristic curve
- AUC:
-
Area under the curve
- DM:
-
Diabetes mellitus
- HTN:
-
Hypertension
- HFNC:
-
High-flow nasal cannula
- NIV:
-
Noninvasive ventilation
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine transaminase
- IL-6:
-
Interleukin-6
- IMV:
-
Invasive mechanical ventilation
- ICU:
-
Intensive care unit
References
Pandita A, Gillani FS, Shi Y, Hardesty A, McCarthy M, Aridi J et al (2021) Predictors of severity and mortality among patients hospitalized with COVID-19 in Rhode Island. PLoS One 16(6):e0252411
Malik P, Patel U, Mehta D, Patel N, Kelkar R, Akrmah M et al (2021) Biomarkers and outcomes of COVID-19 hospitalisations: systematic review and meta-analysis. BMJ Evid Based Med 26(3):107–108
Elshazli RM, Toraih EA, Elgaml A, El-Mowafy M, El-Mesery M, Amin M et al (2020) Diagnostic and prognostic value of hematological and immunological markers in COVID-19 infection: a meta-analysis of 6320 patients. PLoS One 15:e0238160
Stringer D, Braude P, Myint PK, Evans L, Collins JT, Verduri A et al (2021) The role of C-reactive protein as a prognostic marker in COVID-19. Int J Epidemiol 50(2):420–429
Yao Y, Cao J, Wang Q, Shi Q, Liu K, Luo Z et al (2020) d-dimer as a biomarker for disease severity and mortality in COVID-19 patients: a case control study. J Intensive Care 2020:49
Tian W, Jiang W, Yao J, Nicholson CJ, Li RH, Sigurslid HH et al (2020) Predictors of mortality in hospitalized COVID-19 patients: a systematic review and meta-analysis. J Med Virol 92(10):1875–1883
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z et al (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395:1054–1062
Yu H, Qin C, Chen M, Wang W, Tian D (2020) d-dimer level is associated with the severity of COVID-19. Thromb Res 195:219–225
Yuan J, Zou R, Zeng L, Kou S, Lan J, Li X et al (2020) The correlation between viral clearance and biochemical outcomes of 94 COVID-19 infected discharged patients. Inflamm Res 69(6):599-606
Zhao K, Li R, Wu X, Zhao Y, Wang T, Zheng Z et al (2020) Clinical features in 52 patients with COVID-19 who have increased leukocyte count: a retrospective analysis. Eur J Clin Microbiol Infect Dis 39(12):2279–2287
Vasdev S, Stuckless J, Richardson V (2011) Role of the immune system in hypertension: modulation by dietary antioxidants. Int J Angiol 20(04):189–212
Ferlita S, Yegiazaryan A, Noori N, Lal G, Nguyen T, To K et al (2019) Type 2 diabetes mellitus and altered immune system leading to susceptibility to pathogens, especially Mycobacterium tuberculosis. J Clin Med 8(12):2219
Hermann M, Flammer A, Luscher TF (2006) Nitric oxide in hypertension. J Clin Hypertens 8(12 Suppl 4):17–29
Honing ML, Morrison PJ, Banga JD, Stroes ES, Rabelink TJ (1998) Nitric oxide availability in diabetes mellitus. Diabetes Metab Rev 14(3):241–249
Marrazzo F, Spina S, Zadek F, Lama T, Xu C, Larson G et al (2019) Protocol of a randomised controlled trial in cardiac surgical patients with endothelial dysfunction aimed to prevent postoperative acute kidney injury by administering nitric oxide gas. BMJ Open 9(7):e026848
Henry BM, Lippi G (2020) Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int Urol Nephrol 52(6):1193–1194
Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L et al (2020) Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 97(5):829–838
Arnold FW, Fuqua JL (2020) Viral respiratory infections: a cause of community acquired pneumonia or a predisposing factor? Curr Opin Pulm Med 26(3):208–214
Zhang G, Hu C, Luo L, Fang F, Chen Y, Li J et al (2020) Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol 127:104364
Kim D, Quinn J, Pinsky B, Shah NH, Brown I (2020) Rates of co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA 323:2085–2086
Contou D, Claudinon A, Pajot O, Micaëlo M, Flandre PL, Dubert M et al (2020) Bacterial and viral co-infections in patients with severe SARS-CoV-2 pneumonia admitted to a French ICU. Ann Intensive Care 10:119
Musuuza JS, Watson L, Parmasad V, Putman-Buehler N, Christensen L, Safdar N (2021) Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: a systematic review and meta-analysis. PLoS One 16(5):e0251170
Manal M, Mohammed AA, Abdelilah E, Mohammed M, Khaoula J, Houssam B et al (2021) Predictive factors of mortality related to COVID-19: a retrospective cohort study of 600 cases in the intensive care unit of the university hospital of Oujda. Ann Med Surg 69:102711
Vafea MT, Zhang R, Kalligeros M, Mylona EK, Shehadeh F, Mylonakis E (2021) Mortality in mechanically ventilated patients with COVID-19: a systematic review. Expert Rev Med Devices. https://doi.org/10.1080/17434440.2021.1915764
Feinstein MM, Niforatos JD, Hyun I, Cunningham TV, Reynolds A, Brodie D et al (2020) Considerations for ventilator triage during the COVID-19 pandemic. Lancet Respir Med 8(6):e53
Riccioni L, Bertolini G, Giannini A, Vergano M, Gristina G, Livigni S et al (2020) Clinical ethics recommendations for the allocation of intensive care treatments, in exceptional, resource-limited circumstances. Recenti Prog Med 111(4):207–211
Wang Y, Lu X, Li Y, Chen H, Chen T, Su N et al (2020) Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med 201(11):1430–1434
Xu J, Yang X, Yang L, Zou X, Wang Y, Wu Y et al (2020) Clinical course and predictors of 60-day mortality in 239 critically ill patients with COVID-19: a multicenter retrospective study from Wuhan, China. Crit Care 24(1):394
Ferrando C, Suarez-Sipmann F, Mellado-Artigas R, Hernandez M, Gea A, Arruti E et al (2020) Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. Intensive Care Med 46(12):2200–2211
Gupta S, Hayek SS, Wang W, Chan L, Mathews K, Melamed M et al (2020) Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med 180(11):1436–1447
Khalil K, Agbontaen K, McNally D, Love A, Mandalia S, Banya W et al (2020) Clinical characteristics and 28-day mortality of medical patients admitted with COVID-19 to a central London teaching hospital. J Infect 81(3):e85–e89
Acknowledgements
We thank the ICU and ward residents for recruiting the patients. We thank the nursing staff, lab doctors, and technicians for being helpful and cooperative.
Funding
No fund was paid by any organization.
Author information
Authors and Affiliations
Contributions
Conceptualization: AH, MH, and SM. Methodology: MH and MS. Formal analysis: AA. Data curation and software: AA. Validation: AH. Investigation: ER. Writing—original draft preparation: AH, MH, MS, SM, and SI. Writing—review and editing: AH, MH, and MS. All authors: approval of the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the institutional review board of the Ministry of Health, Cairo, Egypt (No: 3- 2021/19).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Assal, H.H., Abdel-hamid, H.M., Magdy, S. et al. Predictors of severity and mortality in COVID-19 patients. Egypt J Bronchol 16, 18 (2022). https://doi.org/10.1186/s43168-022-00122-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43168-022-00122-0