Abstract
Background
Assessment of disease activity in rheumatoid arthritis (RA) is crucial to optimize the response to treatment and prevent radiographic progression. DAS28 is the most commonly used disease activity index, which incorporates either erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP). Several studies showed that using ESR and/or CRP in assessing disease activity falls short of detecting a significant portion of patients with active disease. Calprotectin (CLP) is an interesting protein that was found to be a promising biomarker of disease activity in RA patients’ sera when CRP is normal. This study aimed to measure serum CLP level in 50 RA patients with different grades of disease activity and compare its level with age- and sex-matched control.
Results
In this case–control study, the mean serum CLP level was significantly lower in RA patients (25.94 ± 25.87 ng/ml) compared to the control group values (53.02 ± 77.93 ng/ml), p < 0.001. The measured serum CLP in RA patients was lower than its level in other published studies. No significant difference was found between patients with different disease activity grades in the serum CLP level (H = 4.28, p = 0.23). Serum samples were collected and stored from RA patients over 4 months and from the control subjects over 1.5 months and were stored at –80 °C until analysis was performed according to the manufacturer’s instruction.
Conclusion
The low level of serum CLP among RA patients is most probably due to proteolysis related to storage conditions. Pre-analytic factors like the type of blood sample, whether the sample is fresh or frozen, and duration of storage exert an effect on serum CLP level when measured by enzyme-linked immunosorbent assay.
Similar content being viewed by others
Background
Early diagnosis of rheumatoid arthritis (RA) is the first step to optimal therapeutic success. Identification of prognostic factors has proven to be of comparable importance in guiding treatment decisions and preventing debilitating outcomes [1, 2]. The three main risk factors concerning the prognosis of RA are high disease activity, rheumatoid factor and/or anti-citrullinated protein-peptide antibodies positivity, and the early occurrence of structural damage [2, 3].
Patients with high disease activity for a longer time are at increased risk of mortality, and effective control of disease activity decreases the death rate [4]. Furthermore, fluctuations in disease activity were found to be directly related to changes in radiologic progression. The response to treatment in RA patients after 1 year could be predicted by the disease activity of the first 3 months [5, 6]. Therefore, an accurate assessment of disease activity is necessary to optimize response to treatment and prevent radiographic progression. The most commonly used disease activity index is the Disease Activity Score of 28 joints (DAS28)—a modified version of the disease activity score that uses a count of 28 swollen and tender joints, with a score ranging from 0 to 9.4, and incorporates either erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP) [7,8,9,10].
Even though it has been proven that DAS28 can be used to objectively evaluate a patient’s response to treatment and that it is as valid and reliable as more comprehensive joint counts in trials and clinics [7,8,9], several studies showed that the use of ESR and or CRP in assessing disease activity falls short of detecting a significant portion of patients with active disease [10,11,12]. The need to develop a more reliable and accurate panel of biomarkers of disease activity in RA is mandatory. Calprotectin (CLP), an interesting protein that was first discovered in 1980 [13], was found to be a potential biomarker of disease activity in RA patients [14,15,16]. In one study, serum CLP was shown to be a convenient biomarker when CRP is normal or hard to interpret in patients with medications that suppress interleukin-6 [14]. It was also suggested that since serum CLP is mainly released through passive mechanisms such as necrotic cells and the formation of neutrophil extracellular trap, it could be used as a useful marker of neutrophil activation in comparison to CRP, which is secreted by hepatocytes in response to inflammatory cytokines [14].
Serum CLP was put under the spotlight to investigate its ability to fill this gap. A study conducted in 2018 concluded that serum CLP correctly distinguishes RA patients with active disease despite normal or low CRP [17]. Furthermore, another study aiming to explore the association of CLP with clinical and ultrasound-established disease activity in RA found that serum CLP could be considered a superior predictor of ultrasound-detected synovitis than CRP [18]. Foel et al. compared the role of serum CLP to other laboratory investigations, including ESR and CRP, as a predictive marker of the stability of remission when methotrexate (MTX) was withdrawn in juvenile idiopathic arthritis patients. They stated that a normal serum level of CLP in patients in a clinically inactive state was proven helpful in deciding on safe MTX withdrawal [19], and higher CLP level was related to the risk of relapse after MTX discontinuation [20, 21]. An article published in 2021 found that CLP was significantly correlated not only with disease activity, functional status, and ultrasonographic findings but also with radiographic damage that was assessed by the modified Larsen score [22]. The role of CLP in reflecting disease activity more accurately than the traditional acute-phase reactants puts CLP as a good research biomarker for disease activity in RA.
Aim of the study
This study was designed to measure serum CLP level in 50 RA patients with different grades of disease activity and compare its level with 45 age- and sex-matched control.
Methods
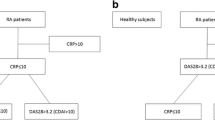
This case–control study included 50 RA patients > 18 years who fulfilled the 2010 ACR/EULAR classification criteria [18], and 45 age- and sex-matched healthy persons constituted the control group. The inclusion criteria were patients > 18 years diagnosed with RA using ACR/EULAR classification criteria. The exclusion criteria were other rheumatic disorders and other diseases known to cause elevation in serum CLP like diabetes mellitus, inflammatory bowel diseases, lung fibrosis, or glomerulonephritis. Smoking, infections, and cancers were additional exclusion criteria. Patients and control groups were recruited over 6 months. Patients were recruited first over 4 months with most (> 90%) being recruited in the first 2 months. Controls were recruited over the last 1.5 months.
All participants were told about the nature of the research, and informed consent was taken from all of them. The study was approved by the local ethics committee.
All the recruited patients had a detailed history taking, anthropometric measurement, and musculoskeletal examination. Assessment of disease activity was done by the disease activity scores: the DAS28-CRP and DAS-ESR [23]. The selection to use two disease activity scores is based on the fact that DAS28-CRP has been shown to give an overall slightly lower score than the ESR. And some claimed that DAS28-CRP underestimates disease activity [24].
According to the DAS28 score, disease activity in RA patients was graded as follows [25]: high disease activity (> 5.1), moderate disease activity (3.2–5.1), low disease activity (2.6–3.2), and in remission (< 2.6).
The laboratory investigations of both groups included the CRP, ESR, and serum CLP—using enzyme-linked immunosorbent assay (ELISA). For the CLP measurement, venous samples were collected and left to clot for 30 min, and then serum was separated by centrifugation. The serum was stored at − 80 °C until analysis was performed. All reagents and samples were prepared and stored according to the manufacturer’s instructions (Human Calprotectin ELISA Kit—Bioassay Technology Laboratory EH). Samples from the patients were collected and stored for a period of approximately 4 to 6 months, while the control group samples were stored for less than 1 and a half months before analysis was performed.
Statistical analysis
Data were entered into the computer and analyzed using IBM SPSS software package version 20.0. Quantitative data were expressed using mean and standard deviation. The distributions of quantitative variables were tested for normality using the Kolmogorov–Smirnov test, Shapiro–Wilk test, and D'Agstino test. Also, histogram and QQ plot were used for the vision test. For normal data distribution, parametric tests were used. For abnormally distributed data, nonparametric tests were used. Significance test results are quoted as two-tailed probabilities. The significance of the obtained results was specified at the 5% level.
Results
The mean age in the RA group was 39.84 ± 8.04 years [23–55 years]. The mean age in the control group was 36.96 ± 9.4 years [25–54 years]. No significant difference between RA and control groups was found as regards age (t = 1.646, p = 0.103). All participants were females.
The mean disease duration was 8 ± 5.68 years (ranging from 0.50 to 25.0 years). The duration of morning stiffness ranged from 0.0 to 4.0 h. The tender joint count ranged from 0 to 28, and swollen joints ranged from 0 to 21.
Most RA patients—37 patients (74%), were on methotrexate (MTX) with a recommended dose ranging from 12.5 to 25 mg per week. Nonsteroidal anti-inflammatory drugs were used in only 4% of patients. Twenty-four patients (48%) were on steroids. Only 7 (14%) patients were on biological disease-modifying antirheumatic drugs (bDMARDs) in the form of etanercept or golimumab.
The mean ESR level was 34.12 ± 14.77 mm/h; only 4% (2 patients) had an ESR level less than or equal to 10 mm/h, while the remaining 96% (48 patients) had an elevated ESR level above 10 mm/h. The mean CRP level was 8.15 ± 9.81 mg/l. Forty-two percent (21 patients) had a CRP level of less than or equal to 3 mg/l, while 58.0% (29 patients) had a high CRP level of more than 3 mg/l. The DAS28-CRP ranged from 1.51 to 6.98 with a mean of 4.45 ± 1.39. The grading of the disease activity using DAS28-CRP and DAS28-ESR is shown in Fig. 1.
Serum CLP level in the studied RA patients ranged from 6.07 to 142.5 ng/ml with a mean of 25.94 ± 25.87. Its level in the control group ranged from 8.62 to 382.4 ng/ml with a mean of 53.02 ± 77.93. Serum CLP was significantly lower in patients compared to the control group (U = 689.50, p < 0.001). Moreover, the measured serum CLP in RA patients in the current study was lower than the measured values detected by previous studies (Table 1).
The mean value of serum CLP did not differ significantly among patients with different grades of disease activity measured by either DAS28-CRP or DAS28-ESR (H = 4.28, 4.83 p = 0.23, 0.185, respectively) (Table 2).
RA patients who received MTX had a significantly lower serum CLP level (22.95 ± 21.58) than MTX naive patients (34.46 ± 35.06) (U = 147.50, p = 0.040).
Discussion
The association of CLP with rheumatic diseases has obtained great attention in recent years, and many studies have studied the role of CLP, specifically in RA [14, 16, 31,32,33]. They found the mean level of serum CLP in RA patients to be significantly higher compared to healthy controls [14, 16, 33]. As opposed to these studies, the current work showed a significantly lower serum CLP level in RA patients compared to healthy controls (25.94 ng/ml vs. 53.02 ng/ml, respectively).
The mean value of serum CLP in our control group was 53.02 ± 77.93. The serum CLP levels measured in different studies in healthy persons showed a wide range with a mean as low as 34.90 ± 4.85 ng/ml and as high as 847.45 [26, 34], which makes our control results located in this range and could be accepted.
The unexpectedly low level of serum CLP of 25.94 ng/ml in the RA group requires vigorous research to find a reasonable explanation. The average serum CLP level of RA patients reported by Aghdashi et al. was 347.12 ± 203.60 ng/ml in their flare-up phase and 188.04 ± 23.58 ng/ml in the phase of remission [35]. Moreover, Gernert et al. demonstrated a serum CLP level of 4155.5 ng/ml in active RA patients as compared to 1040.0 ng/ml in non-active RA [36]. Many other studies showed a different range of serum CLP levels in RA patients, all of which presented a higher serum CLP level in RA patients than in healthy control [27,28,29, 37].
Interestingly, two studies carried out on patients with hypertension and axial spondyloarthropathy (AxSpa) showed significantly lower level of serum CLP in patients than in healthy controls [30, 38]. The first study was led by Bayrakci et al. to investigate serum CLP levels in newly diagnosed hypertensive patients [30]. The second study was conducted by Genre et al. to explore the role of serum CLP in AxSpa [38]. Both studies showed significantly lower CLP level in the patient group compared to the control group. The mean serum CLP levels were 242.8 ng/ml in the controls versus 112.6 ng/ml in the hypertensive patient group and 102.3 ± 31.2 ng/mLl in the control group vs. 91.4 ± 26.1 in AxSpa [30, 38].
Bayrakci et al. explained their finding by the negative correlation between serum CLP level and serum uric acid and calcium levels [30]. Indeed, RA is considered to be a rare cause of hypercalcemia and hyperuricemia [39, 40]. This is an unlikely explanation for low serum CLP levels in our studied patients.
Genre et al. explained why serum CLP was significantly lower in AxSpa patients compared to healthy individuals. They attributed the decreased serum CLP to the local accumulation of the CLP molecules in the synovial fluid [38, 41]. Indeed, De Rycke et al. demonstrated that CLP levels in the synovium of autoimmune arthritis patients are 20 times higher than that in the serum [42]. Similarly, Levitova et al. suggested that low serum CLP levels mirror local-synovial inflammation [41]. It has also been suggested that neutrophils and monocytes migrate from the circulation to the inflamed sites [41, 43]. No synovial samples have been taken from the RA patients in the current study; hence, this explanation is possible but remains unproven in RA.
The impact of MTX treatment on serum CLP level was an additional important factor while trying to explain the low level of serum CLP. Thirty-seven of our patients (74%) were on MTX. Those patients had a significantly lower level of serum CLP (22.95 ng/ml) than patients who were not on MTX (34.46 ng/ml). This suggests that MTX might affect the serum CLP level. A similar finding was reported by Jonsson et al., who found a significant decrease in median CLP after 12 months of treatment with MTX in RA patients [15]. Similarly, Andres et al. showed a reduction in the level of serum CLP in patients with recent-onset RA after three months of conventional drugs [44]. Moreover, Nielsen et al. found that RA patients who are well-established on MTX show a significantly lower level of CLP compared to patients new to MTX [45]. Patro et al. stated that the decrease in serum CLP levels could be due to a particular action of MTX on the myeloid cells— he main producers of CLP [46], and is not necessarily related to improvements in the disease activity. Given that the current study is cross-sectional and pre- and posttreatment levels of CLP were not compared, relating low serum CLP levels to MTX administration cannot be confirmed. More importantly, in different studies that assess CLP levels after MTX administration, the drop in CLP was significant compared to pre-treatment levels but not to levels below the control group values. Accordingly, significantly low serum CLP level in the patient group compared to control group values in the current study is unlikely explained by MTX administration. The effect of bDMARDs on serum CLP could not be studied here, as only seven patients received bDMARD.
In this study, no significant difference in serum CLP levels across the different disease activity scores was found. According to Bayrakci et al., other causes than inflammation could cause low CLP level, which could be misleading in reflecting the underlying inflammation [30].
Possible explanations for the low level of serum CLP in our patients can be driven by Bayrakci et al. suggestion that serum CLP might have been broken down at the site of inflammation (in vivo) or during storage (in vitro) [30]. Similarly, Hoskin et al. reported that vigorous proteolysis of oxidized CLP is bound to occur both at the site of inflammation or during the storage of clinical samples, and this should be considered when interpreting CLP level as it will underestimate the true inflammation level [47].
As a crucial part of the innate immune response, when neutrophils are stimulated, they produce reactive oxygen species such as superoxide, hydrogen peroxide, and hypochlorous acid. Hypochlorous acid is thought to be the main oxidant responsible for the in vivo oxidation of CLP [47, 48]. Even though these neutrophil-derived oxidants act as a first-line defense mechanism against pathogens, excessive or misplaced neutrophil activation causes large amounts of nonselective oxidative and proteolytic stress that renders CLP more vulnerable to proteolysis [49,50,51,52,53].
Interestingly, in 2022, Teagan et al. reported the degradation of CLP and the liberation of 6 specific peptides from oxidized CLP in the airways of children diagnosed with cystic fibrosis [54]. These interesting findings led the authors to steer away from the idea of using intact CLP as a biomarker of inflammation and rather suggested the use of the resulting peptides as a diagnostic biomarker that reflects neutrophil numbers [54]. Indeed, they concluded that the underestimated level of CLP is not restricted to their cystic fibrosis patients only but can also occur in any disease where neutrophils release CLP and huge amounts of oxidants [47, 54]. The key role of neutrophils and the excessive production of oxidants, especially hypochlorous acid, in RA have long been established [55,56,57].
In vivo proteolysis cannot be confirmed in the current work. Moreover, if the low CLP level in the current work is related to in vivo proteolysis, it remains unexplained why this proteolysis was found in our patients and not in other studies that found significantly high level of CLP that correlated with disease activity at clinical (by DAS 28) and structural (by ultrasound) levels in RA.
The explanation for the unexpectedly low level of CLP in our patients is mostly related to the in vitro proteolysis of CLP or the handling of samples. The patient group samples were stored for a period of approximately 4 to 6 months for most patients, while the control group samples were stored for less than 1 and a half months. Even though Bayrakci et al. did not mention the duration of storage of their samples, they highlighted that the samples were stored for some time before analysis which might have contributed to the low serum CLP level in their hypertensive patients [30]. Moreover, one of their recommendations was to review the methods used in the measurement of CLP, specifically for stored samples [30].
Furthermore, when collecting samples, Bettner et al. did not only match serum samples of RA patients and control samples by age, sex, and race but also the duration of sample storage was matched [58]. This suggests that the duration of sample storage may affect the serum CLP levels.
None of the studies exploring the role of serum CLP in RA mentioned the duration of storage; only the temperature at which the samples were stored was stated (− 80 °C), like our study [17, 18, 59]. More studies are required to investigate the stability of CLP under − 80 °C for 6 months—the storage conditions recommended by our ELISA Kit—Bioassay Technology Laboratory Human CLP ELISA Kit [60]. In support of our suggestion regarding the impact of storage duration on serum CLP stability, a recently published study in 2022 assessed serum canine CLP and found that it is unstable at 16 weeks of storage at − 80 °C and concluded a safe storage duration of 8 weeks [61].
Another important pre-analytical factor that influences the level of CLP is the presence of anticoagulants [62, 63]. Ethylenediamine tetraacetic acid (EDTA) in the plasma samples ensures the stability of CLP, which is not the case in the serum samples [62]. EDTA’s calcium-binding method of anticoagulation inhibits the release of CLP from monocytes and thus provides a stabilizing effect on CLP levels in plasma samples [64, 65]. Since coagulation is necessary for obtaining serum, the stabilizing effect of EDTA was not present in serum samples, and therefore, CLP levels measured in serum can provide misleading results [64].
Pedersen et al. proved that CLP level in plasma is not affected by either time or temperature of storage [66]. They also concluded that neutrophil activation resulting from clotting or centrifugation (which occurs in handling serum samples) must be avoided to prevent CLP in vitro proteolysis [66]. When comparing CLP collected from serum with CLP from plasma of RA patients, Nordal et al. found that CLP measured in plasma correlated more strongly with clinical disease activity markers than serum did [64]. Interestingly, no difference between EDTA plasma and serum in fresh, nonfrozen samples was found. The authors hence concluded that any difference that occurs happens after sampling and during processing [67].
Limitation of the study
The main limitation of the work is being a single-center study. Further, multicenter studies are required to detect different pre-analytic factors affecting CLP level and to explore the stability of CLP under various storage conditions.
Conclusion
This study showed a significantly lower level of serum CLP in RA patients than in healthy controls. Serum CLP is an unstable molecule that is vulnerable to proteolysis during sample preparation and storage for the ELISA test. CLP should preferably be measured in EDTA plasma samples and not in serum-frozen samples. Attention to the pre-analytic factors is mandatory when dealing with CLP. Also, an accurate understanding of the causes of CLP degradation should be put into consideration.
Availability of data and materials
All data and materials are presented in the main manuscript.
Abbreviations
- CHNS:
-
China Health and Nutrition Survey
- ACR/EULAR:
-
American College of Rheumatology European League Against Rheumatism
- AxSpa:
-
Axial spondyloarthropathy
- bDMARDs:
-
Biological disease-modifying antirheumatic drugs
- CLP:
-
Calprotectin
- CRP:
-
C-reactive protein
- DAS 28:
-
Disease Activity Score with 28 joint count
- DAS 28-CRP:
-
Disease Activity Score incorporates CRP
- DAS 28-ESR:
-
Disease Activity Score incorporates ESR
- EDTA:
-
Ethylenediamine tetraacetic acid
- ELISA:
-
Enzyme-linked immunosorbent assay
- MTX:
-
Methotrexate
- RA:
-
Rheumatoid arthritis
References
Albrecht K, Zink A (2017) Poor prognostic factors guiding treatment decisions in rheumatoid arthritis patients: a review of data from randomized clinical trials and cohort studies. Arthritis Res Ther 19(1):68
Smolen JS, Aletaha D, McInnes IB (2016) Rheumatoid arthritis. Lancet 388(10055):2023–2038
Smolen JS, Landewé RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A et al (2020) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 79(6):685–699
Listing J, Kekow J, Manger B, Burmester GR, Pattloch D, Zink A et al (2015) Mortality in rheumatoid arthritis: the impact of disease activity, treatment with glucocorticoids, TNFα inhibitors and rituximab. Ann Rheum Dis 74(2):415–421
Welsing PM, Landewé RB, van Riel PL, Boers M, van Gestel AM, van der Linden S et al (2004) The relationship between disease activity and radiologic progression in patients with rheumatoid arthritis: a longitudinal analysis. Arthritis Rheum 50(7):2082–2093
Aletaha D, Funovits J, Keystone EC, Smolen JS (2007) Disease activity early in the course of treatment predicts response to therapy after one year in rheumatoid arthritis patients. Arthritis Rheum 56(10):3226–3235
Wells G, Becker JC, Teng J, Dougados M, Schiff M, Smolen J et al (2009) Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis 68(6):954–960
Prevoo ML, van Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL (1995) Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 38(1):44–8
Fuchs HA, Brooks RH, Callahan LF, Pincus T (1989) A simplified twenty-eight-joint quantitative articular index in rheumatoid arthritis. Arthritis Rheum 32(5):531–7
Orr CK, Najm A, Young F, McGarry T, Biniecka M, Fearon U et al (2018) The utility and limitations of CRP, ESR and DAS28-CRP in appraising disease activity in rheumatoid arthritis. Front Med (Lausanne) 5:185
Sokka T, Pincus T (2009) Erythrocyte sedimentation rate, C-reactive protein, or rheumatoid factor are normal at presentation in 35%-45% of patients with rheumatoid arthritis seen between 1980 and 2004: analyses from Finland and the United States. J Rheumatol 36(7):1387–1390
Wolfe F, Michaud K (1994) The clinical and research significance of the erythrocyte sedimentation rate. J Rheumatol 21(7):1227–1237
Fagerhol M, Dale I, Anderson T (2009) Release and quantitation of a leucocyte derived protein (L1). Scand J Haematol 24:393–398
Jarlborg M, Courvoisier DS, Lamacchia C, Martinez Prat L, Mahler M, Bentow C et al (2020) Serum calprotectin: a promising biomarker in rheumatoid arthritis and axial spondyloarthritis. Arthritis Res Ther 22(1):105
Jonsson MK, Sundlisæter NP, Nordal HH, Hammer HB, Aga AB, Olsen IC et al (2017) Calprotectin as a marker of inflammation in patients with early rheumatoid arthritis. Ann Rheum Dis 76(12):2031–2037
Wang Y, Liang Y (2019) Clinical significance of serum calprotectin level for the disease activity in active rheumatoid arthritis with normal C-reactive protein. Int J Clin Exp Pathol 12(3):1009–1014
Hurnakova J, Hulejova H, Zavada J, Komarc M, Cerezo LA, Mann H et al (2018) Serum calprotectin may reflect inflammatory activity in patients with active rheumatoid arthritis despite normal to low C-reactive protein. Clin Rheumatol 37(8):2055–2062
Hurnakova J, Zavada J, Hanova P, Hulejova H, Klein M, Mann H et al (2015) Serum calprotectin (S100A8/9): an independent predictor of ultrasound synovitis in patients with rheumatoid arthritis. Arthritis Res Ther 17(1):252
Foell D, Frosch M, Schulze zur Wiesch A, Vogl T, Sorg C, Roth J (2004) Methotrexate treatment in juvenile idiopathic arthritis: when is the right time to stop? Ann Rheum Dis 63(2):206–8
Foell D, Wulffraat N, Wedderburn LR, Wittkowski H, Frosch M, Gerss J et al (2010) Methotrexate withdrawal at 6 vs 12 months in juvenile idiopathic arthritis in remission: a randomized clinical trial. JAMA 303(13):1266–1273
Abildtrup M, Kingsley GH, Scott DL (2015) Calprotectin as a biomarker for rheumatoid arthritis: a systematic review. J Rheumatol 42(5):760–770
Radwan A, Allam A, Radwan A (2021) The relationship of serum calprotectin with disease activity, functional status, ultrasonographic findings and radiological damage in rheumatoid arthritis patients. Egypt Rheumatol. 43:147–151
Inoue E, Yamanaka H, Hara M, Tomatsu T, Kamatani N (2007) Comparison of Disease Activity Score (DAS)28- erythrocyte sedimentation rate and DAS28- C-reactive protein threshold values. Ann Rheum Dis 66(3):407–409
Greenmyer JR, Stacy JM, Sahmoun AE, Beal JR, Diri E (2020) DAS28-CRP cutoffs for high disease activity and remission are lower than DAS28-ESR in rheumatoid arthritis. ACR Open Rheumatol 2(9):507–511
Rakieh C, Nam JL, Hunt L, Hensor EM, Das S, Bissell LA et al (2015) Predicting the development of clinical arthritis in anti-CCP positive individuals with non-specific musculoskeletal symptoms: a prospective observational cohort study. Ann Rheum Dis 74(9):1659–1666
Bartáková E, Štefan M, Stráníková A, Pospíšilová L, Arientová S, Beran O et al (2019) Calprotectin and calgranulin C serum levels in bacterial sepsis. Diagn Microbiol Infect Dis 93(3):219–226
Torgutalp M, Yayla ME, Eroglu DS, Dincer ABK, Yurteri EU, Okatan IE et al (2021) Serum calprotectin is indicating clinical and ultrasonographic disease activity in rheumatoid arthritis, even with normal C-reactive protein levels. Mediterr J Rheumatol 32(1):56–65
Tweehuysen L, den Broeder N, van Herwaarden N, Joosten LAB, van Lent PL, Vogl T et al (2018) Predictive value of serum calprotectin (S100A8/A9) for clinical response after starting or tapering anti-TNF treatment in patients with rheumatoid arthritis. RMD Open 4(1):e000654
El-Kady RAE-H, Fathy A, Othman T, Hafez E (2021) Could potentially calprotectin be a promising biomarker to oracle biologic therapy response in rheumatoid arthritis? Egypt Rheumatol Rehabil 48(1):46
Bayrakci N, Ozkan G, Kara SP, Yilmaz A, Guzel S (2022) Serum calprotectin level as an inflammatory marker in newly diagnosed hypertensive patients. Int J Hypertens 2022:6912502
Carrión M, Juarranz Y, Martínez C, González-Álvaro I, Pablos JL, Gutiérrez-Cañas I et al (2013) IL-22/IL-22R1 axis and S100A8/A9 alarmins in human osteoarthritic and rheumatoid arthritis synovial fibroblasts. Rheumatology (Oxford) 52(12):2177–86
Baillet A, Trocmé C, Berthier S, Arlotto M, Grange L, Chenau J et al (2010) Synovial fluid proteomic fingerprint: S100A8, S100A9 and S100A12 proteins discriminate rheumatoid arthritis from other inflammatory joint diseases. Rheumatology 49(4):671–682
Shumnalieva RVT, Kachakova D et al (2017) 03.04 Serum and synovial concentration of calprotectin in rheumatoid arthritis patients. Ann Rheum Dis 76:A31
Jung SY, Park YB, Ha YJ, Lee KH, Lee SK (2010) Serum calprotectin as a marker for disease activity and severity in adult-onset Still’s disease. J Rheumatol 37(5):1029–1034
Aghdashi MA, Seyedmardani S, Ghasemi S, Khodamoradi Z (2019) Evaluation of serum calprotectin level and disease activity in patients with rheumatoid arthritis. Curr Rheumatol Rev 15(4):316–320
Gernert M, Schmalzing M, Tony HP, Strunz PP, Schwaneck EC, Fröhlich M (2022) Calprotectin (S100A8/S100A9) detects inflammatory activity in rheumatoid arthritis patients receiving tocilizumab therapy. Arthritis Res Ther 24(1):200
Nordal HH, Brun JG, Hordvik M, Eidsheim M, Jonsson R, Halse AK (2016) Calprotectin (S100A8/A9) and S100A12 are associated with measures of disease activity in a longitudinal study of patients with rheumatoid arthritis treated with infliximab. Scand J Rheumatol 45(4):274–281
Genre F, Rueda-Gotor J, Remuzgo-Martínez S, Corrales A, Mijares V, Expósito R et al (2018) Association of circulating calprotectin with lipid profile in axial spondyloarthritis. Sci Rep 8(1):13728
McInnes IB, Schett G (2011) The pathogenesis of rheumatoid arthritis. N Engl J Med 365(23):2205–2219
Motlaghzadeh Y, Bilezikian JP, Sellmeyer DE (2021) Rare causes of hypercalcemia: 2021 update. J Clin Endocrinol Metab 106(11):3113–3128
Levitova A, Hulejova H, Spiritovic M, Pavelka K, Senolt L, Husakova M (2016) Clinical improvement and reduction in serum calprotectin levels after an intensive exercise programme for patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis. Arthritis Res Ther 18(1):275
De Rycke L, Baeten D, Foell D, Kruithof E, Veys EM, Roth J et al (2005) Differential expression and response to anti-TNFalpha treatment of infiltrating versus resident tissue macrophage subsets in autoimmune arthritis. J Pathol 206(1):17–27
Turina MC, Yeremenko N, Paramarta JE, De Rycke L, Baeten D (2014) Calprotectin (S100A8/9) as serum biomarker for clinical response in proof-of-concept trials in axial and peripheral spondyloarthritis. Arthritis Res Ther 16(4):413
Andrés Cerezo L, Mann H, Pecha O, Pleštilová L, Pavelka K, Vencovský J et al (2011) Decreases in serum levels of S100A8/9 (calprotectin) correlate with improvements in total swollen joint count in patients with recent-onset rheumatoid arthritis. Arthritis Res Ther 13(4):R122
Nielsen UB, Bruhn LV, Ellingsen T, Stengaard-Pedersen K, Hornung N (2018) Calprotectin in patients with chronic rheumatoid arthritis correlates with disease activity and responsiveness to methotrexate. Scand J Clin Lab Invest 78(1–2):62–7
Patro PS, Singh A, Misra R, Aggarwal A (2016) Myeloid-related protein 8/14 levels in rheumatoid arthritis: marker of disease activity and response to methotrexate. J Rheumatol 43(4):731–737
Hoskin TS, Crowther JM, Cheung J, Epton MJ, Sly PD, Elder PA et al (2019) Oxidative cross-linking of calprotectin occurs in vivo, altering its structure and susceptibility to proteolysis. Redox Biol 24:101202
Storkey C, Davies MJ, Pattison DI (2014) Reevaluation of the rate constants for the reaction of hypochlorous acid (HOCl) with cysteine, methionine, and peptide derivatives using a new competition kinetic approach. Free Radic Biol Med 73:60–66
Pillinger MH, Abramson SB (1995) The neutrophil in rheumatoid arthritis. Rheum Dis Clin North Am 21(3):691–714
Yang X, Wang Y, Shang Z, Zhang Z, Chi H, Zhang Z et al (2021) Quinoline-based fluorescent probe for the detection and monitoring of hypochlorous acid in a rheumatoid arthritis model. RSC Adv 11(50):31656–31662
Zhang S, Ning L, Song Z, Zhao X, Guan F, Yang XF et al (2022) Activatable near-infrared fluorescent organic nanoprobe for hypochlorous acid detection in the early diagnosis of rheumatoid arthritis. Anal Chem 94(15):5805–5813
Wu P, Xiong H (2022) An acid-enhanced OFF-ON fluorescent probe for the detection of hypochlorous acid in rheumatoid arthritis. Talanta 247:123584
Wu P, Zhu Y, Chen L, Tian Y, Xiong H (2021) A fast-responsive off-on near-infrared-II fluorescent probe for in vivo detection of hypochlorous acid in rheumatoid arthritis. Anal Chem. 93(38):13014–21
Edwards TS, Dickerhof N, Magon NJ, Paton LN, Sly PD, Kettle AJ (2022) Formation of calprotectin-derived peptides in the airways of children with cystic fibrosis. J Immunol 208(4):979–990
Sampson AP (2000) The role of eosinophils and neutrophils in inflammation. Clin Exp Allergy 30(Suppl 1):22–27
Cascão R, Rosário HS, Souto-Carneiro MM, Fonseca JE (2010) Neutrophils in rheumatoid arthritis: more than simple final effectors. Autoimmun Rev 9(8):531–535
Wright HL, Moots RJ, Edwards SW (2014) The multifactorial role of neutrophils in rheumatoid arthritis. Nat Rev Rheumatol 10(10):593–601
Bettner LF, Peterson RA, Bergstedt DT, Kelmenson LB, Demoruelle MK, Mikuls TR et al (2021) Combinations of anticyclic citrullinated protein antibody, rheumatoid factor, and serum calprotectin positivity are associated with the diagnosis of rheumatoid arthritis within 3 years. ACR Open Rheumatol 3(10):684–689
Hurnakova J, Hulejova H, Zavada J, Hanova P, Komarc M, Mann H et al (2017) Relationship between serum calprotectin (S100A8/9) and clinical, laboratory and ultrasound parameters of disease activity in rheumatoid arthritis: a large cohort study. PLoS ONE 12(8):e0183420
Bioassay Technology Laboratory EH (2021) Human Calpotectin ELISA kit
Kostanjšak T, Bojanić K, Čičak H, Gotić J, Vrbanac Z, Šimundić A-M et al (2022) Is canine calprotectin in serum stabile after storage at low temperature? BMC Vet Res 18(1):451
Van Hoovels L, Vander Cruyssen B, Bogaert L, Van den Bremt S, Bossuyt X (2019) Pre-analytical and analytical confounders of serum calprotectin as a biomarker in rheumatoid arthritis. Clin Chem Lab Med 58(1):40–49
Mylemans M, Nevejan L, Van Den Bremt S, Stubbe M, Cruyssen BV, Moulakakis C et al (2021) Circulating calprotectin as biomarker in neutrophil-related inflammation: pre-analytical recommendations and reference values according to sample type. Clin Chim Acta 517:149–155
Nordal HH, Fagerhol MK, Halse AK, Hammer HB (2018) Calprotectin (S100A8/A9) should preferably be measured in EDTA-plasma; results from a longitudinal study of patients with rheumatoid arthritis. Scand J Clin Lab Invest 78(1–2):102–108
Chang ZL, Beezhold DH (1993) Protein kinase C activation in human monocytes: regulation of PKC isoforms. Immunology 80(3):360–366
Pedersen L, Birkemose E, Gils C, Safi S, Nybo M (2018) Sample type and storage conditions affect calprotectin measurements in blood. J Appl Lab Med 2(6):851–856
Velayutham R, Nair PP, Adole PS, Mehalingam V (2021) Association of serum calprotectin with peripheral neuropathy in patients with type 2 diabetes mellitus. J Family Med Prim Care 10(4):1602–1606
Acknowledgements
Not applicable.
Funding
No funding resources for this study.
Author information
Authors and Affiliations
Contributions
SS, gathering of data, interpretation of results, and writing of the research. LM, the idea of the research and the gathering of participants. GA, clinical assessment, analysis of data, and writing the research. NA, perform the laboratory tests and analysis of data. HM, analysis of data and writing of the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The ethics committee formally approved this study of the Alexandria University Faculty of Medicine study (FWA 00018699/0106927, date: 20 October 2021). The nature of the study was made clear to the participants, and written informed consent was given by each participant.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Tawab, S.S., Moharram, L.M., Younis, G.A. et al. Study of serum calprotectin level in rheumatoid arthritis: unexpected low level and possible explanations. Egypt Rheumatol Rehabil 51, 4 (2024). https://doi.org/10.1186/s43166-023-00226-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43166-023-00226-5