Abstract
Background
COVID-19 has caused various implications on physical and mental health of human beings. It included several symptoms involving the auditory vestibular system. This study aims to investigate the impact of active COVID-19 infection on central and peripheral auditory pathways.
Method
Two groups of subjects were involved in the research: Group I consisted of 45 individuals with no history of COVID-19, while Group II included 41 individuals who were diagnosed with COVID-19 through RT-PCR testing.
The audiological battery used in this study included Pure tone audiometry, Digit in Noise test, Dichotic CV test, and Pitch pattern test, all of which were administered online. Testing was conducted in two phases for both groups. Phase 1 testing took place within the first 7 days of a positive RT-PCR result, while Phase 2 testing occurred within a week of a negative RT-PCR test. Additionally, participants completed a questionnaire to provide information on general health conditions and their otological symptoms.
Results
The results from Phase 1 testing revealed that Group II exhibited statistically lower scores in all the audiological tests compared to Group I (control group). However, during Phase 2 testing, this significant difference was no longer observed between both groups.
Conclusion
Based on the findings, it can be inferred that the audiological pathway was compromised during the active infection stage of COVID-19. Therefore, this study highlights changes in performance concerning tests that assess the central auditory system during the infection period.
Trial registration
SH/IRB/RP/24. Registered 12 January 2021.
Similar content being viewed by others
Background
COVID-19 is a global pandemic characterized by initial symptoms such as fever, coughing, sore throat, shortness of breath, exhaustion, and malaise. In severe cases, it can progress to pneumonia, acute respiratory distress syndrome (ARDS), and multi-organ failure, with the latter stage posing a significant risk of mortality. The recovery process can be arduous [1]. Among the many unanswered questions about COVID-19, understanding its potential long-term health effects on those who have contracted the disease remains a critical area of investigation. Notably, there is a concern regarding persistent or permanent anosmia (loss of sense of smell) as a sequelae of coronavirus infection [2]. Beyond the respiratory system, other organs may also be susceptible to enduring health effects. Moreover, there are indications that COVID-19 may have implications on mental health, even in ways seemingly unrelated to the primary respiratory symptoms [2]. The understanding of these long-term health consequences is essential for comprehensive management and care for individuals affected by COVID-19.
The well-established association between certain viruses like measles, mumps, and meningitis and their ability to cause hearing loss highlights the potential impact of viral infections on auditory health. Moreover, there is a known connection between auditory neuropathy and Guillain–Barre syndrome, a condition that has been linked to coronavirus infection [3]. In individuals affected by COVID-19, a diverse range of audio-vestibular symptoms has been reported, including hearing loss (both conductive and sensorineural), tinnitus, rotatory vertigo, otitis externa, and vague earache. However, due to the limited availability of comprehensive case reports on COVID-19 and its effects on hearing abilities, the quality of evidence remains assessed as fair or low.
The virus is known to exert a significant impact on the central nervous system (CNS), as evidenced by the presence of ACE2 receptors in the cerebral cortex and brain stem. Cases of meningitis and encephalitis in certain patients indicate the viral invasion of the CNS. This invasion can lead to a depression of reflexes in the brain stem, particularly those involved in detecting oxygen deprivation. Neurological symptoms may manifest alone or in combination with respiratory or other symptoms, with more severe cases being more likely to experience neurological manifestations. These symptoms could be attributed to altered oxygen and carbon dioxide levels and may include dizziness, headaches, confusion, and delirium. Delirium, in particular, is frequent and can result in memory loss and long-term cognitive decline. It is worth noting that the use of benzodiazepines as sedatives, due to a shortage of commonly used sedatives like propofol and dexmedetomidine, might exacerbate delirium. Postmortem examinations of the brains of deceased individuals with COVID-19 have shown hypoxic alterations, although encephalitis or other virus-related changes are infrequent [3]. Understanding the potential neurological implications of COVID-19 is of paramount importance, as it can provide crucial insights for proper patient management and care in cases where the virus affects the central nervous system and auditory system. Further research and comprehensive case studies are needed to elucidate the full extent of COVID-19’s impact on neurological and auditory health.
In recent times, there has been considerable research on the association between COVID-19 and peripheral hearing difficulties. However, investigations into the impact of COVID-19 on the central auditory system, encompassing aspects such as binaural processing, speech in noise, and temporal processing, have been limited. Furthermore, most of the existing studies have predominantly focused on the effects observed post-COVID-19 infection, weeks, or even months after recovery.
Given these gaps in the literature, our study aimed to address whether COVID-19 infection had any discernible effect on both the peripheral and central auditory systems during the active infection phase. Additionally, we sought to investigate whether any observed effects on auditory function subsided after the individual’s recovery from the infection. By examining these aspects, we aimed to contribute to a more comprehensive understanding of the relationship between COVID-19 and auditory health, particularly in the context of both active infection and post-recovery phases.
Methods
This study employed an experimental research design, utilizing a standard group comparison paradigm. The participants were randomly selected from a quarantined facility that housed individuals diagnosed with COVID-19, both in isolation and under observation. Before undergoing the tests, the participants were provided with a consent form through an online modality. Additionally, a questionnaire was administered to the participants in the form of a Google form, aimed at gathering information on their current symptoms, including ear pain, itching, discharge, vertigo, nose blockage, and headache, with the specific purpose of ruling out middle ear involvement since immittance testing was not feasible during their quarantine period. Those subjects who exhibited any of the above-mentioned symptoms were excluded from the study.
A total of 86 subjects were selected to participate in the study and were divided into two groups. Group I served as the control group, comprising individuals with no positive history of COVID-19, while Group II consisted of individuals with a confirmed history of COVID-19. Group I comprised 45 individuals (21 males and 24 females) with a mean age of 25.75 years (SD = 9 years), while Group II comprised 41 individuals (20 males and 21 females) with a mean age of 26.33 years (SD = 8.5 years). The confirmation of SARS-CoV-2 infection in Group II was based on a real-time reverse transcription-polymerase chain reaction (Rt-PCR) test conducted on nose, throat, or sputum samples. Notably, all participants had no prior history of otological complaints such as reduced hearing sensitivity, ear pain, otorrhea, tinnitus, or any underlying medical conditions like diabetes, hypertension, and vertigo. Furthermore, they reported no history of noise exposure or intake of ototoxic drugs.
This study implemented audiological testing in two distinct phases. Phase 1 testing was performed within 1 week of the participants’ receipt of positive Rt-PCR results, during which they were isolated and manifested active symptoms of COVID-19. Subsequently, Phase 2 testing took place within 1 week of the participants testing negative for the virus, specifically occurring within 14 to 20 days from the first day of diagnosis.
Both Group I, consisting of individuals clinically considered normal, and Group II, comprising individuals who tested positive for COVID-19, underwent the same testing phases within identical time frames. Consequently, all subjects in Group II underwent audiological assessments both during their active COVID-19 infection and after recovery, allowing for a meaningful comparison with the control group (Group I).
The audiological tests included the widely used Pure tone audiometry (PTA) to assess hearing sensitivity. Additionally, the study encompassed examinations of the central auditory processing, comprising the following specific tests:
Dichotic CV (DCV): This test evaluates binaural integration, which refers to the brain's ability to process different sounds simultaneously presented to both ears. It provides insights into the brain's capacity to integrate auditory information effectively. If participants correctly report the stimuli presented to one ear, it is considered a single correct response. Similarly, if they accurately respond to stimuli presented in both ears, it is scored as double correct. It is the double correct scores that accounts for the measure of auditory capacity.
-
Pitch pattern test (PPT): This test assesses temporal patterning, which pertains to the brain’s ability to perceive patterns in auditory stimuli, such as recognizing melodies or rhythms. It aids in understanding the temporal processing capabilities of the auditory system.
-
Digit in noise (DIN) test: The DIN test evaluates auditory closure, which refers to the ability to comprehend speech in noisy environments. This examination simulates real-life listening situations and allows for an evaluation of auditory function in challenging acoustic conditions.
The objective of conducting these tests during both the active infection and post-recovery stages was to analyze any potential effects of COVID-19 on both the peripheral and central auditory systems. The study aimed to determine if there were any observable changes in auditory function during the infection period and whether such effects persisted or subsided after the individual’s recovery from the virus. The comprehensive analysis of these findings contributes to a better understanding of the possible implications of COVID-19 on auditory health and functioning.
For the initial phase of the test, the participants of Group II were quarantined in a government guest house; thus, they were contacted over the phone and written consent was taken through Google form. A questionnaire was sent to each participant via email/WhatsApp, where the details regarding the symptoms were captured. It included a total of 11 symptoms. Information regarding audiological symptoms such as the presence of aural fullness, ear pain, tinnitus, and vertigo; and general medical signs and symptoms such as fever, sore throat, cough and cold, anosmia and ageusia, breathing difficulty, and feeling of anxiety and depression. A total score out of 11 symptoms was calculated to check the correlation between symptoms and the scores obtained in PTA, DCV, PPT, and DIN tests.
The participants received detailed instructions on the test procedure and were guided on how to conduct the online tests remotely. Pure tone audiometry (PTA) and Digit in noise (DIN) tests were administered using the “Hearing Test app,” available on the Google Play store. This app, when used with headphones on mobile devices, has demonstrated excellent compatibility with pure tone audiometry, making it suitable for widespread hearing monitoring, screening tests, and epidemiological investigations [4]. The app included a calibration feature at the beginning of the test. Participants were advised to perform the tests in a quiet environment with minimal background noise.
Air conduction measurements were conducted at octave frequencies ranging from 250 Hz to 8 kHz, and the average of four frequencies (500 Hz, 1 kHz, 2 kHz, and 4 kHz) was calculated. The DIN test, also within the app, utilized ten digits delivered by a male native speaker lector. The digits comprised both monosyllabic (2, 3, 5, 6) and disyllabic (0, 1, 4, 7, − 10) elements. The preloaded masking noise in the app was generated by digitally filtering white noise [5]. The DIN threshold was determined based on the dB SNR (signal-to-noise ratio) at which 50% correct scores were obtained. Subsequently, the participants completed the Dichotic CV (DCV) and Pitch pattern test (PPT). Audio files for these tests were shared with the participants via email. The DCV task involved thirty pairs of standardized CV segments. The score for the DCV test was calculated in terms of single correct scores (SC) for each ear and double correct scores (DC). On the other hand, the PPT comprised 21 practice items and 30 test items. Each test item consisted of a pattern of 3-tone bursts, with durations of 500 ms each, separated by 300 ms intervals between tones. The tone frequencies used were 880 Hz (Low) and 1430 Hz (High) in six different combinations (HHL, LLH, HLH, LHL, HLL, LHH). Participants were instructed to perform the PPT using a headphone set at their most comfortable level, preferably around 60% or more of the total volume. Participants manually noted their responses, which were subsequently shared with the author for analysis.
Results
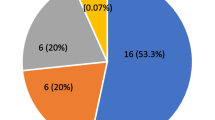
Illustrated in Fig. 1 are the findings pertaining to the array of otological and medical symptoms displayed within Group II. The analysis indicates a predominant presentation of symptoms such as fever, cough, cold, sore throat, and breathing difficulties. Notably, a relatively lower proportion of participants exhibited otological symptoms. The questionnaire administered encompassed a comprehensive spectrum of 11 distinct symptoms, which were aggregated to ascertain their potential correlations with the outcomes of audiological assessments.
Otological and related symptoms. The chart represents the percentage of participants of Group II (COVID-19 positive) showing otological symptoms such as aural fullness, otorrhea, reduced hearing sensitivity, tinnitus, and vertigo; general symptoms such as fever, sore throat, cough and cold, anosmia and ageusia, breathing difficulty, and anxiety/depression
Depicted in Fig. 2 are the average PTA results of Group I and Group II across both testing phases, with red denoting right ear results and blue representing left ear results. Group I, the control group, displayed notably improved auditory thresholds compared to Group II. Likewise, Figs. 3, 4, and 5 illustrate scores from the DIN, DCV, and PPT tests, succinctly capturing the performance outcomes of these auditory assessments. These visuals succinctly communicate the observed differences in auditory function between the two groups, providing valuable insights into the study’s focal conditions.
The data was assessed for normality using Shapiro–Wilk’s test, revealing that it adhered to a normal distribution (p > 0.05). A paired sample t-test was conducted to compare results within each group across different testing phases. For Group II participants, there were significant differences between Phase 1 and Phase 2 scores, with Phase 2 demonstrating improved outcomes in all tests [PTA right ear—t(40) = 3.677, p = 0.001; PTA left ear—t(40) = 4.454, p < 0.001; DIN right ear—t(40) = 3.815, p < 0.001; DIN left ear—t(40) = 3.62, p = 0.004; DCV-SC right ear—t(40) = 3.66, p = 0.01; DCV-SC left ear—t(40) = 2.563, p = 0.014; DCV-DC—t(40) = 2.39, p = 0.02; PPT—t(40) = 3.57, p < 0.01]. In contrast, the paired sample t-test within Group I exhibited no significant differences between the two testing phases (p > 0.05).
Moreover, an independent t-test on test scores indicated significant differences in Phase 1 testing for both groups, highlighting impacts during the COVID-19 period [PTA right ear—t(84) = 2.603, p < 0.001; PTA left ear—t(84) = 2.408, p < 0.001; DIN right ear—t(84) = 10.367, p = 0.033; DIN left ear—t(84) = 3.851, p = 0.003; DCV-SC right ear—t(84) = 2.294, p = 0.017; DCV-SC left ear t(84) = 1.41, p = 0.018; DCV-DC—t(84) = 1.59, p = 0.025; PPT—t(84) = 2.053, p = 0.002].
Furthermore, analysis revealed no significant differences in Group I when tested across the two phases (p > 0.05), indicating stability. Additionally, no significant difference emerged between Phase II scores of Group II and Group I (p > 0.05), post-COVID-19 recovery.
Complementing these statistical evaluations, a Pearson correlation test was employed to explore links between otological/medical symptoms and audiological results. Notably, the cumulative symptom score did not exhibit any correlations with audiological tests (p > 0.05). This suggests that the presence of medical and otological symptoms during COVID-19 does not influence peripheral and central hearing outcomes, enhancing our understanding of the relationship between these factors.
Discussion
COVID-19 infection appears to exert a temporary influence on the auditory pathway, potentially attributed to the virus itself or factors such as psychological distress and reduced alertness, as previously discussed. Notably, this auditory impact is transient, recovering in conjunction with the resolution of the infection. Similar to conditions like Meniere’s disease, which induces cognitive deficits such as brain fog and confusion [6], COVID-19 could yield analogous outcomes, as supported by the findings. Sahin and colleagues extensively documented the neuroinvasive and neurotrophic characteristics of the coronavirus. Building upon these attributes, it is conceivable that COVID-19 may instigate auditory neuropathy spectrum disorder (ANSD), characterized by a disconnection between outer hair cells and ascending neural pathways [7]. Approximately 30% of individuals who had COVID-19 have reported experiencing neurological symptoms [8]. It is worth noting that diurnal rhythm could influence results, as the timing of tests may have an impact [9].
Inflammation is proposed as a factor damaging central auditory pathways, potentially leading to future Central auditory processing disorder (CAPD). Another plausible mechanism for CAPD development is linked to the elevated incidence of thrombosis induced by SARS-CoV-2. Blood coagulation and resulting thrombosis can lead to transient ischemia, contributing to both peripheral and central auditory disorders depending on clot location [10]. The observed lack of a significant correlation between disease severity and auditory disorders may be attributed to the use of anticoagulants in severe cases. This same mechanism is posited to be responsible for vertigo and tinnitus in patients post-COVID-19 recovery.
Dichotic tests, including the Dichotic CV test (DCV) utilized in this study, are widely employed for diagnosing Central auditory processing disorder (CAPD). The DCV used here is resistant to peripheral hearing loss. Binaural integration was evaluated, requiring patients to freely repeat consonant–vowel syllables presented to both ears. This demanding test revealed poor results in a significant portion of patients. Dichotic tests demonstrate high sensitivity to disorders of the auditory cortex and corpus callosum, with lower sensitivity to brainstem-level disorders. Existing data also suggests an influence of the left frontal cortex on dichotic test results. Studies also used the Random Gap Detection Test (RGDT) to assess auditory temporal resolution, crucial for speech comprehension. Poor RGDT results correlated with compromised post-COVID-19 cognitive abilities, supported by low Montreal Cognitive Assessment (MoCA) consistent with findings from other researchers [11]. Although cognitive functions were not directly assessed in the current study, similar results were found in tests of CAPD.
Viral infections primarily impact intracochlear structures, though some can affect the auditory brainstem. The mechanisms behind peripheral auditory damage involve direct viral harm to critical components such as the organ of Corti, stria vascularis, and spiral ganglion. Additionally, the immune response targeting virally expressed proteins and secondary bacterial infections can contribute to this damage. An understanding of these mechanisms informs clinical strategies for addressing viral-induced auditory impairment [5]. A notable case study involving a 60-year-old COVID-19 patient underscores the urgency of audiological assessment for those with hearing loss and neurological symptoms. Bilateral hearing loss and tinnitus emerged post-intensive care treatment, with MRI scans revealing cochlear and meningeal inflammation. Despite the administration of ototoxic medication, the symmetrical hearing loss and MRI findings point to virus-triggered inflammation as the probable cause. Unlike COVID-19-associated anosmia due to direct viral damage, this case emphasizes the importance of prompt audiological evaluation [12]. Regarding the audiological impact of COVID-19, a threshold increase was observed post-infection, but it lacked clinical significance. Adjusting for age and assessment timing eliminated the statistical significance. Moreover, no significant connections were established between hearing changes and other COVID-19-related factors [13].
Thus, this study highlights the necessity of considering potential audiological effects in individuals with a history of COVID-19. Clinicians should be vigilant when assessing these patients, given the potential influence of the infection phase on auditory performance. This awareness can guide the development of appropriate rehabilitation strategies for temporary auditory deficits. Encouragingly, Phase 2 findings suggest a recovery post-COVID-19, with no significant differences in audiological test scores between groups. This suggests that the auditory impact of COVID-19 on peripheral and central auditory systems might be transient and reversible. Consequently, any potential auditory effects are likely to be temporary.
Conclusion
In conclusion, COVID-19 infection temporarily affects the auditory pathway, potentially resulting from the virus itself or factors like psychological distress and reduced alertness, as discussed earlier. Importantly, this auditory impact is not permanent; it recovers in tandem with the resolution of the infection. This study’s comprehensive exploration of auditory responses to COVID-19 underscores the intricate interplay between viral infection, auditory function, and the subsequent recovery process. While the infection phase may induce brief auditory deficits, the recovery phase demonstrates a restoration of auditory function. These insights significantly enhance our broader comprehension of how COVID-19 influences overall health and well-being, furnishing essential information for both clinicians and researchers in their endeavors.
Future directions
A potential avenue for further research involves expanding the study to encompass a larger and more diverse sample size. It is worth noting that while the current study conducted by the PTA utilized an app-based testing approach, there is room for improvement in terms of accuracy when compared to conventional methods.
To enhance the comprehensiveness of the study, additional measures such as Impedance, Otoacoustic emissions (OAE), and Auditory evoked potentials (AEP) could be incorporated. These measures can provide valuable insights into various auditory pathways and contribute to a more comprehensive understanding of the auditory impact on individuals.
The evaluation of cognitive function in patients was not conducted before the onset of COVID-19, which could be considered in future studies using MoCA/MMSE etc.
Considering technological advancements and the availability of protective equipment, there is a promising opportunity to perform a broader range of auditory tests physically on COVID-19 patients presenting with active symptoms. This approach could offer a more holistic assessment and contribute to the refinement of our understanding of the auditory effects associated with the virus.
Availability of data and materials
The datasets and materials used in this study are available upon request.
References
Singhal T (2020) A review of coronavirus disease-2019 (COVID-19). Indian J Pediatr 87(4):281–286. https://doi.org/10.1007/S12098-020-03263-6/METRICS
Hopkins C, Alanin M, Philpott C, Harries P, Whitcroft K, Qureishi A, Anari S, Ramakrishnan Y, Sama A, Davies E, Stew B, Gane S, Carrie S, Hathorn I, Bhalla R, Kelly C, Hill N, Boak D, Nirmal Kumar B (2021) Management of new onset loss of sense of smell during the COVID-19 pandemic-BRS Consensus Guidelines. Clin Otolaryngol 46(1):16–22. https://doi.org/10.1111/COA.13636
Jain U (2020) Effect of COVID-19 on the organs. Cureus 12(8):e9540. https://doi.org/10.7759/CUREUS.9540
Masalski M, Grysiński T, Kręcicki T (2018) Hearing tests based on biologically calibrated mobile devices: comparison with pure-tone audiometry. JMIR Mhealth Uhealth 6(1):e10
Mustafa MWM (2020) Audiological profile of asymptomatic COVID-19 PCR-positive cases. Am J Otolaryngol 41(3):102483. https://doi.org/10.1016/J.AMJOTO.2020.102483
Bhattacharyya R, Barman A, Antony F (2023a) Influence of BPPV and Meniere’s disease on cognitive abilities: a questionnaire-based study. J Otol https://doi.org/10.1016/j.joto.2023.11.001.
Sahin AR, Erdogan A, Agaoglu PM, Dineri Y, Cakirci AY, Senel ME, Okyay RA, Tasdogan AM (2020) 2019 novel coronavirus (COVID-19) outbreak: a review of the current literature. EJMO 4(1):1–7
Ahmad I, Rathore FA (2020) Neurological manifestations and complications of COVID-19: a literature review. J Clin Neurosci 77:8–12
Bhattacharyya R, Upadhya SS, Jargar R, Kv N (2023) Exploring the consequences of the diurnal preference on auditory spatial and working memory tasks. Biol Rhythm Res 54(9):548–562
García-Callejo FJ, Balaguer-García R, Lis-Sancerni MD, Ruescas-Gómez L, Murcia-López M (2022) Blood viscosity in COVID-19 patients with sudden deafness. Acta Otorrinolaringologica (English Edition) 73(2):104–112
Boboshko MY, Garbaruk ES, Vikhnina SM, Golovanova LE, Ogorodnikova EA, Rabchevskaya AV, Zhilinskaia EV (2022) The new coronavirus infection (COVID-19) and hearing function in adults. J Otorhinolaryngol Hear Balance Med 3(2):5.
Degen C, Lenarz T, Willenborg K (2020) Acute profound sensorineural hearing loss after COVID-19 pneumonia. Mayo Clin Proc 95(8):1801–1803. https://doi.org/10.1016/j.mayocp.2020.05.034
Taitelbaum-Swead R, Pinhas A, Cohen Tsemah S, Wechsler H, Chordekar S (2023) Is COVID-19 to blame for sensorineural hearing deterioration? A Pre/Post COVID-19 Hearing Evaluation Study. Laryngoscope 133(8):1976–1981
Acknowledgements
We would like to express our gratitude to the HoD Audiology for their valuable contributions and support throughout this research. We also acknowledge the Director of All India Institute of Speech and Hearing for providing access to the necessary resources that facilitated this study.
Funding
There was no source of any external funding for the research.
Author information
Authors and Affiliations
Contributions
All the authors have significant contribution and participation in this research. Conceptualization, R.B., S.U., P.P.; methodology, R.B.; formal analysis, S.U.; investigation, P.P.; resources, R.B., S.U., P.P.; data curation, R.B., S.U.; writing—original draft preparation, S.U.; writing—review and editing, R.B., P.P; supervision, P.P; All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was taken from the AIISH Institutional Ethical Review Board with reference number SH/IRB/RP/24, registered December 1, 2021.
Informed consent was obtained from all the participants involved in the study. Participants were provided with detailed information about the study's purpose, procedures, and potential risks, and they voluntarily agreed to participate by signing a consent form. Confidentiality and anonymity measures were implemented to protect the privacy of the participants, ensuring that their identities are not disclosed.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bhattacharyya, R., Upadhya, S.S. & Prabhu, P. Effect of COVID-19 on peripheral and central hearing abilities. Egypt J Otolaryngol 40, 12 (2024). https://doi.org/10.1186/s43163-024-00567-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43163-024-00567-8