Abstract
Objective
We aim to describe the clinical features and therapeutic management of necrotizing otitis externa (NOE) with negative culture.
Patients and methods
We included all patients with NOE, who were treated in the period between 2008 and 2020 in our department.
Results
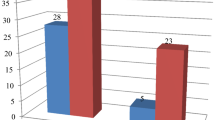
A total of 25 cases of NOE were included. The result of the culture was negative in 13 cases (52%). All patients received a local treatment prior to hospitalization, and eight patients (61.5%) received oral antibiotic. A sampling of the otorrhea was done for all patients. Fungal serology was performed for six patients; it was positive in two cases. The prescribed first-line was ciprofloxacin in combination with ceftazidime for 10 patients, while it was based on the use of imipenem with ciprofloxacin for 2 patients and one patient only received ciprofloxacin. An improvement was noted in 10 cases (77%). The second-line treatment in the three cases of resistance was imipenem with ciprofloxacin in one case. For the two patients with a positive aspergillus serology, one patient received teicoplanin, fusidic acid, imipenem, and voriconazole and the other patient received voriconazole. The total duration of the treatment was a minimum of 6 weeks. An improvement was noted in all cases, and recurrence was noted in 3 cases.
Conclusion
In our study, there were no clinical or radiological specificities noted in NEO with negative culture. Sampling must be repeated. Fungal origin should be suspected in refractory forms and empiric antifungal treatment may be useful.
Similar content being viewed by others
Background
Necrotizing otitis externa (NOE) is a severe and progressive infection; it begins in the external auditory canal (EAC) and spreads to adjacent structures [1]. It usually affects the immunocompromised patients, especially elderly patients with diabetes mellitus [2 ]. It is characterized by an important mortality risk that varies between 5.9 and 24% [2].
Pseudomonas aeruginosa is the most reported microbiological agent with a percentage ranging from 47 to 65% [2, 3]. However, negative culture results are increasing during these last 10 years; according to a systematic review of literature, it occurred in 12.6% of cases [2].
Despite the several publications, the diagnosis, prognosis, and treatment of NOE are still challenging and remain controversial. Only a few studies were interested in NOE with negative culture.
We aim in this study to describe the clinical features and therapeutic management of NOE with negative culture.
Methods
We included all patients with NOE who were treated in our department in the period between 2008 and 2020. The diagnosis of NOE was made according to the following diagnostic criteria of Cohen and Friedman [4]:
-
1.
Painful external otitis not responsive to outpatient treatment of at least 1 week
-
2.
Clinical findings of otorrhea, edema, and exudates of the EAC and/or granulation tissue in the EAC
-
3.
And/or positive findings on temporal bone scan: mainly osteolysis
-
4.
An immunocompromised condition: elderly, diabetes
We collected data concerning the clinical and radiologic features, the treatment strategy, and the follow-up of all patients. Patients with an incomplete medical documentation were excluded. Sampling of otorrhea was done using a swab in all cases. All patients were hospitalized and received intravenous antibiotic therapy associated to a local treatment. Regular monitoring of blood glucose levels in all diabetic patients was performed with glycemic control. Regular monitoring of renal and hepatic function was done once a week for all patients. An improvement was defined as the disappearance of otalgia and the regression of the local and biological inflammation process [2].
Results
We collected a total of 25 cases of NOE. The result of swab culture was negative in 13 patients with a rate of 52%, including 6 males and 7 females. The mean age of patients was 62.5 years [43-84]. Eleven patients had diabetes (84.6%). Two patients had UCNT (undifferentiated carcinoma of nasopharyngeal type) of nasopharynx treated with concomitant radiotherapy and chemotherapy.
Severe otalgia was noted in all cases, with a nocturnal accentuation in 5 cases (38.7%). Otorrhea was the second most common symptom (69%) followed by headache (30.7%) (Table 1). The duration of symptoms ranged from 7 to 180 days, with a median of 25.5 days.
According to the findings of the examination, ten patients (77%) had edema and granulations in the EAC and four patients (30.7%) had polyps in the EAC. A facial palsy was noted in 2 cases (15.3%).
We should note that the sample has been repeated two times in 4 cases and three times for two patients, and the results were still negative.
Candida and Aspergillus serologies were performed for six patients, and they were positive for Aspergillus in two cases.
Only one patient presented a hyperleukocytosis (7.7%). Erythrocyte sedimentation rate (ESR) was elevated in 84.6% of cases, and C-reactive protein (CRP) level was high in 61.5% of cases.
CT (computed tomography) scan was performed for all patients while MRI (magnetic resonance imaging) was performed for 2 patients. They revealed a tympanal bone erosion in 9 cases (69.2%) (Fig. 1), a spreading to the temporomandibular joint in two cases (15.3%) and a soft-tissue involvement in 3 cases (23%). SPECT-CT (single-photon emission computed tomography) using technetium-99 was performed for two patients and showed high tissue activity at the temporal bone, the mastoid, and the skull base (Fig. 2).
A biopsy was performed in all cases with granulation tissue or polyps in the EAC; our aim was to exclude malignancy. The histopathological examination showed features of inflammatory granulation tissue in all cases.
All patients received a local treatment prior to hospitalization while eight patients (61%) received an oral antibiotic.
In all cases, a daily local treatment was performed; it included the cleansing of the EAC with the application of topical antiseptic agents. The prescribed first-line was ciprofloxacin in combination with ceftazidime for 10 patients, while it was based on the use of imipenem with ciprofloxacin for 2 patients and one patient only received ciprofloxacin. An improvement was noted in ten cases (77%).
We noted three cases of resistance and who received ceftazidime in association with ciprofloxacin as a first-line treatment. One patient was then treated using imipenem with ciprofloxacin as the second-line treatment. For two remaining patients, the aspergillus serology was positive, so one patient received teicoplanin, fusidic acid, imipenem, and voriconazole while the other patient received voriconazole. The total duration of the treatment was a minimum of 6 weeks [5,6,7,8,9,10,11,12,13,14,15]. The mean length of hospitalization was 30.5 days [6–100].
One patient received a hyperbaric oxygen therapy because of the unfavorable evolution under antibiotic therapy. She received 20 sessions with an improvement of the otalgia and the local condition.
As a result of this treatment, an improvement of the symptomatology and the local condition of the EAC were noticed in all cases.
Antibiotic therapy was extended for more than 6 weeks in 4 patients. Among these patients, three were female. Three patients had diabetes including one patient who had a degenerative complication of diabetes. Two patients had a peripheral facial paralysis complicating the NOE. The treatment was prolonged for 3 months in the two patients with a positive aspergillus serology.
In the two patients with a history of UCNT, the symptomatology started either 2 months or 4 months following the end of the treatment. One of them required the extension of the treatment for more than 6 weeks. We summarized in Table 2 the clinical and therapeutic features of patients.
No patient required a surgical debridement or a mastoidectomy. Recurrence was noted in 3 cases (23%). We followed the same therapeutic protocol the cases of recurrence.
Discussion
Pseudomonas aeruginosa is the most isolated microbiological agent [5, 16]. A significant increase in the frequency of culture-negative results is observed over the last decade as stated in a literature review: it represents 12.6% of culture results [2]. Speilemn et al. [16] found a negative culture in 25% of cases. Kaya et al. [6] found that the swab cultures of eight (32%) patients did not yield any growth. To the best of our knowledge, we report the highest percentage of negative-culture NOE (52%). Some authors explain the absence of germs to a prior antibiotic therapy; others suggest that patients who present with a refractory culture-negative NOE, fungal disease should be suspected [8]. In such cases, Gruber et al. [8] suggested to perform a polymerase chain reaction (PCR) from a surgical sample; indeed, in their study concerning three patients with a negative culture, PCR assays were performed using pan-bacteria and pan-fungi protocol. In all three samples, a positive result for a fungal pathogen was noted and followed by a successful targeted therapy. A recent study published by Abou Talbian et al. [9] showed that the PCR test on ear aspiration specimens, for 120 patients with otitis externa, was more sensitive and more rapid, as well as less cumbersome in the detection and identification of fungal and bacterial agents.
Aspergillus and Candida serology can help in the diagnosis of fungal NOE. In fact, when positive, it points to the presence of an invasive fungal infection. But we should note that antibody formation may be delayed, reduced, or absent in patients with defective immune systems which presents a limit to this test [10].
So, we propose to repeat microbiological samples before initiating treatment and to indicate fungal serology in the cases of NOE with a negative culture.
The management of NOE generally begins with the use of antipseudomonal penicillin (piperacillin-tazobactam) or ceftazidime in association with fluoroquinolones (ciprofloxacin), as the initial drugs of choice. Subsequently, appropriate antibiotics are administered based on the culture and sensitivity [5, 16]. For the majority of authors, in the case of culture-negative NOE, the first-line treatment is ceftazidime and ciprofloxacin. In the series of Djalilian et al. [11], all patients with a negative culture were treated with a 6-week course of intravenous ceftazidime or aztreonam (for penicillin-allergic patients), oral ciprofloxacin, and topical aminoglycoside drops; this strategy has shown an efficiency in 100% of cases. In our study, 77% of patients were treated by ciprofloxacin and ceftazidime, and this management protocol has shown an efficiency in 70% of cases.
Los et al. [12] suggest that the association of an oral Fluoroquinolone with intravenous ceftazidime is the best initial choice, despite the increased resistance of the Pseudomonas. In their study, they compared the results of the two groups of patients with a NOE: those who had a positive culture treated with adapted antibiotics and those who had a negative culture treated with the association of ciprofloxacin and ceftazidime. They did not find a significant difference in the evolution of the disease between both groups.
The role of empiric antifungal treatment was showed in a large cohort published by Hasibi et al. [13]. Indeed, in this study which included 224 cases of NOE, 127 patients (56%) were improved by an empirical antibacterial treatment (ciprofloxacin with either a carbapenem or ceftazidime). The rest of patients (97 cases), who did not respond to antibacterial drugs, received an empirical antifungal treatment; it was efficient in 89.6% of cases. According to this study, this therapeutic strategy was effective in 95.5% [13].
The minimum duration of treatment in our study was 6 weeks based on intravenous antibiotics followed by oral antibiotics. Although many authors have reported rapid symptomatic response with Quinolones, it is still recommended to continue treatment for 6 to 8 weeks [2, 5]. Some authors recommend to extend the treatment to a duration varying between 6 months to 1 year, mainly in fungal NOE [13]. The treatment may stop if Gallium-67 scintigraphy shows a resolution of the infection [2, 16].
According to Amaro et al. [14], hyperbaric oxygen can be used with some benefit as an adjunct to antimicrobials for patients who failed conventional treatments and in severe cases. However, a Cochrane review found that no randomized, controlled trials had been performed to support this treatment [15].
In line with the finding of Byun et al. [2], there was a significant decrease in extensive surgery in the latter decade, he suggested that the advent of quinolone antibiotics has essentially eliminated the need of local debridement. On the other hand, Peled et al. [17] recorded an efficiency of the surgery in 55% of cases. According to this study, the surgery is justified in a in non-responsive patients who received an initial treatment period of at least 2 weeks.
Based on our findings and on the literature, we then propose this protocol of management in the cases of NOE with negative culture (Fig. 3).
Conclusion
NOE with a negative culture does not have specific clinical or radiological features. To identify the germ, we propose to systematize and repeat microbiological samples before initiating treatment, to minimize the prescription of probabilistic ambulatory antibiotic therapy and to prioritize tissue samples. Fungal serology can guide diagnosis. In refractory forms; a fungal agent should be suspected and empiric antifungal treatment may be useful.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Mahdyoun P, Pulcini C, Gahide I, Raffaelli C, Savoldelli C, Castillo L et al (2013) Necrotizing otitis externa: a systematic review. Otol Neurotol 34(4):620–629
Byun YJ, Patel J, Nguyen SA, Lambert PR (2020) Necrotizing otitis externa: a systematic review and analysis of changing trends. Otol Neurotol 41(8):1004–1011
Arsovic N, Radivojevic N, Jesic S, Babac S, Cvorovic L, Dudvarski Z (2020) Malignant otitis externa: causes for various treatment responses. J Int Adv Otol 16(1):98–103
Cohen D, Friedman P (1987) The diagnostic criteria of malignant external otitis. J Laryngol Otol 101(3):216–221
Prasanna Kumar S, Ravikumar A, Somu L, Nazrin MI (2013) Malignant otitis externa: an emerging scourge. J Clin Gerontol Geriatr 4:128–131
Kaya İ, Sezgin B, Eraslan S, Öztürk K, Göde S, Bilgen C et al (2018) Malignant otitis externa: a retrospective analysis and treatment outcomes. Turk Arch Otorhinolaryngol 56(2):106–110
Mani N, Sudhoff H, Rajagopal S, Moffat D, Axon PR (2007) Cranial nerve involvement in malignant external otitis: implications for clinical outcome. Laryngoscope 117(5):907–910
Gruber M, Roitman A, Doweck I, Uri N, Shaked-Mishan P, Kolop-Feldman A et al (2015) Clinical utility of a polymerase chain reaction assay in culture-negative necrotizing otitis externa. Otol Neurotol 36(4):733–736
Aboutalebian S, Ahmadikia K, Fakhim H, Chabavizadeh J, Okhovat A, Nikaeen M et al (2021) Direct detection and identification of the most common bacteria and fungi causing otitis externa by a stepwise multiplex PCR. Front Cell Infect Microbiol 11:644060
Richardson MD, Iain D (2017) Aspergillus serology: have we arrived yet. Med Mycol 55(1):48–55
Djalilian HR, Shamloo B, Thakkar KH, Najme-Rahim M (2006) Treatment of culture-negative skull base osteomyelitis. Otol Neurotol 27(2):250–255
Loh S, Loh WS (2013) Malignant otitis externa: an asian perspective on treatment outcomes and prognostic factors. Otolaryngol Neck Surg 148(6):991–996
Hasibi M, Ashtiani MK, Motassadi Zarandi M, Yazdani N, Borghei P, Kuhi A et al (2017) A treatment protocol for management of bacterial and fungal malignant external otitis: a large cohort in Tehran, Iran. Ann Otol Rhinol Laryngol 126(7):561–567
Amaro CE, Espiney R, Radu L, Guerreiro F (2019) Malignant (necrotizing) externa otitis: the experience of a single hyperbaric centre. Eur Arch Otorhinolaryngol 276(7):1881–1887
Phillips JS, Jones SEM (2013) Hyperbaric oxygen as an adjuvant treatment for malignant otitis externa. Cochrane Database Syst Rev (5):CD004617
Spielmann PM, Yu R, Neeff M (2013) Skull base osteomyelitis: current microbiology and management. J Laryngol Otol 127(S1):S8–12
Peled C, Parra A, El-Saied S, Kraus M, Kaplan DM (2020) Surgery for necrotizing otitis externa-indications and surgical findings. Eur Arch Otorhinolaryngol 277(5):1327–1334
Acknowledgements
None
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All the authors of the study made significant contributions with special responsibilities. MAC and IA collected the data. GY wrote the manuscript with support from WT, MT, SK and IC. All the authors have reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Institutional Ethics Committee of Habib Bourguiba Hospital (approval number 09/2022). Study data/information was used for the research purpose only. Owing to the retrospective nature of the study, informed consents from the patients were waived.
Consent for publication
Written informed consent for publication of their clinical details was obtained from the patients. Written consent to anonymously use patient data for scientific purposes is given by all patients admitted to our department.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chaabouni, M.A., Achour, I., Yousfi, G. et al. Culture-negative necrotizing otitis externa: diagnosis and management. Egypt J Otolaryngol 39, 30 (2023). https://doi.org/10.1186/s43163-022-00363-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43163-022-00363-2