Abstract
Background
We analyzed prognostic factors of hypopharyngeal carcinoma and developed a nomogram to predict survival rates in non-metastatic (M0) patients.
Subjects and methods
We included 4068 hypopharyngeal carcinoma patients identified from the Surveillance, Epidemiology, and End Results Program database between 2004 and 2015 in a retrospective cohort study. We analyzed prognostic factors of hypopharyngeal carcinoma using cause-specific and overall survival rates. We developed a nomogram to predict patients’ survival rates by multivariate Cox regression.
Results
Five-year survival rates of all stages between 2004 and 2010 were 25–35%. Radiotherapy pre- and post-surgery was the best modality of treatment according to 1-year and 5-year survival rates. The worst survival was in the posterior wall of the hypopharynx significantly (HRs [95% CI], P) (1.238 [1.045–1.466], P = 0.013). The highest survival rate was for the combination of surgery and radiotherapy pre- and post-surgery compared to radiation pre-surgery (0.532 [0.231–1.225], P = 0.138). Our nomogram revealed a better predictive probability over the 6th AJCC-TNM classification for predicting 5-year overall survival.
Conclusions
The worst survival was old age hypopharyngeal carcinoma patients, with the primary site in the posterior wall of the hypopharynx. The best survival was linked to receiving radiotherapy pre- and post-surgery. Our nomogram revealed a better predictive probability over TNM classification for predicting 1- and 5-year overall survival, which enables clinicians to make better treatment recommendations.
Similar content being viewed by others
Background
Carcinoma of the hypopharynx, particularly squamous cell carcinoma (SCC), has a poor prognosis, especially of the SCC type, which shows the worst among all head and neck (H&N) subtypes [1]. The surgical management of this type of cancer is difficult, besides being functionally debilitating [2]. However, there are various treatment options for hypopharyngeal cancer (HC) patients including radiotherapy or surgery alone or in combination with chemotherapy [3]. HCs, which account for 6.5% of all H&N cancers, are considered rare with an estimated global incidence of 0.8 to 5/100,000 population/year [1, 4, 5]. Five-year survival rates of HC increased significantly from 28 to 34% in years of 1991–2000 and 2001–2010, respectively [6]. Mortality rate is reported to occur in about 6% of whole hospital records in surgically treated patients [7]. On the other hand, another report stated that mortality rates increased from 0.81 to 0.95 (per 100,000) in 1989 and 2013, respectively [6]. Another cause for that is the occurrence of second primary malignancies (SPM) either simultaneously or after identification of a primary lesion [8]. Knowledge of prognostic factors is essential for clinicians in classifying patients thereby influencing treatment plans and patients’ outcomes. The absence of an agreement on the best treatment option for HC shows the urgent need for a clear prognostic tool for oncologists to help in making more evidence-based decisions about treatment [9]. To date, there are no prediction data available to estimate the role of most prognostic factors on overall survival outcome in HC patients. Therefore, we aimed to conduct this study to determine significance of reported prognostic factors in literature as well as to estimate their survival impact individually. Moreover, we aimed to develop a more practical, effective, and valid nomogram for estimating overall survival (OS) in HC patients in order to assist clinicians in predicting patients’ survival rate and evaluating the best treatment option for each patient.
Methods
Ethical consideration
Ethical approval was retrieved from the Surveillance, Epidemiology, and End Results Program (SEER) database in which it granted us access to the detailed patient data of the SEER register [10].
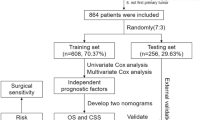
As the predictive ability of the sixth American Joint Committee on Cancer (AJCC)-TNM staging classification was based on the data of patients diagnosed with HC starting from 2004, the SEER [10] database was searched from 2004 to 2015. Finally, in a total of 4068 HC patients with squamous cell carcinoma, data were extracted from the SEER database according to our inclusion criteria. SEER registries contain data from 18 different geographical regions and are known to be representative of 30% of the United States of America (USA) population. We, therefore, analyzed the 1-year and 5-year survival data in patients diagnosed with HC. All cases with histo-pathologically confirmed HC-SCC type, histology recode (8050-8089), and active follow-up were included. We excluded patients with an unknown death certificate or with primary tumors other than HC unless HC was the first occurring malignant lesion.
Outcomes and predictors
The survival time (in months) was our primary outcome in this study. HC patients were followed up over the inclusion period and their vital status was recorded as dead or alive. The survival rates were then calculated for all-cause and cancer-specific mortality. The evaluated predictors were age, sex, race, primary site, clinical staging (Derived SS2000), TNM staging (AJCC 6th edition), and treatment modalities (surgery and radiotherapy with its sequence with surgery and chemotherapy). The primary sites of the HC were defined as pyriform sinus (Ps), post-cricoid (Pc), aryepiglottic fold (Af), posterior wall of the hypopharynx (Ph), overlapping lesions (Ol), and not otherwise specified (NOS).
Statistical analysis
We used uni- and multivariable Cox proportional hazard models to find relationships between patients’ characteristics and survival rates. We then developed a prediction nomogram model, to predict 1- and 5-year OS probability. The development and validation of the nomogram were conducted according to the TRIPOD (transparent reporting of a multivariable prediction model for individual prognosis or diagnosis) statement [11] as shown in Supplementary Table 1.
Construction and validation of the nomogram for predicting survival in M0 hypopharyngeal carcinoma
We used “missForest” for imputation of missing data in SEER [12]. It is a random forest that produces imputed dataset without the need for testing data or performing cross-validations to infer missing data. It is also worth noting that there were other missing variables (radiation and chemotherapy), in which we did not make imputations, as unknown data were mixed with other data. Using SPSS 24.0 [13] for Windows, categorical variables were grouped and then assessed using the χ2 test or Fisher’s exact test. We then used SPSS 24.0 (spilt function) to randomly assign 67% of the patients to the training set and 33% to the validation set. We calculated OS from the date of diagnosis. Covariates with statistical significance (P value < 0.05) were entered into the Cox regression model for multivariable analysis. The nomogram representing the 1-year OS and 5-year OS was formulated using survival [14, 15], root mean square (RMS) [16], and survival ROC packages [17]. The model was assessed with discrimination and calibration. Discrimination value (which indicates the random chance to a perfect ability to accurately discriminate between the model and outcome) ranges from 0.5 (poor discrimination) to 1.0 (perfect discrimination). The nomogram’s predicted probabilities were then compared to the actual probabilities for calibration. The calibration was estimated using bootstrap-adjusted C-index with 2000 resamples in the training group (internal validation). We also performed a receiver operating characteristic (ROC) analysis for censored survival data to estimate the area under the curve (AUC) for a better assessment of the accuracy of the model [18]. Due to the importance of independent validation, the discrimination power, calibration, and AUC were also checked for the validation group and compared with the training group. Additionally, using the quartiles of the nomogram total points, we divided the patients of the training set into four groups. The median survival time (MST) of each group was then plotted using the Kaplan-Meier survival curve and all curves were compared to each other using the log-rank test.
Results
Patients’ characteristics
Table 1 shows patients’ characteristics for all analytic samples (4068). According to HC primary sites, 52.8% of the patients had their cancer in the PS. The average age of the whole population was 62 years. According to 6th AJCC staging, most patients were in stage IVA (45%). Around 79% of patients received radiotherapy, 70% received chemotherapy, and 19% undergo surgery. At the study cutoff, 67% of the patients were dead, with 50.7% of the death cases being caused by this cancer, specifically.
1-year and 5-year survival rates across the follow-up period
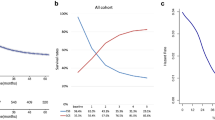
For the 5-year survival rates of the whole sample population over the years between 2004 and 2010, the survival rates were between 25% and 35% and were similar over the years (Supplementary Fig. 1). One-year and 5-year survival rates were estimated across various treatment modalities (Fig. 1). The combination of surgery and radiotherapy (pre- and post-surgery) had the highest survival rates, followed by pre-surgical radiation. Survival rates by primary site were quite similar but somewhat Pc site was a bit better in terms of survival rates than other sites (Fig. 2).
Survival analysis
The results of univariable and multivariable Cox proportional hazard models are shown in Table 2. According to primary sites of HC, Ph indicated the worst survival compared to PS with the highest HRs (1.238 [1.045–1.466], P = 0.013) (Table and Fig. 2). An increase in each stage of T, N, and AJCC was associated with increasing the hazards of death (Supplementary Figs. 2 and 3). According to treatment modalities, surgery combined with radiotherapy (pre- and post-surgery) had significantly better survival compared to pre-surgical radiation with HRs (0.532 [0.231–1.225], P = 0.138) (Supplementary Table 2). The results of comparing each combination of T, N, and M stage in each stage of stage 3, 4A, and 4B (Supplementary Fig. 4) are as follows: In stage 3, the multivariable Cox revealed significantly higher hazards of T3/N0/M0 and T3/N1/M0 compared to T1/N1/M0 with HRs (1.644 [1.114–2.426], P = 0.012) and (1.547 [1.033–2.316], P = 0.034), respectively. In stage 4A, all the combinations of T2, T3, and T4a revealed higher hazards compared to T1/N2/M0, whereas T4a/N2/M0 predicted the worst association with mortality with HRs (2.216 [1.684–2.915], P < 0.001). Within stage 4B, the three combinations of T4b/N3/M0, T3/N3/M0, and T4a/N3/M0 had higher hazards of mortality as compared to T1/N3/M0, with HRs (3.736 [1.726–8.088], P < 0.001), (2.571 [1.248–5.297], P = 0.010), and (2.372 [1.129–4.985], P = 0.023), respectively (Supplementary Table 3).
Except for gender, all factors indicated variable degrees of significant association with HC death in both all-cause and cancer-specific mortality. Advanced ages increased significantly predicted mortality (hazard ratios [95% confidence interval], P value) (HRs [95% CI], P) (HRs 1.023, 95% CIs [1.019–1.028], P < 0.001). Patients in black races had a significant association with mortality as compared to whites with HRs (1.341 [1.206–1.492], P < 0.001) (Supplementary Fig. 2). No significant differences in survival were noted regarding gender. As compared to married HC patients, all patients who were divorced, single, and widowed had significantly higher mortality compared to married HC patients (Table 2).
Nomogram prediction model’s results and its validation
A total of 3639 patients with non-metastatic (M0) HC were eligible for final analysis. We assigned 2547 (70%) patients to the training set and 1092 (30%) to the validation set. The demographic, clinicopathologic, and therapeutic features of patients in the training and validation sets are summarized in Supplementary Table 4.
Using the data from the training set, we identified some independent prognostic factors which were used to build the model. Variables that were considered significant in the univariable Cox analysis were entered into the multivariable analysis: age, race, primary site, grade, T, N, surgery, radiation, and chemotherapy, and furtherly incorporated into the nomogram (Supplementary Table 4).
Using the previously mentioned variables, scores were created on the point scale. By adding up the total score to get the total point scale, we could draw a straight line down to determine the estimated 1-year and 5-year OS probability (Fig. 3).
The calibration plot, based on the data from the training and validation sets (Supplementary Fig. 5A-C) for the probability of 5-year OS, indicated a good agreement between the predicted nomogram and actual observation. The nomogram was found to have a C-index of 0.696 compared to the 6th AJCC-TNM classification’s score of 0.587 (P < 0.01) for predicting the 5-year OS. Regarding the validation set, the C-index was 0.687 in comparison to 0.609 of the AJCC-TNM classification (P < 0.01). The nomogram revealed prognostic accuracy with ROC-AUC at 0.690 and 0.698 in the training and validation sets, respectively (Supplementary Figure 5B-D).
As regards stratifying the risk of patients, MST was 11 months (95% CI, 10 to 13 months), 31 months (95% CI, 26 to 40 months), 56 months (95% CI, 41 to 83 months), and 120 months (95% CI, 96 to 150 months) based on quartiles of the nomogram’s total points (lowest to 114, 114 to 124, 124 to 134, 134 to highest), respectively, with significant difference among groups (P value < 0.0001) (Supplementary Fig. 6).
Discussion
Our population had a 5-year OS rate of 25–35%, which is comparable to results of other reports where OS rates ranged from 10 to 60% [19, 20]. Another study reported survival rates staging from I to IV to be 100%, 66.6%, 53.9%, and 33.3%, respectively [21]. Such low survival rates in our analysis could be related to late presentation with loco-regionally advanced disease and high number of patients having nodal metastasis at the time of diagnosis, which goes in line with previously published literature [19, 20]. Although optimal treatment for early HC-SCC has not yet been established, treatment options have included surgery and radiotherapy with or without chemotherapy.
Radiotherapy and surgery may have been the treatment of choice in terms of functional preservation. Surgery alone was reserved for early disease, while surgery followed by radiotherapy is the treatment of choice in HCs when there was cartilage involvement or when there is an external spread of the disease (T4a). Kim et al. found that the combination of surgery and postoperative radiotherapy was superior to radiotherapy alone in terms of both overall and disease-free survival rates [22]. The results of a phase III clinical study by the Radiation Therapy Oncology Group showed that postoperative radiation provides better loco-regional control over preoperative radiotherapy; however, absolute survival did not change between both arms [23]. Surprisingly, after 2 years, distant metastases and secondary primaries became the main failure pattern, especially in the postoperative radiotherapy arm, which leads to an increase in the number of unrelated deaths.
Therefore, we investigated other treatment options. Interestingly, in our study, we found that radiotherapy (combined pre- and postoperative radiotherapy), when given in conjunction with surgery, showed superiority over preoperative radiotherapy alone as regards 1-year and 5-year survival rates. Even though two previous SEER reports on treatment options in HC lacked data related to chemotherapy [24, 25], we investigated the clinical significance of adding chemotherapy to the treatment regimen and slightly better survival rates were observed in the chemotherapy + surgery+ radiotherapy arm than in the surgery and radiotherapy arm. The literature shows quite contradictory findings in terms of survival, where Pingree et al. [26] found that surgery alone resulted in better survival than the combination of surgery and radiotherapy findings, while another study reported high survival rates in favor of combined therapy [27]. This is probably due to the recruitment of cases with better intrinsic prognosis. The impact of chemotherapy on overall survival remains debatable, where some studies revealed better outcomes when it is combined with surgery and radiotherapy than without surgery [28, 29]. This goes in line with our results, which showed that surgery combined with radio- and chemotherapy resulted in a very good 1-year survival rate of nearly 80% and 5-year survival rate of nearly 30–40%. A similar observation was noted when chemotherapy is given first followed by preoperative radiotherapy and finally surgery [30].
In concordance with other studies, we demonstrated no significant difference based on gender [31, 32].
Moreover, our analysis indicated that marriage was associated with better survival accounting for a protective impact, which was documented in many cancer types [33, 34].
Our results also revealed that the black race poses a higher risk of mortality compared to other races. The higher HRs of ACM in blacks were hypothesized to be strongly associated with comorbidity as a major contributing factor, and second primary malignancy could have played a role [35]. Carvahal suggested that blacks tended to have a higher risk of death based on the complexity related to their health access and social determinants [36].
Nomograms, considered as a graphical representation of prediction models, allow oncologists to combine various prognosticators to be able to predict prognosis and assess the risk of death with more precision [37, 38]. Lately, a nomogram with a validated high accuracy has been developed to help oncologists predict the site of origin of SCC confined to cervical LNs according to several predictors [39]. In the new era, nomograms have been widely used as prognostic models, where it enables the integration of personalized medicine into clinical practice and individualized prediction of clinical events [37, 40].
The nomogram that we have developed to predict 5-year survival rates of patients who were diagnosed with non-metastatic HC was validated showing good discrimination and predictive ability with a good C-index of 0.696. It is user-friendly, not only for oncologists but also for patients and their families. So basically, the larger the C-index (as you move to the right), the better the prognosis. Oncologists could use it to predict the survival rate based on given datasets of variables. It could be interpreted backward by patients to decide the most appropriate treatment based on the prognosticators presented in the nomogram. So, a black patient < 60 years of age with localized HC in the Pc area and a well-differentiated tumor with T1 N0 stage, who underwent the combined therapy of surgery, radiotherapy, and chemotherapy or surgery with radiotherapy, gets a total of 100 nomogram points and his/her 5-year survival probability is calculated to be 0.80. It is noteworthy that patients who have a bad prognosis according to this predictive model are not necessarily unfit for surgery and radiation. Our nomogram was validated internally with AUC (0.694), where these results reflect good concordance and favorable goodness of fit [41].
Our study has a few limitations, SEER is based on individual physician reporting and does not report comorbidity data. Even though it reports data representing national patterns of cancer care, having a large population base, the data is also subjected to inconsistencies. Finally, our model might lack the presence of other prognostic variables that were not present in the SEER database such as smoking but it showed good fitness according to the present prognostic variables. Despite these limitations, we were keen to concentrate on the following points, which helped us to improve our results.
Our nomogram exhibited superiority over TNM classification in both training and validation groups (P < 0.01). The SEER database is comprised of 18 registries covering many hospitals and nearly 30% of the total population across the USA, so heterogeneity of the data allowed our model to be broadly used for decision-making in clinical practice by both oncologists and patients. No previous SEER study combined all clinical prognosticators especially chemotherapy—which affects treatment—and analyzed them to predict OS, ACM, and CSM. Also, previous SEER studies did not incorporate or detect the prognosis of specific TNM staging subdivisions that are referenced in the American Cancer Society site. The last two SEER studies conducted on HC included only patients till 2003 and 2008; however, in our case, we gained access to patients’ data in the SEER database till 2015. This has helped us include all updated cutoff points—dead/alive—of patients, which made our results more dependable, more reliable, and more valid.
Conclusions
Patients with hypopharyngeal carcinoma, older age, and primary site in the posterior wall of the hypopharynx resulted in worse survival compared to younger age and pyriform sinus. The combination of surgery and radiotherapy (pre- and post-surgery) was associated with improved survival compared to pre-surgical radiation alone. Our validated nomogram exhibits a good degree of applicability and accuracy, making it highly effective in assisting clinicians in making more precise survival evaluations based on available treatment modalities.
Availability of data and materials
Data will be available upon reasonable request.
Abbreviations
- NOS:
-
Not otherwise specified
- SD:
-
Standard deviation
- IQR:
-
Interquartile range
- AJCC:
-
American Joint Committee on Cancer
- Ps:
-
Pyriform sinus
- Pc:
-
Post-cricoid
- Af:
-
Aryepiglottic fold
- Ph:
-
Posterior wall of the hypopharynx
- Ol:
-
Overlapping lesions
- HC:
-
Hypopharyngeal carcinoma
- SEER:
-
Surveillance, Epidemiology, and End Results Program
- OS:
-
Overall survival
- SCC:
-
Squamous cell carcinoma
- USA:
-
United States of America
- TRIPOD:
-
Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the curve
- MST:
-
Median survival time
References
Carvalho AL, Nishimoto IN, Califano JA, Kowalski LP (2005) Trends in incidence and prognosis for head and neck cancer in the United States: a site-specific analysis of the SEER database. Int J Cancer 114(5):806–816
Bova R, Goh R, Poulson M, Coman WB (2005) Total pharyngolaryngectomy for squamous cell carcinoma of the hypopharynx: a review. Laryngoscope 115(5):864–869
Kajanti M, Mauntylau M (1990) Carcinoma of the hypopharynx a retrospective analysis of the treatment results over a 25-year period. Acta Oncol 29(7):903–907
Curado MP, Hashibe M (2009) Recent changes in the epidemiology of head and neck cancer. Curr Opin Oncol 21(3):194–200
Ligier K, Belot A, Launoy G, Velten M, Bossard N, Iwaz J et al (2011) Descriptive epidemiology of upper aerodigestive tract cancers in France: incidence over 1980–2005 and projection to 2010. Oral Oncol 47(4):302–307
Petersen JF, Timmermans AJ, van Dijk BAC, Overbeek LIH, Smit LA, Hilgers FJM et al (2018) Trends in treatment, incidence and survival of hypopharynx cancer: a 20-year population-based study in the Netherlands. Eur Arch Otorhinolaryngol 275(1):181–189
Nouraei SA, Dias A, Kanona H, Vokes D, O'Flynn P, Clarke PM et al (2017) Impact of the method and success of pharyngeal reconstruction on the outcome of treating laryngeal and hypopharyngeal cancers with pharyngolaryngectomy: a national analysis. J Plast Reconstr Aesthet Surg 70(5):628–638
Rennemo E, Zätterström U, Boysen M (2008) Impact of second primary tumors on survival in head and neck cancer: an analysis of 2,063 cases. Laryngoscope 118(8):1350–1356
Hoffman HT, Karnell LH, Shah JP, Ariyan S, Brown GS, Fee WE et al (1997) Hypopharyngeal cancer patient care evaluation. Laryngoscope 107(8):1005–1017
Sun W, Jiang YZ, Liu YR, Ma D, Shao ZM (2016) Nomograms to estimate long-term overall survival and breast cancer-specific survival of patients with luminal breast cancer. Oncotarget. 7(15):20496–20506
Collins GS, Reitsma JB, Altman DG, Moons KG (2015) Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMC Med 13(1):1
Stekhoven DJ, Bühlmann P (2012) MissForest--non-parametric missing value imputation for mixed-type data. Bioinformatics (Oxford, England) 28(1):112–118. https://doi.org/10.1093/bioinformatics/btr597.
Nie NH, Bent DH, Hull CH (1970) SPSS: statistical package for the social sciences. McGraw-Hill New York
Therneau T (2022) A package for survival analysis in R. R package version 3.3-1. https://CRAN.R-project.org/package=survival
Therneau TM, Grambsch PM (2000) Modeling survival data: extending the cox model. Springer, New York. ISBN 0-387-98784-3
Jr FEH. rms: regression modeling strategies. 5.1-2 ed. 2018. https://cran.r-project.org/web/packages/rms/index.html
Heagerty PJ, Saha-Chaudhuri pbP. survivalROC: time-dependent ROC curve estimation from censored survival data. 1.0.3 ed. 2013. http://www2.uaem.mx/r-mirror/web/packages/survivalROC/survivalROC.pdf
Liang W, Shen G, Zhang Y, Chen G, Wu X, Li Y et al (2016) Development and validation of a nomogram for predicting the survival of patients with non-metastatic nasopharyngeal carcinoma after curative treatment. Chinese J Cancer 35(1):98
Arriagada R, Eschwege F, Cachin Y, Richard J (1983) The value of combining radiotherapy with surgery in the treatment of hypopharyngeal and laryngeal cancers. Cancer. 51(10):1819–1825
Featherstone CJ, Clarke S, Jackson MA, Shannon KF, McNeil EB, Tin MM et al (2004) Treatment of advanced cancer of the larynx and hypopharynx with chemoradiation. ANZ J Surg 74(7):554–558
Milisavljevic D, Stankovic M, Zivic M, Popovic M, Radovanović Z (2009) Factors affecting results of treatment of hypopharyngeal carcinoma. Hippokratia. 13(3):154–160
Kim S, Wu HG, Heo DS, Kim KH, Sung MW, Park CI (2001) Advanced hypopharyngeal carcinoma treatment results according to treatment modalities. Head Neck 23(9):713–717
Tupchong L, Phil D, Scott CB, Blitzer PH, Marcial VA, Lowry LD et al (1991) Randomized study of preoperative versus postoperative radiation therapy in advanced head and neck carcinoma: long-term follow-up of RTOG study 73-03. Int J Radiat Oncol Biol Phys 20(1):21–28
Kuo P, Chen MM, Decker RH, Yarbrough WG, Judson BL (2014) Hypopharyngeal cancer incidence, treatment, and survival: temporal trends in the United States. Laryngoscope. 124(9):2064–2069
Newman JR, Connolly TM, Illing EA, Kilgore ML, Locher JL, Carroll WR (2015) Survival trends in hypopharyngeal cancer: a population-based review. Laryngoscope, 125(3):624–9. https://doi.org/10.1002/lary.24915
Pingree TF, Davis RK, Reichman O, Derrick L (1987) Treatment of hypopharyngeal carcinoma: a 10-year review of 1, 362 cases. Laryngoscope 97(8):901–904
Carifi VG, Ohanion ML, David AB, Greenlaw R, Rush BF (1974) Results of high dose radiation and surgery in the treatment of advanced cancer of the head and neck. Am J Surg 128(4):580–582
Gupta T, Chopra S, Agarwal JP, Laskar SG, D'Cruz AK, Shrivastava SK et al (2009) Squamous cell carcinoma of the hypopharynx: single-institution outcome analysis of a large cohort of patients treated with primary non-surgical approaches. Acta Oncol 48(4):541–548
Persky MS, Daly JF (1981) Combined therapy vs curative radiation in the treatment of pyriform sinus carcinoma. Otolaryngol Head Neck Surg 89(1):87–91
Higuchi E, Iizuka K, Shoda H, Takeichi N (1996) Outcome of hypopharyngeal cancer. Nihon Jibiinkoka Gakkai Kaiho 99(9):1185–1189
Roberts JC, Li G, Reitzel LR, Wei Q, Sturgis EM (2010) No evidence of sex-related survival disparities among head and neck cancer patients receiving similar multidisciplinary care: a matched-pair analysis. Clin Cancer Res 16(20):5019–27. https://doi.org/10.1158/1078-0432.CCR-10-0755
Dikshit RP, Boffetta P, Bouchardy C, Merletti F, Crosignani P, Cuchi T et al (2005) Lifestyle habits as prognostic factors in survival of laryngeal and hypopharyngeal cancer: a multicentric European study. Int J Cancer 117(6):992–995
He X-K, Lin Z-H, Qian Y, Xia D, Jin P, Sun L-M (2017) Marital status and survival in patients with primary liver cancer. Oncotarget. 8(39):64954
Inverso G, Mahal BA, Aizer AA, Donoff RB, Chau NG, Haddad RI (2015) Marital status and head and neck cancer outcomes. Cancer. 121(8):1273–1278
Zakeri K, MacEwan I, Vazirnia A, Cohen EE, Spiotto MT, Haraf DJ et al (2014) Race and competing mortality in advanced head and neck cancer. Oral Oncol 50(1):40–44
Carvalho AL (2017) Racial/ethnic differences in head and neck cancer survival rates
Balachandran VP, Gonen M, Smith JJ, DeMatteo RP (2015) Nomograms in oncology: more than meets the eye. Lancet Oncol 16(4):e173–e180
Shariat SF, Karakiewicz PI, Suardi N, Kattan MW (2008) Comparison of nomograms with other methods for predicting outcomes in prostate cancer: a critical analysis of the literature. Clin Cancer Res 14(14):4400–4407
Ali AN, Switchenko JM, Kim S, Kowalski J, El-Deiry MW, Beitler JJ (2014) A model and nomogram to predict tumor site origin for squamous cell cancer confined to cervical lymph nodes. Cancer. 120(22):3469–3476
Low SK, Zayan AH, Istanbuly O, Minh Duc NT, Ebied A, Mohamed Tawfik G et al (2019) Prognostic factors and nomogram for survival prediction in patients with primary pulmonary lymphoma: a SEER population-based study. Leuk Lymphoma:1–11
Hosmer DW Jr, Lemeshow S, Sturdivant RX (2013) Applied logistic regression. Wiley
Acknowledgements
Thanks to the Surveillance, Epidemiology, and End Results Program (SEER) for granting us access to the detailed patient data of the SEER register, which without it we would not have been made this original research [10].
Funding
None.
Author information
Authors and Affiliations
Contributions
GMT and AGS were responsible for idea under supervision of NTH. The screening was done by GMT, AA, NLV, AHZ, TMT, TKS, and AE under the supervision of NTH and AGS. Data analysis interpretation and nomogram predictive model were done by GMT, NLV, and AHZ. Tables and figures were done by GMT, NLV, and AHZ. GMT, AA, NLZ, AHZ, TMT, TKS, AE, AGS, and NTH contributed to the manuscript writing and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Surveillance, Epidemiology, and End Results Program (SEER) database retrieved and granted us access to the detailed patient data of the SEER register.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary data.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tawfik, G.M., Vuong, N.L., Zayan, A.H. et al. Prognostic factors impacting survival rates of hypopharyngeal cancer with nomogram prediction: a SEER-based study. Egypt J Otolaryngol 38, 51 (2022). https://doi.org/10.1186/s43163-022-00215-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43163-022-00215-z