Abstract
Introduction
The transformation of acute myeloid leukemia with translocation (16;16) (p13; q22) from AML M2 to acute monocytic leukemia (AML M5) during therapy is a rare clinical occurrence, and this is the first time it has been reported.
Clinical complain
A 19-year-old male patient was admitted for severe fatigue with anemic manifestation and weight loss, for more than 1 month, with exacerbation of the condition in the last 2 days.
Diagnosis
A primary diagnosis was made for AML M2 with t (16;16) (p13; q22) established on bone marrow (BM) morphology. A consequential detection of FLT-3 ITD mutation was done. At day 28 follow-up after induction and maintenance therapy, the diagnosis of AML M2 was maintained with a high bone marrow (BM) blast count, prompting the initiation of a more aggressive treatment protocol. After 1 month of implementing the recent protocol, the patient remains morphologically resistant with a notable transformation of bone marrow infiltration by an abnormal monocytic population (monoblasts and promonocytes). The final diagnosis of transforming FLT3-mutated AML with t (16;16) (p13; q22) was established.
Intervention
After the initial diagnosis of AML M2 with t (16;16) (p13; q22), the patient received the 3 + 7 induction protocol. The 2nd induction protocol initiated after the second evaluation and morphological resistance was the FLAG Adrian protocol. The 3rd protocol after transformation to AML M5 was 1 cycle of the MEC protocol. Anti-FLT3 treatment was considered.
Outcomes
The patient was maintained on the 3rd protocol of chemotherapy. Unfortunately, he was admitted to the ICU unit complaining of neutropenic fever and severe sepsis where he died before final re-evaluation and the anti-FLT3 treatment initiation.
Conclusion
AML with t (16;16) (p13; q22) characterized by favorable outcome. However, identifying additional chromosome abnormality or genetic aberration, especially FLT3 gene mutation, is recognized as an important factor influencing final disease outcome. Therefore, early detection of FLT3 mutations will allow comprehensive disease course prediction and targeted therapy that might achieve longer and more durable remissions.
Similar content being viewed by others
Introduction
One of the most common recurrent chromosomal abnormalities of AML is t (16;16) (p13; q22) which is commonly presented with the M4Eo subtype of AML with fewer cases reported in the AML M2, M5 subtypes, and in cases with myelodysplastic syndrome (MDS) with higher risk features with favorable outcome [1]. When treated with high-dose cytarabine, it achieves a high rate of complete remission (CR) and favorable overall survival (OS) [2]. However, additional chromosome abnormality or genic aberration, especially FLT3 gene mutations, were identified as an important factor influencing final disease outcome [3]. According to recently published studies, a scarcity of AML subtypes can submit to myeloid subtype transformation during chemotherapy or develop secondary therapy-related leukemia, with subsequential unresponsiveness to chemotherapy with an unfavorable prognosis [4]. Therefore, proper clinical diagnosis and treatment of AML with t (16;16) (p13; q22) remain challenging. Here, we will study a case of AML with t (16;16) (p13; q22) and FLT-3 mutation transformed during induction and maintenance therapy including a review of the current literature.
Case report
A 19-year-old male with no former history of exposure to industrial toxins or radioactive substances. On June 5, 2023, he was admitted for severe fatigue with anemic manifestation for 10 days, with exaggeration of symptoms in the last 2 days. On primary clinical inspection, the patient appeared pale with no other physical abnormality or hepatosplenomegaly.
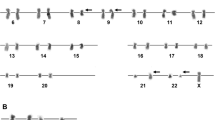
The findings of the initial CBC analysis were as follows: WBC count, 34.19 × 109/L; Hb concentration, 6.9 g/dL; platelet count, 22 × 109/L; abnormal eosinophils with both eosinophilic and basophilic granules (Eos-basophils) were 10%; and immature cells were 70%. BM examination revealed blast cells (89.2%) with ovoid-shaped nuclei, visible nucleoli, and scanty amounts of blue finely granular cytoplasm with a population of 30% abnormal Eos-basophils. In addition, erythropoiesis was inhibited, and megakaryopoiesis was absent with no platelets seen (Fig. 1). FCM of the BM sample revealed the presence of blast cells that were positive for CD34, HLA-DR, CD117, cMPO, CD13, CD33, CD56, and CD123, but negative for B-cell markers (CD19, CD20), T-cell (CD2,3, 5) and monocytic markers (CD14, CD64, CD11c), another population of granulocytic precursors (mostly abnormal Eos-basophils) with brighter CD45 expression were positive for CD13/CD33, CD11c, CD123 and negative for CD 14, CD117, CD34. Karyotyping revealed a male karyotype with an abnormal clone expressing 46, XY, t (16;16). FISH on BM showed negativity for t (8;21) (q22; q22) and t (9;22) (q34; q11). Molecular detection of FLT3-ITD by PCR was done and showed positivity.
Established on former findings, the patient was diagnosed with FLT3-mutated AML M2 with t (16;16) (p13; q22),
After the initial diagnosis of AML M2 with t (16;16) (p13; q22), the patient received the 3 + 7 protocol (3 days adriamycin 50 mg/m2 and 7 days ARAC 100 mg/m2/12 h).
On July 10, 2023, the patient was sent back for his first re-evaluation after day 28 of starting therapy; CBC analysis was as follows: WBCs count, 119.68 × 109/L; Hb concentration, 6.9 g/dL; platelet count, 15 × 109/L; blast cells were 75%; and no abnormal eosinophils. BM examination revealed blast cells (85%) exhibiting similar morphology as the initial ones, and abnormal eosinophils were scarce (Fig. 2). FCM analysis of the BM sample demonstrated the presence of blast cells with positivity for CD34, HLA-DR, CD117, cMPO, CD13, and CD33 with additional CD64 but negative CD56 and CD123 and aberrant display of CD5.
These findings were consistent with a resistance case of FLT3-mutated AML M2 with t (16;16) (p13; q22), with aberrant expression of CD5.
The 2nd induction protocol initiated after the second evaluation was the FLAG Adrian (fludarabine 30 mg/m2/day for 5 days, ARAC 2 gr/m2/day for 5 days, adriamycin 25 mg/m2/day instead of zavidose for 3 days and G-CSF starting from day 1).
On August 13, 2023, a second re-evaluation was done; CBC analysis was as follows: WBCs count, 6.35 × 109/L; Hb concentration, 10.5 g/dL; platelet count, 35 × 109/L; and the abnormal monocytic population was present (52%) of which 12% were immature cells. BM evaluation revealed infiltration by 80% atypical monocytic population (monoblast, promonocytes, and atypical monocytes) with a convoluted nucleus, finely dispersed chromatin, visible nucleoli, and abundant amounts of blue finely granular vacuolated cytoplasm (Fig. 3). FMC analysis of the BM sample showed a monocytic population in different stages of maturation (promonocytes and monocytes) that were positive for CD34, HLA-DR, cMPO, CD 14, CD64, CD36, CD11C, CD13, and CD33 consistent with acute monocytic leukemia (AML M5).
Based on these data, the patient was diagnosed as a resistant case of FLT3-mutated AML with t (16;16) (p13; q22) in morphological and phenotypic transformation from AML M2 to AML M5.
Finally, a third protocol, one cycle of the MEC (mitoxantrone 8 mg/m2/day, etoposide 100 mg/m2/day, and cytarabine 1 g/m2/day from day 1 to day 6) was started. Combination therapy with anti-FLT3 treatment was considered.
Unfortunately, on September 30, 2023, the patient was admitted to the ICU unit complaining of neutropenic fever and severe sepsis where he died before final re-evaluation and the anti-FLT3 treatment initiation.
Discussion
It is rare for AML with t (16;16) (p13; q22) to submit to subtype transformation during therapy, and there is no obvious explanation to prove an underlying mechanism. The outcome for treatment-related secondary myeloid leukemias is often poor; nevertheless, the pathophysiology of these high-risk group leukemia remains debatable; 3 main hypotheses have been postulated:
-
1-
Chemotherapeutic agents with cytotoxic properties such as topoisomerase inhibitors, alkalinizing agents, and anthracyclines lead to treatment-related MDS or AML since they act by alternating the primary and secondary DNA structure [4].
-
2-
Occurrence of t (16;16) (p13; q22) in myeloblast, since it can be presented as AML M4, M2, or M5, it may affect multiple clones in the initial phase of the disease, but obscured by the presiding one. After the application of chemotherapy and elimination of the dominant clone (myeloblast), the other under-expressed clones (monoblast and promyelocytes) have a better opportunity for proliferation and progression [5].
-
3-
The primitive leukemic cells’ inherited plasticity can lineate the potential for reprogramming under lineage-specific pressure [4], for instance, the presence of specific mutations such as FLT-3 ITD which induces responsiveness to therapy and force leukemic cell proliferation [6]. Accumulating evidence has proved that FLT3 mutational status changes along with the disease course. This mutational evolution, besides identifying FLT3-ITD as a poor prognostic indicator, spotlights the need to identify FLT3-ITD status at diagnosis and relapse [7].
In our case, it was troublesome to differentiate between therapy-related secondary myeloid leukemia occurrence and recurrence of AML with t (16;16) (p13; q22) with a series of transitions. However, accurate initial genetic and molecular diagnosis for cases of AML especially earlier detection of FLT3 mutation status will help provide a convenient prediction of the patient’s disease course and might enable targeted treatment or combination therapies that may help patients with FLT3-mutated AML achieve longer and more durable remissions.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AML:
-
Acute myeloid leukemia
- BM:
-
Bone marrow
- IPT:
-
Immunophenotyping
- FMC:
-
Flow cytometry
- CBC:
-
Complete blood picture
- FISH:
-
Fluorescence in situ hybridization
- FLT3-ITD:
-
FMS-like tyrosine kinase-3 internal tandem duplication
- CR:
-
Complete remission
- OS:
-
Overall survival
References:
Yang JJ, Park TS, Wan TS (2017) Recurrent cytogenetic abnormalities in acute myeloid leukemia. Cancer Cytogenet Methods Protoc 1541:223–245
Lv L, Yu J, Qi Z (2020) Acute myeloid leukemia with inv (16) (p13. 1q22) and deletion of the 5’MYH11/3’CBFB gene fusion: a report of two cases and literature review. Mol Cytogenet 13:1–6
Eghtedar A, Borthakur G, Ravandi F, Jabbour E, Cortes J, Pierce S, ... & Garcia-Manero G (2012) Translocation characteristics (16; 16) (p13; q22) acute myeloid leukemia. Am J Hematol 87(3)317
Sun Y, Wang C, Sun Y, Wang J, Rong C, Wu A, ... & Sheng L (2021) Transformation from acute promyelocytic leukemia to acute myeloid leukemia with a CEBPA double mutation: a case report and review of the literature. Medicine 100(5):e24385
Quessada J, Cuccuini W, Saultier P, Loosveld M, Harrison CJ, Lafage-Pochitaloff M (2021) Cytogenetics of pediatric acute myeloid leukemia: a review of the current knowledge. Genes 12(6):924
Kiyoi H, Kawashima N, Ishikawa Y (2020) FLT3 mutations in acute myeloid leukemia: therapeutic paradigm beyond inhibitor development. Cancer Sci 111(2):312–322
Daver N, Schlenk RF, Russell NH, Levis MJ (2019) Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia 33(2):299–312
Acknowledgements
The authors thank the patient who volunteered and agreed to participate in this study. The authors thank their professors and colleague in Hematology subunits of Clinical pathology and Internal medicine departments for their excellent technical support. This work was carried out within the framework of the diagnostic network for “Hematological malignancies” in Ain Shams University Hospitals, Cairo, Egypt.
Funding
This work was supported by personal funding.
Author information
Authors and Affiliations
Contributions
S.Z. conceived and designed the case report. S.Z. and H.H. were responsible for data curation and methodology. S.Z. preformed final data analysis and interpretation. Writing manuscript done by S.Z. Review and editing manuscript were done by S.Z. and H.H. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics committee of Ain Shams University; written informed consent was obtained from the patient's relatives for publication of this report and any accompanying images.
Consent for publication
Written informed consent was obtained from the patient's relatives for publication of this report and any accompanying images.
Competing interests
The authors have no conflict of interest to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zaiema, S.E.G.E., Hafez, H.M. Unpredicted transformation of acute myeloid leukemia with translocation (16;16) (p13; q22): a case report and review of the literature. Egypt J Intern Med 36, 28 (2024). https://doi.org/10.1186/s43162-024-00295-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43162-024-00295-8