Abstract
Diabetes mellitus is a common metabolic disorder characterized by chronic hyperglycemia and disturbance of carbohydrate, fats, and protein metabolism. Type 2 diabetes mellitus results from reduced insulin secretion, decreased glucose utilization, and increased glucose production, which results in hyperglycemia. Hypertension further increases the risk of cardiovascular diseases, including coronary heart disease (CHD), congestive heart failure (CHF), ischemic and hemorrhagic stroke, renal failure, and peripheral arterial disease (PAD). Angiotensin receptor blockers (ARBs) are very effective antihypertensive drugs. This study was done to find the effects of two different angiotensin receptor blockers on various biochemical markers in type-2 diabetes mellitus patients.
Methods
This was a prospective interventional study, comparing two ARBs Azilsartan and telmisartan, involving 76 patients with type 2 diabetes mellitus and hypertension.
Results
Both drugs controlled blood pressure equally. The study showed that improvement in fasting plasma glucose was more with Azilsartan as compared to Telmisartan but their mean difference is not statistically significant (p > 0.05). The improvement in post-prandial plasma glucose and HbA1C was more with Telmisartan as compared to Azilsartan but only mean HbA1C was statistically significant (p < 0.05).
Conclusions
Telmisartan has a better impact on HbA1c reduction than Azilsartan, as a part of the pleotropic effect of ARBs.
Similar content being viewed by others
Introduction
Diabetes mellitus is a common metabolic disorder described by chronic hyperglycemia and disturbance of carbohydrate, fats, and protein metabolism. Type 2 diabetes mellitus results from reduced insulin secretion, decreased glucose utilization, and increased glucose production, which results in hyperglycemia [1]. It is characterized by defects in insulin secretion, action, or both. The International Diabetes Federation (IDF) reported that at present there are 415 million people worldwide affected by T2DM, aged between 20 and 79 years old, the global prevalence being 8.8% and it is anticipated that in 2040 their number will grow up to 642 million with a prevalence of 10.4% [2].
Hypertension is one of the leading causes of the global burden of disease. Raised blood pressure affects more than one billion individuals and causes an estimated 9.4 million deaths per year as per The World Heart Federation. Hypertension further increases the risk of cardiovascular diseases, including coronary heart disease (CHD), congestive heart failure (CHF), ischemic stroke, hemorrhagic stroke, renal failure, and peripheral arterial disease (PAD) [3]. Antihypertensive therapy reduces the risks of cardiovascular and renal disease, but a large proportion of the hypertensive population is either untreated or inadequately treated.
Angiotensin receptor blockers (ARBs) are very efficient antihypertensive drugs. ARBs exert their effect through blockage of the type 1 angiotensin II receptor and quite possibly through stimulation by the type 2 angiotensin II receptor [4]. ARBs have been found to be beneficial in the treatment of heart failure and diabetic nephropathy, besides hypertension [5]. ARBs have appeared as being very effective and perhaps superior to other antihypertensive drugs in the prevention of de novo or recurrent stroke [6]. Their stroke-protective effects include their anti-atherogenic, anti-diabetic, anti-platelet aggregating, hypouricemic, and atrial anti-fibrillatory actions. All these actions make the ARBs a true pleotropic class of drugs. There had been a large no of studies in Western literature which have established the pleotropic effect of angiotensin receptor blockers, but very few studies have looked into the new angiotensin receptor blockers like Azilsartan [7, 8].
There is no head-to-head comparison between telmisartan and Azilsartan, to look at their pleotropic effect on diabetes; hence, an attempt was made to compare the pleotropic effect of these two drugs.
Methodology
This study was a prospective, interventional, open-labelled hospital-based study. This study was conducted from November 2017 to July 2019 after getting approval from the Institutional Ethics Committee, J. N. Medical College, and patients were enrolled after getting informed consent. A detailed history and examination were carried out for every patient who entered in study as per a pre-designed proforma.
A total of 62 subjects were recruited and admitted in the wards of the Department of Medicine, and Rajiv Gandhi Center of Diabetes and Endocrinology, J.N. Medical College, AMU. Our inclusion criteria were as follows:
-
A.
Criteria for diagnosing diabetes mellitus: Fasting glucose levels of ≥ 126 mg/dl (7.0 mmol/l) or 2-h PG ≥ 200 mg/dl (11.1 mmol/l) during OGTT (75 g) or random blood glucose ≥ 200 mg/dl or HbA1c ≥ 6.5% (48 mmol/mol) or current treatment with hypoglycemic therapy.
-
B.
Hypertension: SBP/DBP > 140/ 90 mm of Hg
Inclusive criteria were age > 18 years, diagnosed case of type 2 diabetes
Mellitus with hypertension, all patients were on equal dose statin, and equal doses of oral hypoglycemic agents. Exclusion criteria were age < 18 years and patients with unstable angina, secondary hypertension, renal insufficiency/renal artery stenosis, and chronic kidney disease.
The drugs were administered in the recommended doses: Telmisartan 80 mg once a day and Azilsartan 40 mg once a day. Parametric variables were analyzed by Student’s t test, non-parametric variables were analyzed by chi-square test. The correlation was analyzed by regression analysis. Complete blood count (CBC), renal function test and serum electrolyte, blood sugar—fasting and post-prandial, HbA1c, lipid profile (total serum cholesterol, triglycerides, high-density lipoprotein, low-density lipoprotein, very low-density lipoprotein) was done in all patients.
The patients were divided into two groups: Group 1 patients on telmisartan and group 2 patients on Azilsartan.
Results
The basic parameters of 62 patients are summarized in Table 1. The mean HbA1c difference (post-treatment - pretreatment) in the Telmisartan group was 1.522%, while in Azilsartan group it was 0.212%. The mean HbA1c difference between the telmisartan and Azilsartan groups was 1.309 and is statistically significant p = 0.000, which is summarized in Table 2.
The mean fasting blood sugar difference (post-treatment-pretreatment) in the telmisartan group was 30.290, while in Azilsartan, it was 15.032. The impact on fasting blood sugar was more with telmisartan in comparison to Azilsartan. The difference between Telmisartan and Azilsartan is 15.258, which is statistically not significant P = 0.391.
The mean post-prandial blood sugar difference (post-treatment-pretreatment) in Telmisartan is 49.548, and this difference is statistically significant P = 0.000. The mean blood sugar (PP) difference in Azilsartan is 26.838, which is statistically significant with P = 0.004. The impact on postprandial blood sugar is more with telmisartan in comparison to Azilsartan. The mean blood sugar (PP) difference between the two study groups is 22.70, and it is not statistically significant P = 0.115.
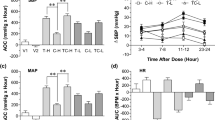
Blood pressure was controlled optimally in both groups. The difference between both the groups was not significant, which is shown in Fig. 1.
Discussion
This study revealed that blood sugar (fasting) improves at 6 months follow-up in both the treatment group, more in the Telmisartan group in comparison to the Azilsartan group. There is no significant difference in blood sugar (fasting) between these two drugs (Table 2). Similar results were observed in earlier studies. [6, 9,10,11,12] Post-prandial blood sugar improved in both Telmisartan and Azilsartan groups, more in the Telmisartan group (Table 2). HbA1C improves in both Telmisartan as well as Azilsartan after 6 months as compared to baseline. Improvement was more with Telmisartan (Table 2).
They all suggest the beneficial effects of Azilsartan and telmisartan in improving insulin sensitivity. In one of the animal studies, it was found that Azilsartan is very much effective in reducing insulin resistance [11]. One of the studies on diabetic KK-AY mice found that Azilsartan was superior to Candesartan in improving glucose intolerance, insulin sensitivity, and inducing adipocyte differentiation [6].
Telmisartan has been found to increase insulin sensitivity in non-diabetic patients with essential hypertension [13]. Telmisartan, an angiotensin II receptor antagonist, is also a partial PPARγ agonist. In male Sprague Dawley rats, fed a high-fat and high-carbohydrate diet for 3 months, telmisartan given along with the diet reduced weight gain as well as glucose, insulin, and triglyceride levels [7, 13]. Adiponectin has been shown to have insulin-sensitizing properties in murine models and studies have shown that some of the beneficial effects of peroxisome proliferator–activated receptor γ (PPAR γ) activation are mediated by the increase in adiponectin levels [14, 15].
Telmisartan, an angiotensin II type 1 (AT1) receptor antagonist, is a partial PPARγ nuclear receptor agonist [13] It is also established that telmisartan reduces insulin resistance in Hypertensive patients with metabolic syndrome [8] Telmisartan significantly reduces plasma glucose in a double-blind, parallel-group, randomized study on patients with metabolic syndrome [13]. Another study showed improvement in all the three indices of glucose and insulin metabolism like fasting plasma glucose, OGTT, and HbA1C [13]. Similar results were found in another study of telmisartan [14].
This study had a few limitations. The number of patients in our study is small, compared to various studies across the globe. Because our study was conducted at a single center, a tertiary care hospital, hence our results cannot be generalized to the general population, necessitating the need for further large-scale studies.
Conclusion
Telmisartan has a better impact on the glycemic profile in diabetic patients, in comparison to Azilsartan, as a part of its pleotropic effects.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CHD:
-
Coronary heart disease
- CHF:
-
Congestive heart failure
- PAD:
-
Peripheral arterial disease
- ARBs:
-
Angiotensin receptor blockers
- IDF:
-
International diabetes federation
- T2DM:
-
Type 2 diabetes mellitus
- OGTT:
-
Oral glucose tolerance test
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- CBC:
-
Complete blood count
- PPARℽ:
-
Peroxisome proliferator-activated receptor gamma
References
Diabetes mellitus: diagnosis, classification, and pathophysiology | Harrison’s Principles of Internal Medicine, 19e | AccessMedicine | McGraw Hill Medical. Available from: https://accessmedicine.mhmedical.com/content.aspx?bookid=1130§ionid=79752868. [Cited 2022 Oct 30]
Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH et al (2017) IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract 128:40–50. Available from: (https://pubmed.ncbi.nlm.nih.gov/28437734/) [Cited 2022 Oct 30].
Iwai M, Chen R, Imura Y, Horiuchi M (2007) TAK-536, a new AT1 receptor blocker, improves glucose intolerance and adipocyte differentiation. Am J Hypertens 20(5):579–586
Kajiya T, Ho C, Wang J, Vilardi R, Kurtz TW (2011) Molecular and cellular effects of azilsartan. J Hypertens 29(12):2476–2483
Kusumoto K, Igata H, Ojima M, Tsuboi A, Imanishi M, Yamaguchi F et al (2011) Antihypertensive, insulin-sensitising and renoprotective effects of a novel, potent and long-acting angiotensin II type 1 receptor blocker, azilsartan medoxomil, in rat and dog models. Eur J Pharmacol 669(1–3):84–93
Iwai M, Chen R, Imura Y, Horiuchi M (2007) TAK-536, a new AT1 receptor blocker, improves glucose intolerance and adipocyte differentiation. Am J Hypertens 20(5):579–86. Available from: (https://pubmed.ncbi.nlm.nih.gov/17485025/) [cited 2022 Oct 30]
Benson SC, Pershadsingh HA, Ho CI, Chittiboyina A, Desai P, Pravenec M et al (2004) Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARgamma-modulating activity. Hypertension 43(5):993–1002 Available from: (https://pubmed.ncbi.nlm.nih.gov/15007034/) [Cited 2022 Oct 30]
Derosa G, Cicero AFG, D’Angelo A, Ragonesi PD, Ciccarelli L, Piccinni MN et al (2006) Telmisartan and irbesartan therapy in type 2 diabetic patients treated with rosiglitazone: effects on insulin-resistance, leptin and tumor necrosis factor-alpha. Hypertens Res 29(11):849–56 Available from: (https://pubmed.ncbi.nlm.nih.gov/17345784/) [Cited 2022 Oct 30]
Kajiya T, Ho C, Wang J, Vilardi R, Kurtz TW (2011) Molecular and cellular effects of azilsartan: a new generation angiotensin II receptor blocker. J Hypertens 29(12):2476–83. Available from: (https://pubmed.ncbi.nlm.nih.gov/21986624/) [Cited 2022 Oct 30]
Kusumoto K, Igata H, Ojima M, Tsuboi A, Imanishi M, Yamaguchi F et al (2011) Antihypertensive, insulin-sensitising and renoprotective effects of a novel, potent and long-acting angiotensin II type 1 receptor blocker, azilsartan medoxomil, in rat and dog models. Eur J Pharmacol. 669(1–3):84–93 Available from: (https://pubmed.ncbi.nlm.nih.gov/21816148/) [Cited 2022 Oct 30]
用于治疗增殖性障碍的1h-吡唑并[3,4-b]吡啶衍生物及其药物组合物. 2014 Aug 18;
Tarikuz Zaman AKM, McLean DL, Sobel BE (2013) The efficacy and tolerability of azilsartan in obese insulin-resistant mice with left ventricular pressure overload. J Cardiovasc Pharmacol 62(4):381–7 Available from: (https://pubmed.ncbi.nlm.nih.gov/23921308/) [Cited 2022 Oct 30]
Abdelsaid M, Coucha M, Ergul A (2014) Cerebrovasculoprotective effects of azilsartan medoxomil in diabetes. Transl Res 164(5):424–432
Sharma AM, Staels B (2007) Review: Peroxisome proliferator-activated receptor gamma and adipose tissue–understanding obesity-related changes in regulation of lipid and glucose metabolism. J Clin Endocrinol Metab. 92(2):386–95 Available from: (https://pubmed.ncbi.nlm.nih.gov/17148564/) [Cited 2022 Oct 30]
Sharabi Y, Oron-Herman M, Kamari Y, Avni I, Peleg E, Shabtay Z et al (2007) Effect of PPAR-gamma agonist on adiponectin levels in the metabolic syndrome: lessons from the high fructose fed rat model. Am J Hypertens 20(2):206–10 Available from: (https://pubmed.ncbi.nlm.nih.gov/17261469/) [Cited 2022 Oct 30]
Acknowledgements
I am thankful to Dr. Hamid Ashraf and Dr. Mohd Aslam for helping me in complication and analysis of data.
Funding
None.
Author information
Authors and Affiliations
Contributions
SIF collected all the data and under the guidance of SFH and SSS. SIF and RA analyzed data and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was started after getting the ethical clearance from the institutional ethical committee of Jawaharlal Nehru Medical College, Aligarh Muslim University, Aligarh, UP, India. Proper consent was also obtained from every patient before enrolment in study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bhati, S.I., Haque, S.F., Siddiqi, S.S. et al. Effects of two different angiotensin receptor blockers on blood glucose level and HbA1c in type-2 diabetes mellitus patients with hypertension. Egypt J Intern Med 35, 72 (2023). https://doi.org/10.1186/s43162-023-00256-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43162-023-00256-7