Abstract
Background
The devastating adverse effects of interferon (IFN) for the treatment of hepatitis C virus (HCV) lead to the emerging of direct acting antiviral agents (DAAs). This investigation was undertaken to assess safety and efficacy of two Egyptian DAA protocols for HCV: sofosbuvir (SOF)/daclatasvir (DCV)/simeprevir (SMV)/ribavirin (RBV) and sofosbuvir (SOF)/ombitasvir (OMB)/paritaprevir (PTV)/ritonavir (RTV)/RBV for 12 weeks in treatment-experienced HCV Egyptian patients.
Methods
It is a retrospective study where 139 patients, out of 400 patients, were divided according to their documented treatment protocol into two groups (Gp1: SOF/DCV/SMV/RBV and Gp2: SOF/PTV/OMB/RTV/RBV). All patients’ physical examination, disease history, laboratory baseline, and end of treatment data were collected from their profiles, evaluated and compared.
Results
Gp1 and Gp2 regimens had achieved sustained virologic response rates (SVR12) of 96.6% and 95.1%, respectively. Hemoglobin, ALT, and AST had decreased significantly (P < 0.05) in the two groups. Total bilirubin level had increased significantly in Gp1 and Gp2 (P = 0.002 and < 0.001, respectively). Creatinine level had increased significantly (P = 0.002) in Gp1 at end of treatment, while Gp2 remained unchanged. Headache and fatigue were the most common side effects in both protocols.
Conclusions
SOF/DCV/SMV/RBV and SOF/PTV/OMB/RTV/RBV regimens achieved high similar efficacy in Egyptian treatment-experienced HCV patients. Even though the outcome was with tolerable side effects, a better treatment regimen was recommended to abate these side effects for the welfare of Egyptian HCV patients.
Similar content being viewed by others
Background
Hepatitis C virus (HCV) is one of the most distressing blood-transmitted pathogens where global prevalence is estimated to be 177.5 million HCV infected adults [1]. Chronic hepatitis C (CHC) infection is currently the most common cause of chronic liver disease in the USA, and HCV is commonly associated with liver transplantation among adults [2]. In Egypt, 93% of HCV patients are infected by genotype 4 (G4) [3].
The continuous evolving of HCV quasispecies leads to virus escape from host immune responses and applied antivirals [4]. Moreover, no vaccine was developed to prevent or control HCV infection [5, 6]. Yet, the use of antiviral medications had been considered the only alternative for controlling the HCV epidemic [7].
Peg-interferon (PEG-IFN) alfa, either 2a or 2b, and ribavirin (RBV) were the standard and the only available treatment regimen for HCV till 2011. Unfortunately, this course of treatment had not only shown suboptimal efficacy but also patients suffered from severe side effects. The major adverse events of the standard interferon and ribavirin therapy were anemia, neutropenia, and thrombocytopenia [8].
The introduction of direct acting antiviral (DAA) agents for the treatment of HCV infections has assured a promising cure in the majority of patients with a short duration of well-tolerated regimens. According to the Egyptian protocols, interferon-free therapies included sofosbuvir (SOF)-based and non-SOF-based regimens. Both proved excellent sustained virological response (SVR12) rates and an acceptable safety profile, independent of liver fibrosis and previous treatment experience [9]. HCV G4 is a historically difficult to treat genotype with lower rates of sustained virological response (SVR) even with DAA treatment [10].
In December 2014, the first interferon-free DAA regimen was approved for therapy for treating naïve and experienced HCV G1 patients; the regimen was effective and well tolerated [11]. In a multicenter PEARL-1 trial (2015), a combination of ombitasvir (OMB), paritaprevir (PTV), and ritonavir (RTV) plus RBV was used for 467 HCV G4 patients. The outcome had revealed a high SVR12 in treatment-naïve patients and treatment-experienced patients as well [12]. The same trend was observed in AGATE I trial (2014–2015) where 120 HCV G4 patients from Austria, Belgium, Canada, France, Germany, Greece, Italy, and USA were recruited for a multicenter study to assess the combination of OMB and PTV plus ribavirin in chronic HCV G4 infection [13], while AGATE II study (2016) has investigated the feasibility of using the same treatment combination in HCV G4 treatment-naive or treatment-experienced with interferon-based regimens in Egyptian patients recruited from 5 academic and hepatology centers in Egypt [14]. Moreover, The Canadian AMBER study had concluded that OMB, PTV, RTV ± dasabuvir (DSV) ± RBV regimen had demonstrated high efficacy in difficult to treat 565 HCV G1 and G4 patients with liver cirrhosis or non-responders [15].
Worldwide, Egypt had the highest documented prevalence of HCV among nations. In 2015, > 6% of the Egyptian population had been tested HCV antibody positive, and the rate of infection was increased with age reaching 27.6% in those aged 55–59 years. It was associated with an economic burden; hence, HCV elimination became a national health priority [16].
As a response to this epidemic, the Egyptian government through the Egyptian Ministry of health (2016) had launched a comprehensive large program for controlling the HCV endemic. The program had provided easy access and free of charge antiviral medications’ protocols. This program was able to treat about two million HCV patients with high success rates [17].
When comparing number of cases who experienced DAA treatment failure to total number of HCV cases, we will find small percentage of DAA failure cases. But total number of HCV cases who are still receiving treatment is growing [18]. Therefore, more studies are needed to identify causes of non-response trying to avoid failure of treatment [19].
There are different regimens had been tried since the introduction of DAAs for HCV. Recently, two regimens have been used for treatment-experienced HCV Egyptian patients. Therefore, the current study aimed to assess and compare the efficacy and safety of the two DAAs regimens utilized in the treatment-experienced HCV patients. According to their documents, patients were divided into 2 groups: Gp1 and Gp2. Baseline and end of treatment (12 weeks) data of viral load, complete blood count, and kidney and liver function tests were reported. SVR12 was tested after 12 weeks of treatment completion (24 weeks) and collected.
Materials and methods
Patient selection
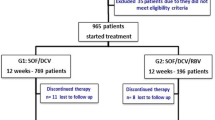
Treatment-experienced HCV patients who visited the HCV Research and Treatment Unit in Faculty of Medicine, Ain Shams Research Institute (MASRI), Cairo, Egypt, from January 2017 to November 2019 were tested for eligibility of the treatment protocol. Out of 400 patients, only 139 were enrolled in this study according to the inclusion criteria, exclusion criteria, and the availability of complete data (Fig. 1). Informed consents were obtained from all patients included in each protocol. All procedures followed were in accordance with the ethical standards of the Faculty of Pharmacy, Egyptian Russian University (human section), with the approval no. ECH-021, and all procedures complied with the Declaration of Helsinki.
Physical examination, full history taking, laboratory data, and abdominal ultrasound data were collected from patients’ profiles to be included in this study. Moreover, the previous treatments for HCV and signs of decompensated cirrhosis were investigated and documented.
Eligible patients met the following criteria (inclusion) according to the guidelines of the Egyptian Ministry of Health [20]:
-
1.
Positive HCV-ribonucleic acid (RNA)
-
2.
Age between 18 years and 65 years, while older patients performed ECG and echocardiography before participating in the study
-
3.
Treatment-experienced patients who had previously received either oral SOF/DCV ± RBV regimen or OMB/PTV/RTV plus RBV regimen but did not achieve SVR12
Patients were excluded for any of the following criteria:
-
1.
Hepatocellular carcinoma (HCC) except after 6 months of disease-free interval
-
2.
Patients classified as CHILD C stage with the CHILD-PUGH classification system
-
3.
Any other malignancy except after 2 years of disease-free interval
-
4.
Pregnant females or unable to use effective contraception
-
5.
Uncontrolled diabetes mellitus (glycated hemoglobin (HbA1c) > 9%)
-
6.
Patients previously received PEG-IFN
-
7.
Patients refused to participate in any of the two protocols
-
8.
Non-compliant patients
-
9.
Known cases of chronic renal failure or impairment
Study design and methods
This study was retrospective, double armed, observational study. Patients were considered into one of the treatment groups (Gp1 or Gp2) according to the received drug regimen.
Gp1 received daily doses of 400 mg sofosbuvir (Augispov®, AUG pharma, Giza, Egypt) plus 60 mg daclatasvir (Augidacla®, AUG pharma, Giza, Egypt) plus 150 mg simeprevir (Olysio®, Janssen, Nasr city, Cairo) plus 15 mg/kg Ribavirin (RBV) (Ribavirin®, Amriya, Alexandria, Egypt) for 12 weeks.
Gp2 received daily doses of 400 mg sofosbuvir (Augispov®, AUG pharma, Giza, Egypt) plus 25 mg ombitasvir, 150 mg paritaprevir, and 100 mg ritonavir (Qurevo®, Abbvie, Cairo, Egypt) plus 15 mg/kg RBV (Ribavirin®, Amriya, Alexandria, Egypt) for 12 weeks.
Decreasing or increasing RBV dose were performed for some patients in both treatment groups according to patient's tolerability.
Assessments
At baseline all patients were assessed for ALT, AST, creatinine, complete blood count (CBC), alpha-fetoprotein (AFP), and pregnancy tests for females at childbearing age. Monthly follow-ups for CBC, liver functions, and kidney functions were performed for assessing patients’ tolerability and incidence of adverse events (AEs). Moreover, adherence was assured by checking the empty pill packs in the monthly visits.
HCV RNA viral load measurements were performed at baseline and after 3 months of treatment completion (24 weeks) to detect achievement of SVR12. SVR12 was defined as HCV RNA levels that are below 15 IU/ml at 12 weeks after the end of treatment.
Patients were asked about any experienced AEs each follow-up visit. Death, life-threatening complications, or patient hospitalization were considered serious AEs.
Statistical analysis
The data were analyzed using the IBM Statistical Package of Social Science (SPSS) (version 26), where the normality of the data was tested using the Kolmogorov-Smirnov test. Numerical data were summarized as means ± standard deviations (SD) or medians and ranges. Medians were used mainly for skewness and not normally distributed data, while qualitative data were described as frequencies and percentages. Comparison between two groups for numerical variables was done using Mann-Whitney U test (nonparametric t-test). Comparison between before and after treatment data was done using Wilcoxon signed ranks. The relation between qualitative data was done using the chi-square test or Fisher’s exact test as appropriate. Probability (P-value) ≤ 0.05 is considered significant. P-value was adjusted due to multiple comparisons.
Results
Patients’ characteristics
Four hundred HCV patients visited the MASRI were investigated for eligibility to be included in this study. Figure 1 shows that 139 HCV patients were recruited according to the inclusion criteria listed above. These patients were allocated to Gp1 or Gp2 according to the received treatment. Gp1 arm included 58 patients while Gp2 arm included 81 patients’ according to their profiles.
The demographics data of all patients are shown in Table 1. Baseline parameters of the patients had revealed comparable results (P > 0.05) between the two treatment groups. Diabetes was confirmed in 26.3% and 17.3% patients in Gp1 and Gp2, respectively. Cirrhotic patients were 17.2% and 12.3% in Gp1 and Gp2, respectively. The same trend was observed for hypertensive patients. None of the patients had encephalopathy or ascites. There was no single patient has stopped the treatment due to serious side effects. One patient in each group had shown liver focal lesion that was further investigated to exclude HCC by triphasic CT which showed benign lesions.
Comparing safety in Gp1 and Gp2
Table 2 presents the data collected from patients after 12 weeks of treatment. It is clear that both treatment groups (Gp1 and Gp2) had shown comparable results. There was no significant difference (P > 0.05) detected among the results of the two groups.
Baseline and end of treatment data
The comparison between the patients’ baseline and end of treatment data in Gp1 are presented in Table 3. A significant decrease (P < 0.001) in ALT, AST, and hemoglobin levels were observed after 12 weeks of treatment. Furthermore, there was a significant increase in creatinine and total bilirubin levels (P = 0.008). Elevation of serum creatinine was an observation noticed in the first group during treatment which was transient as it has been declined again during post treatment follow-up. Ten patients (17.2%) reported fatigue and 12 patients (20.7%) complained of headache.
When comparing patients’ baseline and 12th week data in Gp2 (Table 4), the same trend was observed for ALT, AST, and hemoglobin levels (P < 0.001) as in Gp1. A significant increase (P < 0.001) in bilirubin level was detected after treatment, while there was no significant change in creatinine level (P = 0.28). Fifteen patients (18.5%) complained of mild headache and 20 patients (24.7%) suffered from fatigue.
Efficacy in both treatment groups
After 12 weeks of treatment completion (24 weeks of starting treatment), the viral load was reassessed to evaluate the achievement of SVR12 in Gp1 and Gp2. Figure 2 shows a comparable 24th week viral load level in both groups. The highest negative viral load was observed in Gp1 patients. Six patients in the 2 groups did not achieve SVR12. Two of the 6 patients were cirrhotic, while 3 patients were with abnormal liver echo pattern, and 1 patient had normal liver echo pattern.
Comparable percentages of patients who achieved SVR12 in Gp1 and Gp2. SVR12, sustained virological response; Gp, treatment group. Gp1 received sofosbuvir 400 mg/day plus daclatasvir 60 mg/day plus simeprevir 150 mg/day plus ribavirin 15 mg/kg/day for 12 weeks. Gp2 received sofosbuvir 400 mg/day plus ombitasvir 25 mg, paritaprevir 150 mg and ritonavir 100 mg/day plus ribavirin 15 mg/kg/day for 12 weeks
Discussion
Globally, the introduction of the DAA-based therapies had shown a high safety and efficacy in chronic HCV patients [20]. Despite this promising efficacy of DAAs, treatment failures had been documented, and they were explained by several host, drug, and virus-related factors [19] .
In 2006, Egypt established the National Committee for Control of Viral Hepatitis (NCCVH) for HCV management all over the country via a large network of centers specialized for viral hepatitis diagnosis and treatment [21].
Due to consequent changes in the international HCV treatment guidelines, the Egyptian practice guidelines were consequently modified in 2016 [20]. The NCCVH treatment protocol for CHC depends on the combination of SOF plus DCV with or without RBV as the main treatment regimen. SOF/DCV-based regimens had shown to be safe and effective in Egyptian CHC patients [22]. Several studies stated that HCV 4a subtype is the dominant one in Egypt [23,24,25]. Therefore, we depend on previous studies as cost saving measures.
The results of Gp 1 in this study had revealed that the combination of SOF, DCV, SMV, and RBV has resulted in SVR12 of 96.6%. This is coinciding with Ebada et al., who used the same regimen effectively in treatment-experienced HCV patients [21]. The same trend was reported in a French study which had reported the high SVR12 rates of the regimen in patients previously treated with DAAs [26].
None of the patients in our investigated two patients’ groups (Gp1 and Gp2) had discontinued treatment due to any serious side effect. Regimens’ safety was detected by absence of any patient’s hospitalizations. In accordance with our data, an Egyptian study had reported mild tolerated adverse effects that required no treatment discontinuation [21]. This is on the contrary of a study that reported some treatment discontinuations in retreated French cirrhotic HCV patients who reported severe dyspnea due to pulmonary arterial hypertension that resolved by discontinuation of therapy [26]. This controversy may be attributed to the difference in the ethnic groups in the 2 studies.
The data collected from Gp1 and Gp2 reported significant elevation in total bilirubin levels at end of treatment, but the levels did not exceed 1.2 mg/dl (unfavorable elevated total bilirubin level) [27]. In accordance with Gp1 data, an Egyptian study had reported significant but safe increase in total bilirubin level after administration of SOF/SMV regimen for 12 weeks in cirrhotic and non-cirrhotic patients [28]. This elevated bilirubin levels can be explained that SMV is a known inhibitor of bilirubin transporters; organo-anion transporter polypeptide (OATP1B1), and multidrug resistance protein 2 (MRP2) causing elevated levels of bilirubin [29]. Moreover, a study had reported exclusion of one patient after 4 weeks of treatment due to highly elevated serum total bilirubin (6.4 mg/dl) although achieving SVR12 [21]. A different study, included 10 French treatment-experienced patients, had mentioned an increase in the serum conjugated bilirubin level (up to 169 μmol/l) that led to treatment discontinuation and hospitalization [26]. On the contrary, an Egyptian study had concluded an improvement in total bilirubin levels in cirrhotic patients who received oral DAAs regimens [30].
Gp2 results are in accordance with a Chinese study that reported an increase in serum bilirubin level after administration of SOF, PTV, OMB, and RTV containing regimen in HCV G1b patients [31]. Moreover, an Egyptian case control study had reported an increase in total bilirubin level after administration of regimen used in Gp2 [32]. Also, another Egyptian study had reported marked hyper bilirubinemia after treatment that occurred significantly in HCV patients regardless of patients' age [33].
The Gp1 and Gp2 results in this study had reported statistically significant (P < 0.001) non-clinical drop in hemoglobin levels after 12 weeks of treatment. In accordance with Gp1 results, an Egyptian study had shown a clinically significant non severe decrease in hemoglobin levels in Egyptian patients previously received SOF/DCV-based therapy [21]. Moreover, another investigation had reported that SOF/SMV/RBV combination in treatment-naïve HCV G4 or treatment-experienced patients had reported a significant decline (P < 0.05) in hemoglobin level [34]. Besides, a recent Egyptian study had stated a decrease in hemoglobin levels after 12 weeks of SOF/DCV treatment [35]. Also, Matsumoto et al. described a decrease in hemoglobin levels after administration DCV including antiviral regimen. This observation was explained by a decrease in mean corpuscular volume, serum iron, and serum ferritin levels resulting in iron deficiency [36]. In diverge results to Gp1, a study had reported no significant changes in blood count in HCV G3 patients after a full course of SOF/DCV therapy [37].
In agreement with the Gp2 results, hemoglobin level has decreased significantly after administration of the same regimen in Egyptian case control studies [32, 33]. Moreover, RBV which is used in both treatment regimens has shown to decrease hemoglobin levels in a recent Egyptian study [35].
RBV used in Gp1 and Gp2 exerts its toxicity through an inhibition of intracellular energy metabolism and oxidative membrane damage, leading to an accelerated extravascular hemolysis. Improvement of anemia usually occurs upon RBV dose decrement. This fact was considered during Gp1 and Gp2 monitoring in some patients. Recent data suggest that erythrocyte oxidative defense mechanisms may play an important role in RBV-induced anemia [38].
A significant decrease in ALT and AST levels were observed after treatment in Gp1 and Gp2. In agreement with these results, Said et al. had determined significant decrease in liver aminotransferases levels after 3 months administration of the two regimens [21]. Moreover, the data are in agreement with the findings of El Kassas et al., who reported that ALT levels in CHC patients significantly decreased after the end of DAAs therapy [39].
Creatinine level had increased significantly in Gp1 in this study which was in accordance with a retrospective study that reported a significant increase in creatinine levels after administration of SOF-based regimens in chronic kidney disease patients infected with HCV [40]. Furthermore, an American study had reported a 1-2-fold increase in creatinine from baseline at the end of treatment with SOF-based drug regimen [41]. Besides, a Chinese study in 6 kidney transplant patients with HCV had decreased sofosbuvir’s dose to half in order to stabilize the elevated serum creatinine levels in 25% of patients after 2 days of SOF administration [31].
Conclusions
Both antiviral Egyptian regimens (SOF/OMB/PTV/RTV/RBV and SOF/DCV/SMV/RBV) are of similar safety and efficacy in Egyptian treatment-experienced HCV patients with no need for treatment discontinuation or RBV dose modifications. Even though the outcome was with tolerable side effects, a better treatment regimen was recommended to abate these side effects for the welfare of Egyptian HCV patients.
Availability of data and materials
The datasets used and analyzed during the current study are available from corresponding author upon reasonable request.
Abbreviations
- INF:
-
Interferon
- HCV:
-
Hepatitis C virus
- DAAs:
-
Direct acting antiviral agents
- SOF:
-
Sofosbuvir
- DCV:
-
Daclatasvir
- SMV:
-
Simeprevir
- RBV:
-
Ribavirin
- OMB:
-
Ombitasvir
- PTV:
-
Paritaprevir
- RTV:
-
Ritonavir
- SVR:
-
Sustained virologic response
- Gp:
-
Treatment group
- ALT:
-
Alanine transaminase
- AST:
-
Aspartate transaminase
- CHC:
-
Chronic hepatitis C
- G4:
-
Genotype 4
- PEG-IFN:
-
Peg-interferon
- ECG:
-
Electrocardiogram
- HCC:
-
Hepatocellular carcinoma
- HbA1C:
-
Glycated hemoglobin
- CBC:
-
Complete blood count
- AFP:
-
Alpha-fetoprotein
- AEs:
-
Adverse events
- RNA:
-
Ribonucleic acid
- SD:
-
Standard deviations
- MASRI:
-
Faculty of Medicine, Ain Shams Research Institute
- NCCVH:
-
National Committee for Control of Viral Hepatitis
- OA TP1B1:
-
Organo anion transporter polypeptide
- MRP2:
-
Multidrug resistance protein 2
References
Petruzziello A, Marigliano S, Loquercio G et al (2016) Global epidemiology of hepatitis C virus infection: an up-date of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol 22:7824–7840
El-Kamary SS, Jhaveri R, Shardell MD (2011) All-cause, liver-related, and non-liver-related mortality among HCV-infected individuals in the general US population. Clin Infect Dis an Off Publ Infect Dis Soc Am 53:150–157
Hathorn E, Elsharkawy AM (2016) Management of hepatitis C genotype 4 in the directly acting antivirals era. BMJ open Gastroenterol 3. https://doi.org/10.1136/BMJGAST-2016-000112
Bukh J (2016) The history of hepatitis C virus (HCV): Basic research reveals unique features in phylogeny, evolution and the viral life cycle with new perspectives for epidemic control. J Hepatol 65:S2–S21
Fakhry MM, Abdel-Hamed AR, Abo-elmatty DM et al (2020) A possible novel co-relation of locus 7q11 rs1761667 polymorphism with the severity of preeclampsia in Egyptian pregnant women. Meta Gene 24:100650
Stoll-Keller F, Barth H, Fafi-Kremer S et al (2009) Development of hepatitis C virus vaccines: challenges and progress. Expert Rev Vaccines 8:333–345
Elgharably A, Gomaa AI, Crossey MME et al (2017) Hepatitis C in Egypt - past, present, and future. Int J Gen Med 10:1–6
Dusheiko G (1997) Side effects of alpha interferon in chronic hepatitis C. Hepatology 26(3 Suppl 1):112S–121S. https://doi.org/10.1002/hep.510260720
Charatcharoenwitthaya P, Wongpaitoon V, Komolmit P et al (2020) Real-world effectiveness and safety of sofosbuvir and nonstructural protein 5A inhibitors for chronic hepatitis C genotype 1, 2, 3, 4, or 6: a multicentre cohort study. BMC Gastroenterology 20:47. https://doi.org/10.1186/s12876-020-01196-0
Arias A, Aguilera A, Soriano V et al (2017) Rate and predictors of treatment failure to all-oral HCV regimens outside clinical trials. Antivir Ther 22:307–312
Holmes J, Thompson A (2015) Interferon-free combination therapies for the treatment of hepatitis C: current insights. Hepatic Med Evid Res 7:51
Hézode C, Asselah T, Reddy KR et al (2015) Ombitasvir plus paritaprevir plus ritonavir with or without ribavirin in treatment-naive and treatment-experienced patients with genotype 4 chronic hepatitis C virus infection (PEARL-I): a randomised, open-label trial. Lancet (London, England) 385:2502–2509
Asselah T, Alami NN, Moreno C et al (2019) Ombitasvir/paritaprevir/ritonavir plus ribavirin for 24 weeks in patients with HCV GT4 and compensated cirrhosis (AGATE-I Part II). Heal Sci Rep 2:1–9
Waked I, Shiha G, Qaqish RB et al (2016) Ombitasvir, paritaprevir, and ritonavir plus ribavirin for chronic hepatitis C virus genotype 4 infection in Egyptian patients with or without compensated cirrhosis (AGATE-II): a multicentre, phase 3, partly randomised open-label trial. lancet. Gastroenterol Hepatol 1:36–44
Flisiak R, Janczewska E, Wawrzynowicz-Syczewska M et al (2016) Real-world effectiveness and safety of ombitasvir/paritaprevir/ritonavir ± dasabuvir ± ribavirin in hepatitis C: AMBER study. Aliment Pharmacol Ther 44:946–956
Abdel-Razek W, Hassany M, El-Sayed MH et al (2019) Hepatitis C virus in Egypt: interim report from the world’s largest national program. Clin Liver Dis 14:203–206
Omran D, Alboraie M, Zayed RA et al (2018) Towards hepatitis C virus elimination: Egyptian experience, achievements and limitations. World J Gastroenterol 24:4330–4340
Spaulding AC, Adee MG, Lawrence RT et al (2018) Five questions concerning managing hepa-titis C in the justice system: finding practical solutions for hepatitis C virus elimination. Infect Dis Clin North Am. 32:323–345
Park H, Lo-Ciganic WH, Huang J et al (2022) Machine learning algorithms for predicting direct-acting antiviral treatment failure in chronic hepatitis C: An HCV- TARGET analysis. Hepatology. 00:1–9
El-Akel W, El-Sayed MH, El Kassas M et al (2017) National treatment programme of hepatitis C in Egypt: Hepatitis C virus model of care. J Viral Hepat 24:262–267
Said EM, Abdulaziz BA, El Kassas M et al (2020) High success rates for the use of sofosbuvir/ombitasvir/paritaprevir/ritonavir + ribavirin and sofosbuvir/simeprevir/daclatasvir + ribavirin in retreatment of chronic hepatitis C infection after unsuccessful sofosbuvir/daclatasvir therapy: a real-life exp. Arch Virol 165:1633–1639
Omar H, El Akel W, Elbaz T et al (2018) Generic daclatasvir plus sofosbuvir, with or without ribavirin, in treatment of chronic hepatitis C: real-world results from 18 378 patients in Egypt. Aliment Pharmacol Ther 47:421–431
Tanaka Y, Agha S, Saudy N et al (2004) Exponential spread of hepatitis C virus genotype 4a in Egypt. J Mol Evol 58:191–195
Ray SC, Arthur RR, Carella A et al (2000) Genetic epidemiology of hepatitis C virus throughout Egypt. J Infect Dis 182:698–707
El Raziky M, Fathalah WF, El-akel WA et al (2013) The effect of peginterferon alpha-2a vs. peginterferon alpha-2b in treatment of naive chronic HCV genotype-4 patients: a single centre Egyptian study. Hepat Mon 13(5):e10069
Hézode C, Fourati S, Chevaliez S et al (2017) Sofosbuvir-daclatasvir-simeprevir plus ribavirin in direct-acting antiviral–experienced patients with hepatitis C. Clin Infect Dis 64:1615–1618
Kwak MS, Kim D, Chung GE et al (2012) Serum bilirubin levels are inversely associated with nonalcoholic fatty liver disease. Clin Mol Hepatol 18:383–390
Fontana RJ, Brown RS, Moreno-Zamora A et al (2016) Daclatasvir combined with sofosbuvir or simeprevir in liver transplant recipients with severe recurrent hepatitis C infection. Liver Transplant 22:446–458
Sane RS, Steinmann GG, Huang Q et al (2014) Mechanisms underlying benign and reversible unconjugated hyperbilirubinemia observed with faldaprevir administration in hepatitis C virus patients. J Pharmacol Exp Ther 351:403–412
Elnadry A-ASA, Ghareb M et al (2018) Impact of direct-acting antiviral therapy in Egyptian patients with chronic hepatitis C and liver cirrhosis. Sci J Al-Azhar Med Fac Girls 2:181
Xue Y, Zhang LX, Wang L et al (2017) Efficacy and safety of sofosbuvir and daclatasvir in treatment of kidney transplantation recipients with hepatitis C virus infection. World J Gastroenterol 23:5969–5976
Teama NM, Abdel-Mohsen WA, Ahmed OA et al (2021) Effectiveness and safety of ombitasvir/paritaprevir/ritonavir in treatment of chronic hepatitis C Egyptian hemodialysis patients, case-control study. Egypt Liver J 11:4–11
Othman MAEM, Abd EL Mawjood MMS, Aly MOA et al (2018) Comparison between the effect of two regimens for hepatitis C treatment (Qurevo and ribavirin) and (sofosbuvir, daclatsvir and ribavirin) on patients above and below the age of 60 years. Egypt J Hosp Med 72:5385–5390
Degré D, Sersté T, Lasser L et al (2017) Sofosbuvir in combination with simeprevir +/- ribavirin in genotype 4 hepatitis C patients with advanced fibrosis or cirrhosis: a real-world experience from Belgium. PLoS One 12:e0170933
Ibrahim Mohammed Ebid AH, Ahmed OA, Agwa SH et al (2020) Safety, efficacy and cost of two direct-acting antiviral regimens: a comparative study in chronic hepatitis C Egyptian patients. J Clin Pharm Ther 45:539–546
Matsumoto N, Ikeda H, Shigefuku R et al (2016) Hemoglobin decrease with iron deficiency induced by daclatasvir plus asunaprevir combination therapy for chronic hepatitis c virus genotype 1b. PLoS One 11:4–10
Sperl J, Frankova S, Kreidlova M et al (2017) Combination of sofosbuvir and daclatasvir in the treatment of genotype 3 chronic hepatitis C virus infection in patients on maintenance hemodialysis. Ther Clin Risk Manag 13:733–738
Russmann S, Grattagliano I, Portincasa P et al (2006) Ribavirin-induced anemia: mechanisms, risk factors and related targets for future research. Curr Med Chem 13:3351–3357
El Kassas M, Alboraie M, Mostafa A et al (2018) After successful hepatitis C virus antiviral therapy: it looks that normal alanine aminotransferase level is not the normal. J Clin Lab Anal 32. https://doi.org/10.1002/jcla.22296
Sise ME, Backman E, Ortiz GA et al (2017) Effect of sofosbuvir-based hepatitis C virus therapy on kidney function in patients with CKD. Clin J Am Soc Nephrol 12:1613–1623
Cox-North P, Hawkins KL, Rossiter ST et al (2017) Sofosbuvir-based regimens for the treatment of chronic hepatitis C in severe renal dysfunction. Hepatol Commun 1:248–255
Acknowledgements
The authors are grateful to all participants in the two groups.
Funding
This research received no specific grant from any funding agency in the public, commercial, or nonprofit sectors.
Author information
Authors and Affiliations
Contributions
RSH: Performing the procedure/writing the manuscript/revision of the manuscript. MMF: Data analysis/writing the manuscript/revision of the manuscript. OAA: Writing the manuscript/revision of the manuscript. SAA: Data analysis/revision of the manuscript. MAR: Data analysis/revision of the manuscript. GGN: Conceptualization/performing the procedure/revision of the manuscript. All authors shared in the finalization of the manuscript till it reached its final stage. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All subjects involved in the study signed an informed written consent to participate. This research has an approval no. ECH-021 from the ethical committee of the Egyptian Russian University on June, 2021, and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution’s human research committee.
Consent for publication
Not applicable (the manuscript does not contain any individual person’s data in any form including individual details, images, or videos)
Competing interests
All authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hagag, R.S., Fakhry, M.M., Ahmed, O.A. et al. Assessment of efficacy and safety of two Egyptian protocols for treatment-experienced HCV patients: an observational study. Egypt J Intern Med 34, 40 (2022). https://doi.org/10.1186/s43162-022-00126-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43162-022-00126-8