Abstract
Venous thromboembolism has many risk factors including protein S deficiency, which poses a significant diagnostic challenge as it presents with atypical complaints. A treatable yet potentially fatal condition, acute pulmonary embolism, is currently third most common cause of cardiovascular death. Clinicians should include pulmonary embolism as differential diagnosis in young adults with atypical symptoms with 2 D ECHO findings of the dilated right atrium, right ventricle, and elevated pulmonary artery pressure, and diagnosis is confirmed by computed tomography pulmonary angiography (CTPA). Anticoagulants including NOACs should be initiated promptly to improve the outcome for patients.
Similar content being viewed by others
Introduction
Protein S is a vitamin K-dependent glycoprotein that functions as a cofactor for activated protein C in the degradation of coagulation factors Va and VIIIa. The prevalence of protein S deficiency in the general population is 0.03 to 0.13% and 1 to 13% in individuals with venous thromboembolism and can be inherited or acquired causing a significant risk of thrombosis. We present the case of a young female with atypical presentation of protein S deficiency with no risk factors and was diagnosed and promptly managed with anticoagulants
Case report
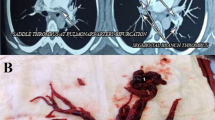
A 25-year-old young, unmarried lady come to the OPD with complaints of persistent stabbing chest pain for the last 7 days. She had no fever, shortness of breath, or any recent trauma. She had no comorbidity, was non-smoker, no contraceptive use, or any prolonged recumbency/recent operation. Examination revealed a blood pressure (BP) of 110/70 mmHg, heart rate (HR) of 110/min, respiratory rate (RR) of 18/min, and oxygen saturation (SpO2) of 97% at room air. Initial laboratory investigations revealed mild normocytic normochromic anemia (Hb 11.7 g/dl) with normal leukocyte and platelet count (TLC 9.71 × 109/L, platelet count 159 × 109/L). Liver function and renal profile were within normal limits. Cardiac enzymes were within the normal range (CPK 55 U/L, CK-MB 0.8 ng/ml, Trop I 0.02 ng/ml). An initial electrocardiogram (ECG) showed sinus tachycardia at a rate of 110 beats per minute. 2D ECHO revealed no regional wall motion abnormality and good left ventricle function; however, RA and RV were dilated and she had mild pulmonary arterial hypertension. The D dimer was raised (6919 ng/ml). Chest X-ray showed a well-defined rounded opacity in the right lower zone likely representing consolidation (Fig. 1). In view of these findings, computed tomography pulmonary angiography (CTPA) was done which revealed the presence of thrombus in the distal main pulmonary trunk, right and left pulmonary, and its ascending and descending branches suggestive of pulmonary thromboembolism (Figs. 2 and 3). Venous Doppler of the lower limb revealed deep vein thrombosis involving left common, external iliac, common, superficial and deep femoral, and popliteal veins with extension into a short saphenous vein (Figs. 4, 5, 6, 7, 8 and 9). She was managed with subcutaneous enoxaparin and was started on oral vitamin K antagonist, warfarin with international normalized ratio (INR) monitoring.
Further investigation showed homocysteine 12.4 micromol/L, factor V Leiden mutation, prothrombin gene mutation, MTHFR gene mutation, antinuclear antibody, and vasculitis panel were all negative, anti-phospholipid antibody IgG 2.18 AU/ml, anti-phospholipid antibody IgM 0.67 AU/ml, antithrombin III 122%, and protein C 91%, but her protein S (free) level was low at 15.1%.
Her chest pain improved and was discharged on day 6 on an oral anticoagulant. She was continued on oral anticoagulant and was doing well on regular follow-ups from the last 6 months.
Discussion
Vitamin K-dependent glycoprotein, protein S, is a cofactor for activated protein C reducing thrombin generation by inactivating procoagulant factors Va and VIIIa, along with enhancing fibrinolysis and inhibiting prothrombin activation [1,2,3,4,5,6]. It is an inherited thrombophilia transmitted in an autosomal dominant manner due to PROS1 gene mutation on the third chromosome causing an increased risk of thromboembolism [7,8,9]. Inherited protein S deficiency has been subdivided into three types I—classic type causing reduced both level and function of protein S, II—reduced protein S function, and III—quantitative reduction in free protein S level along with its function [10]. Pregnancy, oral hormonal contraceptive use, disseminated intravascular coagulation, acute thrombosis, HIV infection, nephrotic syndrome, and liver disease along with PROS1 gene mutation causes reduced protein S level. Protein S deficiency incidence among patients with venous thromboembolism is 2–8%, whereas the 2013 MEGA study showed the frequency of 0.9% [11]. VTE (including DVT and pulmonary embolism), arterial thrombosis, neonatal purpura fulminans, and obstetrical complications are the myriad of presenting manifestations of protein S deficiency. 14.5 years was the median age for first thromboembolic event, and pulmonary embolism incidence was 17% [12]. Suspicion of protein S deficiency should be raised in patients with VTE in a strong family history of VTE or protein S deficiency, or first VTE event before 50 years, unusual site VTE such as portal mesenteric, cerebral vein, and recurrent VTE. Protein S deficiency diagnosis confirmation is very difficult among all hereditary thrombophilias with a preferred approach for screening and testing for true deficiency ID estimating the level of free protein S [13, 14]. The lower level of protein S is more predictive for an increased risk of VTE in asymptomatic patients or having first VTE in the absence of strong family history [15].
Inherited thrombophilias (factor V Leiden mutation, protein C deficiency, antithrombin deficiency, prothrombin G20210A mutation) along with acquired risk factors like immobility, surgery, trauma, cancer, antiphospholipid syndrome, paroxysmal nocturnal hemoglobinuria, disseminated intravascular coagulation, oral contraceptive use, and hormonal changes in pregnancy are the differential diagnosis of VTE which should be evaluated and ruled out. The management of acute VTE includes anticoagulation for at least 3 to 6 months. Anticoagulation continuation depends on whether thrombosis is provoked or unprovoked along with other factors including recurrent thrombosis, life-threatening thrombosis, and unusual site thrombosis. The higher dose of DOAC is used if chosen for a long-term prevention of recurrent VTE, as protein S deficiency is more thrombophilic among the hereditary thrombophilias.
Availability of data and materials
Not applicable.
References
Esmon CT (1992) Protein S and protein C Biochemistry, physiology, and clinical manifestation of deficiencies. Trends Cardiovasc Med. 2(6):214–219
Dahlbäck B (1995) The protein C anticoagulant system: inherited defects as basis for venous thrombosis. Thromb Res. 77(1):1–43
Koppelman SJ, Hackeng TM, Sixma JJ, Bouma BN (1995) Inhibition of the intrinsic factor X activating complex by protein S: evidence for a specific binding of protein S to factor VIII. Blood. 86(3):1062–1071
Hackeng TM, van 't Veer C, Meijers JC, Bouma BN. (1994) Human protein S inhibits prothrombinase complex activity on endothelial cells and platelets via direct interactions with factors Va and Xa. J Biol Chem. 269(33):21051–21058
Koenen RR, Tans G, van Oerle R, Hamulyák K, Rosing J, Hackeng TM (2003) The APC-independent anticoagulant activity of protein S in plasma is decreased by elevated prothrombin levels due to the prothrombin G20210A mutation. Blood. 102(5):1686–1692
Takeyama M, Nogami K, Saenko EL, Soeda T, Nishiya K, Ogiwara K, Yoshioka A, Shima M (2008) Protein S down-regulates factor Xase activity independent of activated protein C: specific binding of factor VIII(a) to protein S inhibits interactions with factor IXa. Br J Haematol. 143(3):409–420
Schwarz HP, Fischer M, Hopmeier P, Batard MA, Griffin JH (1984) Plasma protein S deficiency in familial thrombotic disease. Blood. 64(6):1297–1300
Comp PC, Nixon RR, Cooper MR, Esmon CT (1984) Familial protein S deficiency is associated with recurrent thrombosis. J Clin Invest. 74(6):2082–2088
Comp PC, Esmon CT (1984) Recurrent venous thromboembolism in patients with a partial deficiency of protein S. N Engl J Med. 311(24):1525–1528
Gandrille S, Borgel D, Sala N, Espinosa-Parrilla Y, Simmonds R, Rezende S, Lind B, Mannhalter C, Pabinger I, Reitsma PH, Formstone C, Cooper DN, Saito H, Suzuki K, Bernardi F, Aiach M (2000) Plasma coagulation inhibitors subcommittee of the scientific and standardization committee of the international society on thrombosis and haemostasis. Protein S deficiency: a database of mutations--summary of the first update. Thromb Haemost 84(5):918
Pintao MC, Ribeiro DD, Bezemer ID, Garcia AA, de Visser MC, Doggen CJ, Lijfering WM, Reitsma PH, Rosendaal FR (2013) Protein S levels and the risk of venous thrombosis: results from the MEGA case-control study. Blood. 122(18):3210–3219
Klostermeier UC, Limperger V, Kenet G, Kurnik K, Alhenc Gelas M, Finckh U, Junker R, Heller C, Zieger B, Knöfler R, Holzhauer S, Mesters R, Krümpel A, Nowak-Göttl U (2015) Role of protein S deficiency in children with venous thromboembolism. An observational international cohort study. Thromb Haemost. 113(2):426–433
Simmonds RE, Ireland H, Lane DA, Zöller B, García de Frutos P, Dahlbäck B (1998) Clarification of the risk for venous thrombosis associated with hereditary protein S deficiency by investigation of a large kindred with a characterized gene defect. Ann Intern Med. 128(1):8–14
Smock KJ, Plumhoff EA, Meijer P, Hsu P, Zantek ND, Heikal NM, Van Cott EM (2016) Protein S testing in patients with protein S deficiency, factor V Leiden, and rivaroxaban by North American Specialized Coagulation Laboratories. Thromb Haemost. 116(1):50–57
Lijfering WM, Mulder R, ten Kate MK, Veeger NJ, Mulder AB, van der Meer J (2009) Clinical relevance of decreased free protein S levels: results from a retrospective family cohort study involving 1143 relatives. Blood. 113(6):1225–1230
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
1. Dr. Saurabh Puri: manuscript writing. 2. Dr. Ashok Kumar Grover: clinician. 3. Dr. Ankita Kaur Narula: editing. 4. Dr. Pankaj Nand Choudhry: editing. 5. Dr. Arjun Prem Gupta: proofreading. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Given.
Consent for publication
Given.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Puri, S., Grover, A.K., Narula, A.K. et al. Atypical presentation of pulmonary embolism and deep vein thrombosis due to protein S deficiency in a young female with chest pain. Egypt J Intern Med 34, 37 (2022). https://doi.org/10.1186/s43162-022-00124-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43162-022-00124-w