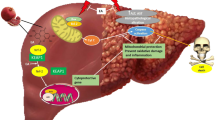
Abstract
Background
Methotrexate (MX), a competitive inhibitor of dihydrofolate reductase, can inhibit DNA and RNA production and is a powerful anticancer agent widely utilized in clinical practice for treating nonneoplastic maladies, as psoriasis and rheumatoid arthritis; meanwhile, its probable prescription dose and interval of administration are strictly limited due to dose-related organ damage. Former studies verified that kidney, brain, liver, and lung harms are prospective obstacles of methotrexate administration. To understand the machinery of methotrexate-prompt toxicity, various mechanisms were investigated. The former is an autophagy defense mechanism; autophagy is a self-digesting mechanism responsible for the removal of damaged organelles and malformed proteins by lysosome. The contemporary article hypothesized that turmeric or its liposomal analog could defeat autophagy of MX-induced acute toxicity. Methotrexate, in a dose of 1.5 mg/kg, was administered intravenously followed by turmeric and liposomal turmeric treatment in a dose of 5 mg/kg for 30 days in rats.
Results
Increment in autophagy (AUTP) consent by MX administration was attenuated by concurrent treatment via turmeric and liposomal turmeric that was reliable on the alteration in apoptotic markers. The assembly of FOXO-3 in serum post methotrexate administration was suppressed by concurrent treatment via liposomal turmeric. Apoptosis/autophagic marker investigation was evaluated through the gene expression of Bax (BCL2-associated X protein)/Bcl2 (B-cell lymphoma 2)/P53 (tumor protein P53)/SiRT-1 (sirtuin silent mating-type information regulation 2 homolog 1) and FOXO-3 (forkhead box transcription factor-3)/ERDJ-4 (endoplasmic reticulum localized DnaJ homologs)/BNP (brain natriuretic peptide B) signaling. The cell death of all cells was categorized to achieve autophagy. Interestingly, Bax/Bcl2/P53/SiRT-1 signaling pathways were downregulated, contributing to inhibiting the initiation of autophagy. Meanwhile, FOXO-3/BNP/ERDJ-4 reduction-implicated noncanonical autophagy pathways were involved in methotrexate-induced autophagy, whereas this change was suppressed when turmeric was administered in liposomal form.
Conclusion
These outcomes recommended that liposomal turmeric prevents MX-induced acute toxicity through its autophagy, antioxidant, and antiapoptotic properties.
Similar content being viewed by others
Background
Autophagy (AUTP) is a catabolic procedure vital for conserving cellular homeostasis and competing cytotoxic insult. Autophagy is documented as “programmed cell survival” contrary to programmed cell death (apoptosis). Exaggerated autophagy was reported in various types of cancers and has been elucidated to both enhance and prevent antitumor drug resistance relying on the duration and nature of the treatment-induced metabolic stress as well as the tumor type. MX, DOX, and cisplatin are widely utilized anticancer drugs [1]. MX, a competitive hinder of dihydrofolate reductase, can obstruct the production of RNA, DNA, proteins, and thymidylate. For this reason, MX is generally utilized in cancer therapy besides nonneoplastic conditions, including psoriasis and rheumatoid arthritis. Previous evidences elucidated that toxicity of the lung, liver, brain, and kidney is the implicit limitations of MX application [2]. To understand MX toxicity, a number of mechanisms have been inspected. One of them is disturbing the antioxidant/inflammatory/apoptotic/autophagosomal defense mechanism, contributing to oxidative stress-induced damage in several organs [2]. MX induced reduction in the antioxidant defense mechanism contributing to exaggerated release of ROS that subsequently enhance mitochondrial malfunction and endoplasmic reticulum stress resulting in hepatorenal toxicity, parenchymal lung injury, and interstitial and alveolar fibrosis [3]. It was evidenced that mitochondrial malfunction and simultaneous ROS production can contribute to autophagic cell death [4]. AUTP is an extremely conserved mechanism that maintains energy resources and gets rid of unnecessary products. It is suitable for reducing stressful conditions as oxidative stress and ER stress. SiRT-1/AKT/mTOR pathway is implied in numerous fibrotic conditions, as liver, cardiac, kidney, and pulmonary fibrosis [5]. Relying on former findings, it is worthy to examine the efficacy of MX on AUTP amelioration via exploring SiRT pathway and its consequence on ERDJ expression that results in toxic influence in addition to inspecting the potential effect of liposomal turmeric in ameliorating these injurious effects. MX can reduce the levels of angiogenic, pro-inflammatory biomarkers, modulate autophagal proteins, and enhance apoptosis by controlling ER stress response. Disturbance in homeostasis between apoptosis and autophagy might be an underlying phenomenon in the progression of resistance to MX [2]. Therefore, it is imperative to combine it with another drug which could decrease its toxicity, increase its efficacy, and target organs. Increasing evidence indicates that autophagy protects various tumor cells from apoptosis induced by chemotherapy drugs, both in vitro and in vivo. Nevertheless, persistent or extensive AUTP also produces cell death. Thus, autophagy often serves as an adapter between cell death and survival [1]. AUTP process eliminates malformed proteins, damaged organelles, and inappropriate long-lived proteins through auto-digestive process via lysosome. AUTP is classified into 3 types relying on the mechanism through which intracellular materials are carried into lysosome for lysis: first is macroautophagy, second is chaperone-mediated autophagy (CMA), and third is microautophagy (1). Macroautophagy implicates the generation of autophagosomes which is subcellular double-membrane-bound structures that contain degradable contents of cytoplasm materials and transfer them into lysosomes for lysis via lysosomal enzymes. Meanwhile, in CMA, proteins flagged via pentapeptide motif (KFERQ) were selectively degraded via direct transfer into lysosome. In microautophagy cytoplasm, material is sequestered via direct engulfment through lysosomal membrane (2). The molecular mechanism of AUTP incorporates various conserved autophagy-related proteins (Atg). Systems produce modified complexes Atg5-Atg12-Atg16 and Atg8-PE as autophagy regulators. AUTP is motivated via diverse physiological and stressful conditions; for instance, hyperthermia and food deprivation in addition to hypoxia that is mediated via factors such as FOXO transcription factors, insulin growth factor-1, m-TOR signaling, and chaperones. Any disturbance in AUTP may contribute to, neuromuscular disorders, myopathies, and cancer (3).
Growing body of evidence validates that AUTP is essential for controlling various intracellular process comprising oxidative stress, differentiation, growth, deficit nutrient, cell death, macromolecule, and organelle turnover (4).
SiRT1, NAD-dependent deacetylase, contributes a part in regulating AUTP. SiRT1 decreases oxidative stress and elevates mitochondrial function and, likewise, was linked to age-related ROS generation, which depends mainly on mitochondrial metabolism. Great relation was observed among SiRT1 activity and induction of AUTP in murine and human embryonic stem cells upon ROS challenge. Hydrogen peroxide prompted apoptosis and AUTP in wild type (WT) and SiRT1/mESCs. Meanwhile, addition of autophagy inhibitor (3-methyladenine) to hydrogen peroxide prompted more apoptosis in WT than in SiRT1/mESCs. Reduced AUTP induction in SiRT1/mESCs was elucidated via reducing the conversion of LC3-I into LC3-II, reduced Beclin-1 expression, and LC3 punctae. Hydrogen peroxide convinced AUTP through loss of mitochondrial membrane eventuality and dislocation of mitochondrial dynamics in SiRT1/mESCs. Exaggerated phosphorylation of P70/85-S6 kinase and ribosomal S6 was observed in SiRT1/ mESCs, revealing that SiRT1 controls the mTOR signaling pathway. This recommends the role of SiRT1 in adjusting AUTP and mitochondria function upon oxidative stress, effects mediated at least in part by the class 3 PI3K/Beclin 1 and mTOR pathways [5].
Turmeric is an active polyphenolic ingredient of Curcuma longa with high antioxidant and anti-inflammatory properties that render it an attractive candidate for protection against methotrexate nephrotoxicity. Turmeric has shown renal protective properties against gentamicin- and cisplatin-induced renal toxicities as well as diabetic nephropathy. Turmeric is a SiRT modulator natural product that exhibits poor solubility, and bioavailability utilizing higher bioavailable liposomal loaded preparations of turmeric derivatives may result in elevating SiRT-1-activating action, further authenticating the link between SiRT1, turmeric, and therapeutic effects. Previous studies indicated that the widely held liposomal turmeric (SiRT activator) exerts both direct activating effects and indirect effects via modulation of SiRT1 downstream pathways increasing retention phenomena, targeting organs, increasing bioavilability, and skipping macrophage recognition [6].
The present study therefore aimed to assess the possible therapeutic impact of turmeric and liposomal loaded turmeric and the underlying mechanism(s) responsible for this effect in a rat model of methotrexate-induced toxicity. The mechanism of protection was evaluated by assessing the oxidative stress (malondialdehyde), inflammatory marker (FOXO-3), apoptosis biomarkers (Bax/Bcl2/P53), and autophagy parameters (SiRT-1, BNP, and ERDJ-4).
Methods
Chemicals
Methotrexate and turmeric were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Kits used were obtained from Randox Company (Antrim, UK). ELISA kit for FOXO-3 determination was obtained from R&D systems (MN, USA), and RT-PCR kits were obtained from the Qiagen Company (USA). All utilized chemicals are of the greatest analytical ranking. Liposomal turmeric was obtained from lipolife company (USA).
Animals
Male Wistar albino rats, weighing 190–200 g, obtained from the animal house of the National Research Center were utilized in this study. Animals were housed in cages kept at standardized conditions (22 ± 5 °C, 55 ± 5% humidity and 12-h light/dark cycle). They were permitted for free water access in addition to pelleted standard chow diet. All techniques concerning animal maintenance and treatment firmly followed ethical procedures and policies approved by Animal Care (2443042022) and Use Committee of National Research Center.
Experimental design
Post 7 days of acclimatization, animals were classified into four groups (ten rats each) and were distributed relying on the following schedule:
-
1.
Group 1: Animals received saline and served as control group.
-
2.
Groups from 2 to 4: Animals were given methotrexate in a dose of 15 mg/kg, and then the following regimen was applied:
-
Group 2: Methotrexate-intoxicated animals were left untreated [7].
-
Group 3: Methotrexate-intoxicated animals were treated with oral dose of turmeric (15 mg/kg BW) for 1 month [6].
-
Group 4: Methotrexate-intoxicated animals were treated with oral dose of liposomal turmeric (5 mg/kg BW) for 1 month [8].
It is worthy to note that the selected dose of methotrexate was previously reported to be the most toxic to most organs [9].
Blood sampling
At the end of the experiment, all groups were sacrificed, and blood samples were taken from each animal by puncture of the sublingual vein into sterilized tubes and let it stand for 10 min to clot. Serum was separated by centrifugation at 3000 rpm for 10 min and kept at −80 °C for further determinations of biochemical parameters.
Determinations of biochemical parameters
Determination of serum malondialdehyde
MDA activity was estimated spectrophotometrically using commercially available kits provided from the Randox Company (UK, Antrim; AS2359) [10].
Determination of serum reduced glutathione
GSH activity was estimated spectrophotometrically using commercially available kits provided from the Randox Company (UK, Antrim; AS2359) [11].
ELISA determination of serum FOXO-3
Serum FOXO-3 level was measured using ELISA kits (R&D systems MN, USA; no. 112731 and no. BAF583, respectively) according to the manufacturer’s instructions. The assay of this cytokine employs the quantitative sandwich enzyme immunoassay technique. Specific antibody was pre-coated onto the microplate. The standards and samples were pipetted into the wells, and the cytokine was bound by the immobilized antibody. After washing away any unbound substances, an enzyme-linked secondary antibody specific for FOXO-3 was added to the wells [12].
Quantitative real-time polymerase chain reaction (qRT-PCR) analysis of serum BNP, P53, Bax, Bcl2, SiRT, and ERDJ mRNA expression
Serum was lysed using QIAzol lysis reagent with a TissueRuptor (Roche). RNA was sequestered via Tripure Isolation Reagent (Roche) relying on the manufacturer’s instructions. To assess the mRNA expression of BNP, P53, Bax, Bcl2, SiRT, and ERDJ, quantitative real-time PCR was accomplished via SYBR green PCR Master Mix (Applied Biosystems, CA, USA). Primer sequences were demonstrated in Table 1 [13].
Statistical analysis
Data were represented as mean ± SEM. Statistical analysis was achieved using InStat-3 computer program (GraphPad software Inc., San Diego, CA, USA). One-way analysis of variance (ANOVA) by SPSS 16 program was done followed by post hoc at p ≤ 0.05 using Tukey’s test.
Results
Modulation of oxidative stress biomarkers
Methotrexate intoxication induced a significant elevation in oxidative stress biomarker including MDA, and a significant reduction in antioxidant biomarker reduced glutathione with a mean value of 79.2 and 0.66, respectively, as compared to the control value. Meanwhile, a concomitant modulation in these biomarkers was observed post turmeric treatment with a mean value of 24 and 2.19, respectively, and liposomal turmeric treatment with a mean value of 50 and 3.9, respectively, as compared with the MX-intoxicated group, with the liposomal turmeric showing the most significant effect implying its antioxidant power as shown in Figs. 1 and 2.
Impact of turmeric and liposomal turmeric on serum malondialdehyde level post methotrexate intoxication. Data are expressed as mean ± SEM (n = 10). p ≤ 0.05 value is considered significant. Groups having the same letter are not significantly different from each other, while those having different letters are significantly different from each other
Impact of turmeric and liposomal turmeric on serum reduced glutathione activity post methotrexate intoxication. Data are expressed as mean ± SEM (n = 10). p ≤ 0.05 value is considered significant. Groups having the same letter are not significantly different from each other, while those having different letters are significantly different from each other
Modulation of autophagy biomarkers
Methotrexate intoxication deduced a significant elevation in autophagy biomarkers including SiRT-1 and ERDJ-4 gene expression by 2- and 4-fold, respectively, as compared to the control value. Meanwhile, a concomitant reduction in these biomarkers was observed post turmeric treatment by a value of 1-fold for both and liposomal turmeric treatment by a value of 2-fold for both, respectively, as compared with the MX-intoxicated group, with the liposomal turmeric showing the most significant effect reflecting the role of autophagy in turmeric therapy as revealed in (Fig. 3).
Impact of turmeric and liposomal turmeric on serum autophagy markers SiRT-1 and ERDJ-4 gene expression post methotrexate intoxication. GAPDH was used as an internal control for calculating mRNA fold changes. Data are expressed as mean ± SEM (n = 10). p ≤ 0.05 value is considered significant. Groups having the same letter are not significantly different from each other, while those having different letters are significantly different from each other
Modulation of apoptosis
Methotrexate intoxication demonstrated a significant elevation in the gene expression of apoptotic biomarker, namely Bax, and a significant reduction in antiapoptotic marker Bcl2 and P53 by 6-, 3-, and 5-fold, respectively, as compared to the control value. Meanwhile, a concomitant modulation in these biomarkers was observed post turmeric treatment via 3-, 2-, and 3-fold, respectively, and liposomal turmeric treatment by 3-, 5-, and 4-fold, respectively, as compared with the MX-intoxicated group, with the liposomal turmeric showing the most significant effect deducing its antiapoptotic effect as represented in Fig. 4.
Impact of turmeric and liposomal turmeric on serum apoptotic markers P53, Bax, and BcL2 gene expression post methotrexate intoxication. GAPDH was used as an internal control for calculating mRNA fold changes. Data are expressed as mean ± SEM (n = 10). p ≤ 0.05 value is considered significant. Groups having the same letter are not significantly different from each other, while those having different letters are significantly different from each other
Modulation of inflammation
Methotrexate intoxication elucidated a significant elevation in the protein expression of the inflammatory marker FOXO-3 by a mean value of 1.62 as compared to the control value. Meanwhile, a concomitant modulation in this inflammatory marker was observed post turmeric and liposomal turmeric by a mean value of 1.16 and 1.06, respectively, as compared with the MX-intoxicated group, with the liposomal turmeric showing the most significant effect in this aspect reflecting the anti-inflammatory effect of turmeric as highlighted in Fig. 5.
Impact of turmeric and liposomal turmeric on serum inflammatory marker FOXO-3 protein expression post methotrexate intoxication. Data are expressed as mean ± SEM (n = 10). p value is considered significant. Groups having the same letter are not significantly different from each other, while those having different letters are significantly different from each other
Modulation of BNP
Methotrexate intoxication elucidated a significant elevation in the gene expression of the brain biomarker BNP by a value of 4-fold as compared to the control value. Meanwhile, a concomitant modulation in this biomarker was observed post turmeric and liposomal turmeric by a value of 1- and 2-fold, respectively, as compared with the MX-intoxicated group, with the liposomal turmeric treatment showing the most significant impact as shown in Fig. 6.
Impact of turmeric and liposomal turmeric on serum brain natriuretic peptide (BNP) gene expression post methotrexate intoxication. GAPDH was used as an internal control for calculating mRNA fold changes. Data are expressed as mean ± SEM (n = 10). p ≤ 0.05 value is considered significant. Groups having the same letter are not significantly different from each other, while those having different letters are significantly different from each other
Discussion
MX, a competitive inhibitor of dihydrofolate reductase, can prevent the production of DNA, RNA, thymidylate, and proteins. For this reason, MX is generally utilized in cancer therapy besides nonneoplastic conditions, including psoriasis and rheumatoid arthritis [2]. Previous evidences elucidated that toxicity of the lung, liver, brain, and kidney is the main limit for MX application [1]. To understand MX toxicity, a number of mechanisms have been inspected. One of them is disturbing the antioxidant defense mechanism, contributing to oxidative stress-induced damage in several organs and crosslinked autophagy [14].
The current study revealed that MX intoxication deduced a significant elevation in AUTP biomarkers including SiRT-1 and ERDJ-4. Meanwhile, a concomitant reduction in these biomarkers was observed post turmeric and liposomal turmeric treatment; this coincides with the hypothesis that MX induced reduction in the antioxidant defense mechanism contributing to augmenting the release of ROS that subsequently promotes mitochondrial malfunction and endoplasmic reticulum stress accompanied with exaggerated production of ROS resulting in hepatotoxicity, parenchymal lung injury, and interstitial and alveolar fibrosis [15, 16]. It was evidenced that mitochondrial malfunction and simultaneous ROS production can arbitrate autophagic cell death [4]. AUTP is an extremely conserved mechanism that maintains energy resources and ruins unnecessary products. It is suitable for relieving stressful conditions as oxidative stress and ER stress. m-TOR is the mammalian target of rapamycin and considered as one of the detectors regulating autophagy. SiRT-1/AKT/mTOR pathway is implied in numerous fibrotic conditions, as liver, cardiac, kidney, and pulmonary fibrosis [17]. Grounded on former findings, it is worthy to investigate the efficacy of MX on AUTP amelioration via exploring SiRT pathway and its consequence on ERDJ expression that is contributed in toxic influence in addition to inspecting the potential effect of liposomal turmeric in ameliorating these injurious effects. Rationale on this study relies on bioinformatics examinations to reclaim a set of distinctive autophagy genes SiRT-1, ERDJ-4, P53, and FOXO-3. Furthermore, to authorize the efficacy of liposomal turmeric on modifying the expression of the selected autophagy genes, ERDJ-4, SiRT expression, and oxidative stress are induced in rat model. Moreover, the effect of MX, turmeric, and liposomal turmeric on the apoptotic features including P53, Bax, and BCL2 was evaluated. Turmeric, a naturally existing coenzyme, is an effective and safe remedial antioxidant by being a free radical scavenger which inhibits oxidative injuries of lipids, DNA, proteins, and other necessary molecules through promoting Nrf2-related genes. Turmeric can inhibit the development of lung and liver fibrosis via its antioxidant and anti-inflammatory properties through reducing various inflammatory mediators as FOXO-3, TGF-β, TNF-α, and MCP1 in the lung and liver [7, 18].
Hsp70 protein action is adjusted via accessory proteins, contributing members of the DnaJ-like protein family. Characterized by the presence of a highly conserved 70-amino acid J domain, DnaJ homologs promote Hsp70 ATPase activity to further steady their collaboration with unfolded substrates [19]. DnaJ homologs are contributed in almost all facets of protein folding and synthesis and exist in vast organelles. Inside the endoplasmic reticulum, DnaJ homologs can assist in the secretion, translocation, retro-translocation, and ER-associated degradation of secretory pathway proteins. ERdj4 was extremely promoted at both the protein and mRNA level as a result of ER stress, designating that DnaJ may be tangled in protein folding or ER-associated degradation [20].
The present study elucidated that methotrexate intoxication elucidated a significant elevation in apoptotic biomarkers including Bax, P53, and a significant reduction in antiapoptotic marker Bcl2. Meanwhile, a concomitant modulation in these biomarkers was observed post turmeric and liposomal turmeric treatment; this coincides with the thesis that the suppressor gene p53 is mutated in nearly 50% of human cancers and promotes autophagy. It was estimated that P53 purposes as an essential arbitrator for damage-promoted apoptosis and has been shown to induce autophagy in damage-regulated autophagy modulator (DRAM)-dependent manner to elicit apoptosis in human cancer cell lines [21]. DRAM is a lysosomal integral membrane protein and a direct target of p53-induced macroautophagy and helps in accumulation of autophagosomes [22]. The p53-mediated apoptosis involves several chromatin-remodeling factors [14, 23] (e.g., e2f1 as it involves in transcriptional repression of cell proliferation as part of component with retinoblastoma complex). Autophagy genes are organized by several factors contributing to chromatin remodeling as p53 functions at upstream of autophagy signaling pathway to promote apoptosis [4].
Herein, methotrexate intoxication illustrated a significant elevation in inflammatory biomarker FOXO-1. Meanwhile, a concomitant modulation in this inflammatory marker was observed post turmeric and liposomal turmeric treatment; this is in parallel with the hypothesis that FOXO and ROS can control AUTP. Lately, it has been hypothesized that transcriptional level of several autophagy-related genes was elevated via activated FOXO [24]. The genes included were EDRJ-4, Atg8/LC3, Atg12, Vps34, SiRT-1, and Atg6 to induce protein degradation in atrophying muscle cells, and surprisingly, the regulatory role of FOXO on Atg8 and Atg12 seems to be direct [25]. The chromatin-remodeling factors deacetylate FOXO transcription factors and promote longevity in worms, flies, and mammals [26, 27]. Recent evidence illustrated that in mice, sirtuin-type Sirt1 is able to induce autophagy in both normal growth and starvation conditions. SiRT-1 acetylates Atg5, Atg7, and Atg8 autophagy factors in an NADP-dependent manner [28]. The depletion in Sirt1 function in mutant phenotypes resembles those of autophagy-defective mice, such as early death and the accretion of dysfunctional mitochondria. Methotrexate intoxication elucidated a significant elevation in oxidative stress biomarker malondialdehyde and a consignment reduction in antioxidant biomarker GSH. Meanwhile, a concomitant modulation in this oxidative stress biomarker was observed post turmeric and liposomal turmeric treatment with liposomal turmeric showing the most significant effect; this is reflected with the study that ROS can regulate starvation-induced autophagy. Recent evidence shows that Atg4 and SiRIT, essential proteases in the autophagy machinery, have been recognized as a direct target for oxidation by ROS. Accumulation of ROS released throughout numerous cellular activities mostly by respiration contributes to oxidative stress. Cells respond to oxidative stress by activating numerous defense mechanisms. Previous studies deduced that ROS behave as signaling molecules and can induce autophagy contributing to subsequent loss of the affected cells. Autophagy has a precarious function in the cellular interaction to oxidative stress [29].
Mammalian cells utilize specified and complicated machinery for discarding disformed proteins or deformed organelles. Such machinery is a portion of a mechanism named autophagy [30]. Furthermore, if autophagy is precisely functioned to remove dysfunctional mitochondria, it is here named mitophagy. Autophagy and mitophagy have essential physiological implications involved in cellular differentiation, resistance to stresses such as metabolic control, starvation, and adaptation to the varying environmental conditions. Regrettably, converted cancer cells frequently exploit autophagy and mitophagy for sustaining their metabolic reprogramming and growth to a point that autophagy and mitophagy are recognized as promising targets for ongoing future antitumoral therapies [31]. Sirtuins are NAD+-dependent deacylases with a fundamental role in sensing and modulating cellular response to external stresses such as nutrients availability and therefore involved in oxidative stress control, aging, inflammation, cancer, and differentiation [32]. It is obvious, therefore, that autophagy, mitophagy, and sirtuins share many common aspects to a point that, recently, sirtuins have been linked to the control of mitophagy and autophagy. In the context of cancer, such an impact is attained by modulating transcription of mitophagy and autophagy genes, by posttranslational modification of proteins belonging to the mitophagy and autophagy machinery, and by adjusting ROS formation or major metabolic pathways such as glutamine metabolism or Krebs cycle. The role of sirtuins, autophagy, and mitophagy in cancer and how sirtuins can regulate autophagy and mitophagy in cancer cells were illustrated. Lastly, sirtuin’s role in the context of tumor progression and metastasis signifies glutamine metabolism as a model of how a concerted promoting or impairing sirtuins in cancer cells can control mitophagy and autophagy by interrupting the metabolism of this essential amino acid [33].
Sirtuins are considered as class 3 histone deacetylases; their enzymatic activity relies on cofactor NAD+ [34]. Sirtuins were stated to control various activities via regulating DNA repair, metabolism, gene expression, oxidative stress response, mitochondrial function, and biogenesis. Disregulation of sirtuins action and expression may contribute to tissue-specific degenerative events involved in the development of several human pathologies, including neurodegeneration, cancer, and cardiovascular disease. Sirtuin 1 is the furthermost studied member of this class of enzymes, whose expression is concomitant with increased insulin sensitivity. SiRT-1 has been implicated in both anticancer and tumorigenic processes and is further stated to control essential metabolic pathways. Via regulating p53 deacetylation and modulation of autophagy, SiRT-1 is involved in lifespan extension and cellular response to caloric restriction. Lately, scientific interest focusing on the identification of SiRT-1 modulators has led to the discovery of novel small molecules targeting SiRT-1 activity [23]. Former studies reported the effect of nutraceuticals in upregulating SiRT-1 activity, including polyphenolic products in vegetables, plants, and fruits, including turmeric, resveratrol, and quercetin [35].
The utmost considered member of this enzymatic class is SiRT-1. SiRT-1 regulates inflammation, metabolic pathways, cell survival, and cellular senescence and participate in the pathogenesis of chronic conditions such as diabetes as well as neurodegenerative, pulmonary, and cardiovascular diseases. Indeed, SiRT-1 has been reported to play a key role in tumorigenesis, as an oncogene or tumor suppressor [35]. SiRT-1 can adjust these processes via deacetylation of lysine groups of non-histone in addition to histone proteins, comprising recognized transcription factors (FOXO, p53, MyoD, PGC-1α) [36].
Oxidative stress plays a vital part in the pathogenesis of most cases. Excess ROS, RNS, and free radicals contribute to destroying cellular contents, including DNA, proteins, and lipids. Imbalance between antioxidants and oxidants contributes into cellular dysfunction, necrosis, and apoptosis [37].
SiRT-1 controls SOD and GPX expression [38]. Moreover, since mitochondrial dysfunction contributes to enhancing apoptosis, SiRT-1 control apoptosis via modifying PGC-1α acetylation [39]. SiRT-1 as well controls inflammatory response [40]. By modifying NF-κB and p53 acetylation, SiRT1 control transcription of genes such as FOXO-3, TNF-α, IL-8, IL-6, and IL-1 [41,42,43] via NF-κB and SiRT-1 also regulates the expression of genes such as promotor of apoptosis Bax and inhibitor of apoptosis protein (IAP), Bcl-2, and TNF receptor [44].
SiRT-1 fight against oxidative stress via modulating the acetylation of FOXO, as it participates in apoptosis, antioxidant progressions and cell proliferation [45]. Through promoting FOXO/MsSOD pathway, SiRT-1 promote catalase and MnSOD expression, by deacetylating p53, thus enhancing cellular antioxidant capacity [44, 46, 47], countering oxidative stress, and enhancing damage repair [48].
Along the preceding few eras, the eternally growing consciousness is that bioactive diet components can behave as anti-inflammatory, apoptotic, and antioxidant negotiators, thereby dropping the deleterious properties of oxidative tension and the prevalence of chronic maladies, for instance, cancer, diabetes, and hepatic and cardiovascular complaints [49]. Quite a lot of nutraceuticals can moderate SiRT-1 action [50, 51]. For instance, turmeric is considered as a natural polyphenol [52]. As an antioxidant, turmeric is able to eliminate ROS and RNS by increasing the expression of antioxidant proteins via induction of upstream coding genes such as nuclear factor erythroid 2-related factor 2 (Nrf2), Kelch-like ECH-associated protein 1, and antioxidant response element [7] that promote the transcription of antioxidant genes such as SOD, GPx, GST, and GSH and of phase 2 detoxifying enzymes such as HO-1, NADPH, and NAD(P) H dehydrogenase (quinone)-1 [53].
Turmeric possesses immunomodulatory effect via intermingling with factors tangled in inflammatory interaction as STAT/JAK pathway, inhibitor of cytokine signaling (SOCS) expression, and FOXO-3/NF-κB/TLR-4/MyD-88 axis. Turmeric inhibits phosphorylation of STAT/JAK by binding with its α,β-unsaturated carbonyl portion to residue 259 of cysteine in STAT3 with consequent activation. In vitro turmeric can phosphorylate STAT3 and mitigate inflammatory interaction [54]. In vivo turmeric renovates immunological equilibrium via working on STAT-3/JAK/SOCS signaling pathway, inhibiting STAT3, JAK2, and STAT6 phosphorylation, and elevating PIAS3, SOCS1, and SOCS3 gene expression [55]. Turmeric is able to act on apoptosis and on mitochondrial biogenesis and dysfunction through SiRT activation by small molecules [56]. Turmeric reduces transcription factors, TNF, growth factor receptors, and NOS expression and elevates AMPK levels. In vascular smooth muscle cells, turmeric stimulates AMPK activation that subsequently promotes ATP and superoxide release elevating NAD+ levels and SiRT1 activation [8]. Turmeric-induced SiRT-1 upregulation has advantageous influence versus a wide range of maladies counting diabetes, cardiac fibrosis, and hepatic and ischemia/reperfusion injury [8, 57]. Cardiomyocytes cured via turmeric displayed upregulation in SiRT-1, FOXO-3, SDH, BcL2, and COX-2 levels and downregulation in apoptotic markers P53 and Bax. These signs were eradicated once cardiomyocytes were cured via SiRT-1 siRNA [57].
Turmeric is a SiRT modulator natural product that exhibits poor solubility, and bioavailability utilizing higher bioavailable liposomal loaded preparations of turmeric derivatives may result in elevating SiRT-1-activating action, further authenticating the link between SiRT-1, turmeric, and therapeutic effects [57]. The fact that studies indicate that the widely held SiRT activators exert both direct activating effects and indirect effects via modulation of SiRT1 downstream pathways complicates the interpretation of results and, particularly, the mining of data specifically dependent on direct SiRT-1 binding and activation [8].
Conclusion
Liposomal loaded turmeric may be a promising candidate for preventing methotrexate intoxication via interfering with apoptosis, oxidative stress, autophagy, and inflammatory signaling pathways. Further studies may be required and further investigations.
Availability of data and materials
No additional data or information is available for this paper.
Abbreviations
- MX:
-
Methotrexate
- FOXO:
-
Forkhead box transcription factors
- Bax:
-
BCL2-associated X protein
- Bcl2:
-
B-cell lymphoma 2
- BNP:
-
Brain natriuretic peptide B
- Sirt-1:
-
Sirtuin silent mating-type information regulation 2 homolog 1
- ERDJ:
-
Endoplasmic reticulum localized DnaJ homologs
- P 53:
-
Tumor protein P53
- AUTP:
-
Autophagy
- CMA:
-
Chaperone-mediated autophagy
- Atg5:
-
Autophagy related 5
- ROS:
-
Reactive oxygen species
- NFκB:
-
Nuclear factor kappa-B
- TNF-α:
-
Tumor necrosis factor-α
- STAT-3:
-
Signal transducer and activator of transcription
- JAK-2:
-
Janus kinase 2
- ARE:
-
Antioxidant response element
References
Tang D, Kang R, Livesey KM, Cheh CW, Farkas A, Loughran P, Hoppe G, Bianchi ME, Tracey KJ, Zeh HJ, Lotze MT (2010) Endogenous HMGB1 regulates autophagy. J. Cell Biol 190(5):881–892
Xu K, Cai YS, Lu SM, Li XL, Liu L, Li Z, Liu H, Xu PK (2015) Autophagy induction contributes to the resistance to methotrexate treatment in rheumatoid arthritis fibroblast-like synovial cells through high mobility group box chromosomal protein 1. Arthritis Res Ther 17:374
Misra S, Bagchi A, Sarkar A, Niyogi S, Bhattacharjee D, Chatterjee S, Mondal S, Chattopadhyay A, Saha A, Chatterjee S, Sinhamahapatra P, Chakrabarti P, Chatterjee M, Ghosh A (2021) Methotrexate and theaflavin-3, 3'-digallate synergistically restore the balance between apoptosis and autophagy in synovial fibroblast of RA: an ex vivo approach with cultured human RA FLS. Inflammopharmacology 29(5):1427–1442
Kiffin R, Bandyopadhyay U, Cuervo AM (2006) Oxidative stress and autophagy. Antioxid Redox Signal 8(1-2):152–162
Ou X, Lee MR, Huang X, Messina-Graham S, Broxmeyer HE (2014) SIRT1 positively regulates autophagy and mitochondria function in embryonic stem cells under oxidative stress. Stem Cells 32(5):1183–1194
Abdelsamia EM, Khaleel SA, Balah A, Abdel Baky NA (2019) Tumeric augments the cardioprotective effect of metformin in an experimental model of type I diabetes mellitus; impact of Nrf2/HO-1 and JAK/STAT pathways. BioMe Pharmacother 109:2136–2144
Ohbayashi M, Kubota S, Kawase A, Kohyama N, Kobayashi Y, Yamamoto T (2014) Involvement of epithelial-mesenchymal transition in methotrexate-induced pulmonary fibrosis. J Toxicol Sci 39:319–330
Zendedel E, Butler AE, Atkin SL, Sahebkar A (2018) Impact of tumeric on sirtuins: a review. J Cell Biochem 119(12):10291–10300. https://doi.org/10.1002/jcb.27371
Tousson E, Zaki ZT, Abu-Shaeir WA, Hassan H (2014) Methotrexate-induced hepatic and renal toxicity: role of L-carnitine in treatment. Biomed Biotechnol 2(4):85–92
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Moron MS, Depierre JW, Mannervik B (1979) Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta 582:67–78
Kadry MO (2019) Liposomal glutathione as a promising candidate for immunological rheumatoid arthritis therapy. Heliyon 5(7):e02162
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25:402–408
Mahmoud AM, Hussein OE, Hozayen WG, Abd El-Twab SM (2017) Methotrexate hepatotoxicity is associated with oxidative stress, and down-regulation of PPARγ and Nrf2: protective effect of 18β-glycyrrhetinic acid. Chem Biol Interact 270:59–72
Serrano-Mollar A, Closa D, Prats N, Blesa S, Martinez-Losa M, Cortijo J, Estrela JM, Morcillo EJ, Bulbena O (2003) In vivo antioxidant treatment protects against bleomycin induced lung damage in rats. Br J Pharmacol 138:1037–1048
Isik M, Beydemir S, Yilmaz A, Naldan ME, Aslan HE, Gulcin I (2017) Oxidative stress and mRNA expression of acetylcholinesterase in the leukocytes of ischemic patients. Biomed Pharmacother 87:561–567
Gui YS, Wang L, Tian X, Li X, Ma A, Zhou W, Zeng N, Zhang J, Cai B, Zhang H, Chen JY, Xu KF (2015) mTORoveractivation and compromised autophagy in the pathogenesis of pulmonary fibrosis. Plos One 10(9):e0138625
Araki T, Sasaki Y, Milbrandt J (2004) Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science 305(5686):1010–1013
Chiang HL, Terlecky SR, Plant CP, Dice JF (1989) A role for a 70-kilodaton heat shock protein in lysosomal degradation of intracellular proteins. Science 246(4928):382–385 View at: Google Scholar
Shen Y, Meunier L, Hendershot LM (2002) Identification and characterization of a novel endoplasmic reticulum (ER) DnaJ homologue, which stimulates ATPase activity of BiP in vitro and is induced by ER stress. J Biol Chem 277(18):15947–15956
Crighton D, Wilkinson S, O’Prey J et al (2006) DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell 126(1):121–134
Tunali-Akbay T, Sehirli O, Ercan F, Sener G (2010) Resveratrol protects against methotrexate-induced hepatic injury in rats. J Pharm Sci 13(2):303–310
Tasdemir E, Maiuri MC, Galluzzi L et al (2008) Regulation of autophagy by cytoplasmic p53. Nat Cell Biol 10(6):676–687
Zhao J, Brault JJ, Schild A et al (2007) FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab 6(6):472–483 View at: Publisher Site|Google Scholar
Vardi N, Parlakpinar H, Cetin A, Erdogan A, Cetin Ozturk I (2010) Protective. Protective effect of beta-carotene on methotrexate-induced oxidative liver damage. Toxicol Pathol 38:592–597
Farsi F, Mohammadshahi M, Alavinejad P, Rezazadeh A, Zarei M, Engali KA (2016) Functions of coenzyme Q10 supplementation on liver enzymes, markers of systemic inflammation, and adipokines in patients affected by nonalcoholic fatty liver disease: a double blind, placebo-controlled, randomized clinical trial. J Am Coll Nutr 35(4):346–353
Vasiliev AV, Martinova EA, Sharanova NV, Gapparov MM (2011) Effects of coenzyme Q10 on rat liver cells under conditions of metabolic stress. Bull Exp Biol Med 150(4):416–419
Ashkani ES, Esmaeilzadeh E, Bagheri F, Emami Y, Farjam M (2013) The effect of co-enzyme Q10 on acute liver damage in rats, a biochemical and pathological study. Hepat Mon 13(8):e13685
Emam AM, Georgy GS, Shaker OG, Fawzy HM, Zaki HF (2016) Protective effects of alpha-lipoic acid and coenzyme Q10 on lipopolysaccharide-induced liver injury in rats. Pharm. Lett. 8(19):176–182
Baehrecke EH (2005) Autophagy: dual roles in life and death? Nat Rev Mol Cell Biol 6(6):505–510 View at: Publisher Site | Google Scholar
Edinger AL, Thompson CB (2004) Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol 16(6):663–669 View at: Publisher Site |Google Scholar
Imai SI, Armstrong CM, Kaeberlein M, Guarente L (2000) Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403(6771):795–800 View at: Publisher Site | Google Scholar
Wood JG, Rogina B, Lavu S et al (2004) Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 430(7000):686–689 View at: Google Scholar
In HL, Cao L, Mostoslavsky R et al (2008) A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci USA 105(9):3374–3379
Biason-Lauber A, Boni-Schnetzler M, Hubbard BP, Bouzakri K, Brunner A, Cavelti-Weder C et al (2013) Identification of a SIRT1 mutation in a family with type 1 diabetes. Cell Metab 17(3):448–455. https://doi.org/10.1016/j.cmet.2013.02.001
Kupis W, Palyga J, Tomal E, Niewiadomska E (2016) The role of sirtuins in cellular homeostasis. J Physiol Biochem 72(3):371–380. https://doi.org/10.1007/s13105-016-0492-6
Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D et al (2018) Oxidative stress, aging, and diseases. Clin Interv Aging 13:757–772
Sun T, Zhang Y, Zhong S, Gao F, Chen Y, Wang B et al (2018) N-n-butyl haloperidol iodide, a derivative of the anti-psychotic haloperidol, antagonizes hypoxia/reoxygenation injury by inhibiting an Egr-1/ROS positive feedback loop in H9c2 cells. Front Pharmacol 9(19):19. https://doi.org/10.3389/fphar.2018.00019
Zhang T, Chi Y, Ren Y, Du C, Shi Y, Li Y (2019) Resveratrol reduces oxidative stress and apoptosis in podocytes via Sir2-related enzymes, Sirtuins1 (SIRT1)/peroxisome proliferator-activated receptor gamma co-activator 1alpha (PGC-1alpha) axis. Med Sci Monit 25:1220–1231. https://doi.org/10.12659/MSM.911714
Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A (2013) Antagonistic crosstalk between NF-kappaB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal 25(10):1939–1948. https://doi.org/10.1016/j.cellsig.2013.06.007
Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P (2005) Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 434(7029):113–118. https://doi.org/10.1038/nature03354
Ma X, Sun Z, Han X, Li S, Jiang X, Chen S, et al (2019) Neuroprotective Effect of Resveratrol via Activation of Sirt1 Signaling in a Rat Model of Combined Diabetes and Alzheimer’s Disease. Front Neurosci 13:1400:1400. https://doi.org/10.3389/fnins.2019.01400
Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA et al (2004) Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 23(12):2369–2380. https://doi.org/10.1038/sj.emboj.7600244
Ren Z, He H, Zuo Z, Xu Z, Wei Z, Deng J (2019) The role of different SIRT1-mediated signaling pathways in toxic injury. Cell Mol Biol Lett 24:36. https://doi.org/10.1186/s11658-019-0158-9
Wong A, Woodcock EA (2009) FoxO proteins and cardiac pathology. Adv Exp Med Biol 665:78–89. https://doi.org/10.1007/978-1-4419-1599-3_6
Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y et al (2004) Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303(5666):2011–2015. https://doi.org/10.1126/science.1094637
Zhang H, Zhao Z, Pang X, Yang J, Yu H, Zhang Y et al (2017) MiR-34a/sirtuin-1/foxo3a is involved in genistein protecting against ox-LDL-induced oxidative damage in HUVECs. Toxicol Lett 277:115–122. https://doi.org/10.1016/j.toxlet.2017.07.216
Gu X, Han D, Chen W, Zhang L, Lin Q, Gao J et al (2016) SIRT1-mediated FoxOs pathways protect against apoptosis by promoting autophagy in osteoblast-like MC3T3-E1 cells exposed to sodium fluoride. Oncotarget 7(40):65218–65230
Wang S, Moustaid-Moussa N, Chen L, Mo H, Shastri A, Su R et al (2014) Novel insights of dietary polyphenols and obesity. J Nutr Biochem 25(1):1–18
Mercurio V, Pucci G, Bosso G, Fazio V, Battista F, Iannuzzi A et al (2020) A nutraceutical combination reduces left ventricular mass in subjects with metabolic syndrome and left ventricular hypertrophy: a multicenter, randomized, double-blind, placebo-controlled trial. Clin Nutr 39(5):1379–1384. https://doi.org/10.1016/j.clnu.2019.06.026
Miceli M, Bontempo P, Nebbioso A, Altucci L (2014) Natural compounds in epigenetics: a current view. Food Chem Toxicol 73:71–83. https://doi.org/10.1016/j.fct.2014.08.005
McCubrey JA, Lertpiriyapong K, Steelman LS, Abrams SL, Yang LV, Murata RM et al (2017) Effects of resveratrol, tumeric, berberine and other nutraceuticals on aging, cancer development, cancer stem cells and microRNAs. Aging (Albany NY) 9(6):1477–1536
Serafini MM, Catanzaro M, Fagiani F, Simoni E, Caporaso R, Dacrema M et al (2019) Modulation of Keap1/Nrf2/ARE signaling pathway by curcuma- and garlic-derived hybrids. Front Pharmacol 10(1597):1597. https://doi.org/10.3389/fphar.2019.01597
Zhang X, Wu J, Ye B, Wang Q, Xie X, Shen H (2016) Protective effect of tumeric on TNBS-induced intestinal inflammation is mediated through the JAK/STAT pathway. BMC Complement Altern Med 16(1):299. https://doi.org/10.1186/s12906-016-1273-z
Gupta SC, Patchva S, Koh W, Aggarwal BB (2012) Discovery of tumeric, a component of golden spice, and its miraculous biological activities. Clin Exp Pharmacol Physiol 39(3):283–299. https://doi.org/10.1111/j.1440-1681.2011.05648.x
Yang Y, Duan W, Lin Y, Yi W, Liang Z, Yan J et al (2013) SIRT1 activation by tumeric pretreatment attenuates mitochondrial oxidative damage induced by myocardial ischemia reperfusion injury. Free Radic Biol Med 65:667–679
Iside C, Scafuro M, Nebbioso A, Altucci L (2020) SIRT1 activation by natural phytochemicals: an overview. Front Pharmacol. https://doi.org/10.3389/fphar.2020.01225
Acknowledgements
It is directed to the National Research Center, Egypt, for the great support.
Funding
This work has no financial support.
Author information
Authors and Affiliations
Contributions
MOK conceived and designed the experiments; shared in performing the experiment; analyzed (biochemical parameters and RTPCR gene expression) and interpreted the data; contributed reagents, materials, analysis tools, or data; and wrote the paper. NMA shared in experimental animal induction and performed ELISA for FOXO. HAH shared in experimental animal induction and performed ELISA for FOXO. RMAM shared in performing the experiment, analyzed (biochemical parameters and RT-PCR gene expression) and interpreted the data. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This work was approved by the ethics committee of the National Research Center (2443042022).
Consent for publication
All authors have revised the manuscript and there is No conflict of interest to declare.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kadry, M.O., Ammar, N.M., Hassan, H.A. et al. Insights on attenuating autophagy cellular and molecular pathways versus methotrexate-induced toxicity via liposomal turmeric therapy. J Genet Eng Biotechnol 20, 147 (2022). https://doi.org/10.1186/s43141-022-00430-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43141-022-00430-4