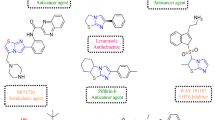
Abstract
Background
To overcome the problem of side effects and toxicity, development of new anticancer agents is needed. Recently, piperidine salicylanilide derivatives with nanomolar epidermal growth factor receptor (EGFR) inhibitory and cytotoxicity activity have been reported. In the present study effect of replacing piperidine in reported piperidine salicylanilide with N-methyl piperazine and changing substituent’s of phenyl ring at other end on anticancer activity have been explored. A series of sixteen methyl piperazine incorporated phenyl benzamide and phenyl methanone derivatives have been synthesized and tested in a panel of three cancer cell lines (adenocarcinomic human alveolar basal epithelial cells (A-549), human colon carcinoma (HCT-116) and human pancreatic carcinoma (MIAPaCa-2)), using gefitinib as standard. Further, to study the probable mechanism, due to their structural similarity with EGFR inhibitors, docking interactions with EGFR active site were observed using Schrodinger suite.
Result
The results indicated that most of the compounds showed promising activity; out of which, compound A-11 was most active having cytotoxicity much better than that of gefitinib. It showed IC50 value of 5.71 µM against A-549 cell line, 4.26 µM against HCT-116 colon cancer line and 31.36 µM against MIAPaCa-2 cell line.
Conclusion
It was found that these compounds fit well in the active site and may be exhibiting anticancer activity via EGFR inhibition.
Graphical Abstract

Similar content being viewed by others
Background
Cancer results from uncontrolled cell growth, and remains one of the most potentially life threatening disease worldwide [1]. As per reports of the International Agency for Research on Cancer, in 2012, 14.1 million new cancer cases were reported, along with 8.2 million cancer deaths worldwide. The global burden of cancer is expected to grow to 21.7 million as new cancer cases and 13 million cancer deaths in 2030 despite the presence of variety of anticancer medicines, development of anticancer agents has received more attention of medicinal chemists, due to occurrence of side effects [2, 3].
EGFR amplification and overexpression are prevalent various cancers including NSCLC, occurring in up to 85% of patients with this type of cancer. Mutations typically arise in exons 18–21, which encompass the kinase domain of the EGFR gene. The majority, around 90%, of these mutations manifest as either exon 21 L858R point mutations or deletions within exon 21. These genetic alterations lead to heightened EGFR kinase activity, resulting in increased downstream signaling. Conversely, most exon 20 insertion mutations are associated with reduced sensitivity to EGFR TKIs. Hence, in order to address resistance, there is a critical need for the development of novel ligands that function as inhibitors of EGFR [4].
In literature, quinazoline and piperazine containing compounds have been reported to inhibit various types of cancers [4]. Quinazoline derivatives are known for EGFR tyrosine kinase (TK) and other kinase inhibition in non small cell lung cancer (NSCLC) and other carcinomas. Gefitinib is one of the quinazoline derivatives which have been used for anticancer activity, especially in lung cancer. Unfortunately, resistance against quinazoline compounds develops within a short span of use [5].
Similarly, piperazine and benzothiazole piperazine derivatives have been evaluated for their anticancer potential against HCT-116 colon cancer cell line [6,7,8]. Piperazine substituted adamantanes have been reported for colon and pancreatic cancer, whereas piperazine methanone shows good potency against human breast adenocarcinoma cell line, MCF-7 and mouse embryonic fibroblast cell lines, NIH3T3 [9, 10]. Moreover, various piperazine derivatives have been patented as lung cancer chemotherapy agents [11]. Benzofuran with N-aryl piperazine derivatives have also been reported for anticancer activity as hybrid compounds [12]. Recently, phenyl piperazine benzoxazole and benzhydryl piperazine derivatives have been shown to inhibit lung, breast and other carcinomas [13,14,15].
Hu et al. have described novel piperidine salicylanilide derivatives having nanomolar EGFR TK inhibitory and cytotoxicity activity (Fig. 1) [16]. Using this structure as basis, scaffold I was considered wherein the piperidine was replaced with N-methyl piperazine and the phenyl ring on the amidic nitrogen was substituted with various electron donating and withdrawing substituent’s, hypothesizing that these changes could enhance the anticancer potential. Further, in scaffold II, the amidic nitrogen was constrained in a piperazine ring and the effect of substituents on the phenyl ring on the other nitrogen was also evaluated.
Materials
For docking, Glide XP of Schrodinger suite was used and physicochemical properties of the compounds were predicted using Chemaxon Jchem for Excel. The docking protocol was validated to govern the reliability and reproducibility of the docking parameters used for the study. Reaction progress was monitored using analytical grade solvents and pre-coated Silicagel G TLC plates (Kieselgel 60F254, Merck) and visualized in UV light. Melting points were measured with Buchi 530 melting point apparatus and were uncorrected. 1H NMR spectra were recorded on a Bruker Avance-400 MHz system using CDCl3 or DMSO as the solvent. Chemical shifts (d) are reported in parts per millions (ppm) relative to TMS as internal standard. FTIR spectra were performed on IR Prestige 21 Shimadzu using KBr as standard. MS were analyzed on MICROMASS Quattro-II LCMS system (Waters Corporation, Milford, USA). All reagents were obtained from commercial suppliers and used without further purification. Synthesized compounds were tested against A-549 (human lung carcinoma), HCT-116 (human colon cancrcinoma) and MIAPaCa-2 (human pancreatic carcinoma) for anticancer potential.
Method
The X-ray structure of EGFR bound with gefitinib as a cocrystallized ligand (pdb id: 2ITO) was obtained from the brookhaven protein database for docking purposes [17]. Docking of the compounds was performed using Glide 5.9. Running on maestro version 9.4, to investigate their putative binding mode in EGFR binding pocket. The Protein preparation wizard within the Schrödinger suite was employed to prepare the designated protein. The protein underwent distinct preprocessing stages, which involved the removal of the substrate co-factor and water molecules lacking hydrogen bonds, followed by the optimization of hydrogen bonds. Subsequently, a charge was assigned, and the energy was minimized, achieving a Root Mean Square Deviation (RMSD) value of 0.30 Å utilizing the Optimized Potentials for Liquid Simulations-2005 (OPLS-2005) force field. The structures of all compounds were drawn using ChemSketch and converted into 3D structures through a 3D optimization tool. The ligands, drawn previously, were geometry optimized using the LigPrep 2.6 module, with partial atomic charges computed through the OPLS-2005 force field. The prepared ligands were then subjected to docking with the prepared protein utilizing the Glide 5.9 module, operating in extra precision mode (XP) [18]. The anticancer activity was measured by using the 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) cell viability assay and Gefitinib was used as positive control.
Synthetic scheme
Total 10 derivatives of 4-(3-(4-methylpiperazin-1-yl)propoxy)-N-phenylbenzamide (scaffold I) and 6 derivatives of (4-phenyl)piperazin-1-yl)(4-(3-(4-methylpiperazin-1-yl)propoxy)phenyl)methanone (scaffold II) were synthesized. All the synthesized analogs were subjected to characterization using infrared spectroscopy (IR), proton nuclear magnetic resonance spectroscopy (1H NMR), and mass spectrometry (MS) to determine their molecular weight.
The compounds were synthesized using Scheme 1.
Initially, N-methyl piperazine was alkylated using 1-bromo-3-chloropropane. Resultant 1-(3-chloropropyl)-4-methyl piperazine was refluxed with methyl 4-hydroxybenzoate in acetonitrile using potassium carbonate for O-alkylation. The methyl 4-[3-(4-methylpiperazin-1-yl) propoxy] benzoate formed was hydrolyzed with base to give 4-[3-(4-methylpiperazin-1-yl)propoxy]benzoic acid, which was then coupled with substituted anilines or phenyl piperazines to give final compounds using hydroxy-O-benztriazole (HOBt) and N-(3-dimethylaminopropyl)-N’-ethylcarbidiimide.HCl (EDC.HCl). Purity of the compounds was checked by High Performance Liquid Chromatography (HPLC). All the intermediates and final compounds were characterized by spectroscopic techniques.
Biological evaluation: cell viability/MTT assay
The reduction of tetrazolium salts was used to examine cell proliferation and growth inhibition. Gefitinib was used as positive control and IC50 (the concentration which resulted cytotoxicity in 50% cells) values are the mean of three independent experiments [19].
For in vitro evaluation, MTT assays for all compounds against three cell lines viz. A-549 human lung carcinoma, HCT-116 human colon cancer and MIAPaCa-2 human pancreatic carcinoma were performed. The DMEM, RPMI-1640, DMEM-F12, were used as culture mediums for A-549 human lung carcinoma, HCT-116 colon cancer and pancreatic MIAPaCa-2 cell lines, respectively. 104 Cells per well were grown in 96-well plates and exposed to different concentrations of various test compounds for 48 h. After 44 h treatment, 20 µl of MTT solution (2.5 mg/ml) was added to each well and incubated at 37 °C for 4 h in a humidified atmosphere containing 5% CO2. In case of suspension cell lines, the plates were centrifuged at 1500 r.p.m. for 15 min, and the supernatant was discarded while in adherent cell lines, the media was removed without centrifugation. The MTT-formazan crystals were dissolved in 150 µl dimethyl sulfoxide. The absorbance was recorded at a wavelength of 570 nm in the microplate reader and cytotoxicity was calculated as % cell growth inhibition.
where At, Ab and Ac are absorbance of test, blank and control, respectively. Concentrations 1 µM, 10 µM, 20 µM, 30 µM, and 50 µM were used for assay.
Results
Spectral data
1-(3-chloropropyl)-4-methylpiperazine: Liquid, 80%, IR (KBr, cm−1): 2954–2858 (Aliphatic-C-H stretching), 1355 (C-N); 1H NMR (400 MHz, Chloroform-d) δ 3.54 (t, J = 3.9 Hz, 2H), 2.61–2.50 (m, 8H), 2.90–2.570 (m, 2H), 2.32 (s, 3H), 1.98 (tt, J = 6.4, 4.0 Hz, 2H).
Methyl-4-[3-(4-methylpiperazin-1-yl)propoxy]benzoate: Liquid, 75%, IR (KBr, cm−1): 3217–3004 (Aromatic CH stretching) 2954–2858 (Aliphatic CH stretching), 1750–1735 (–CO), 1373 (–CN); 1H NMR (400 MHz, Chloroform-d) δ 7.90–7.84 (m, 2H), 7.05–6.99 (m, 2H), 3.99 (t, J = 6.1 Hz, 2H), 3.94 (s, 3H), 2.61 (t, J = 6.4 Hz, 2H), 2.58–2.50 (m, 8H), 2.32 (s, 3H), 1.83 (td, J = 6.2 Hz, 2H).
Methyl-4-[3-(4-methylpiperazin-1-yl)propoxy]benzoic acid: White solid, 70%, (mp: 180–184 °C), IR (KBr, cm−1), 3210–3010 (Aromatic C–H stretching) 2954–2858 (Aliphatic-CH stretching), 1703 (–CO), 1373 (–CN) 1280–1300 (CO ether); 1H NMR (400 MHz, Chloroform-d) δ 7.90–7.84 (m, 2H), 7.05–6.99 (m, 2H), 3.99 (t, J = 6.1 Hz, 2H), 2.61 (t, J = 6.4 Hz, 2H), 2.58–2.50 (m, 8H), 2.32 (s, 3H), 1.83 (p, J = 6.2 Hz, 2H).
A-1: 4-[3-(4-Methylpiperazin-1-yl)propoxy]-N-phenylbenzamide: White solid, 55%, (mp: 134–136 °C), IR (KBr, cm−1), 3344.57 (–NH stretching) 3010–2980 (Aromatic CH stretching), 2881–2852 (Aliphatic CH stretching) 1633 (–CO amide); 1H NMR (400 MHz, Chloroform-d), δ 8.04–7.98 (m, 2H), 7.73–7.67 (m, 2H), 7.36–7.29 (m, 2H), 7.09 (tt, J = 7.0, 1.2 Hz, 1H), 7.00–6.94 (m, 2H), 3.99 (t, J = 6.1 Hz, 2H), 2.61 (t, J = 6.4 Hz, 2H), 2.54 (d, J = 1.2 Hz, 8H), 2.32 (s, 3H), 1.83 (td, J = 6.2 Hz, 2H). 13C-NMR (100 MHz, CDCl3): δ = 164.7, 162.6, 137.8, 128.6, 128.9, 128.2, 128.3, 128.2, 125.8, 121.9, 114.5, 114.6, 73.1, 57.2, 57.9, 58.9, 55.4, 55.9, 46.9, 28.6, 27.02. ESI MS: m/z = 354.21 (M + 1 H)+.
A-2: 4-[3-(4-Methylpiperazin-1-yl)propoxy]-N-(p-tolyl)benzamide: White solid, 58%, (138–140 °C), IR (KBr, cm−1), 3350 (–NH) 3005–2933 (Aromatic CH stretching), 2875–2792 (Aliphatic CH stretching), 1651 (CO); 1H NMR (400 MHz, Chloroform-d), δ 8.04–7.90 (m, 2H), 7.44–7.37 (m, 2H), 7.18–7.12 (m, 2H), 7.00–6.90 (m, 2H), 3.96 (t, J = 6.1 Hz, 2H), 2.61 (t, J = 6.4 Hz, 2H), 2.57–2.51 (m, 8H), 2.31 (d, J = 6.0 Hz, 6H), 1.86 (tt, J = 6.2 Hz, 2H); 13C-NMR (100 MHz, CDCl3): δ = 164.7, 162.6, 137.8, 128.6, 129.9, 128.2, 129.3, 127.2, 125.8, 121.9, 114.5, 114.6, 73.1, 57.2, 57.9, 58.9, 55.4, 55.9, 46.9, 28.6, 27.02, 21.5, ESI MS: m/z = 368.2 (M + 1 H)+.
A-3: 4-[3-(4-Methylpiperazin-1-yl)propoxy]-N-(m-tolyl)benzamide: Off white solid, 52%, (mp: 132–134 °C), IR (KBr, cm−1), 3350 (–NH stretching) 3000–2931 (Aromatic CH stretching), 2875–2767 (Aliphatic CH stretching), 1643 (CO amide); 1H NMR (400 MHz, Chloroform-d), δ 8.03–7.91 (m, 2H), 7.50 (t, J = 1.9 Hz, 1H), 7.44 (ddd, J = 7.7, 1.8, 1.1 Hz, 1H), 7.18 (t, J = 7.9 Hz, 1H), 7.00–6.90 (m, 3H), 3.99 (t, J = 6.1 Hz, 2H), 2.61 (t, J = 6.4 Hz, 2H), 2.57–2.50 (m, 8H), 2.30 (s, 3H), 2.27 (d, J = 0.7 Hz, 3H), 1.81 (p, J = 6.2 Hz, 2H): 13C-NMR (100 MHz, CDCl3): δ = 164.7, 162.6, 138.8, 128.6, 129.9, 128.2, 129.3, 125.2, 125.8, 121.9, 118.5, 114.6, 114.9, 73.1, 56.2, 57.8, 58.9, 55.4, 55.9, 46.9, 28.6, 21.3; ESI MS: m/z = 368.1 (M + 1 H)+.
A-4: N-(2,4-dimethylphenyl)-4-(3-(4-methylpiperazin-1-yl)propoxy)benzamide: White solid, 55%, (mp: 144–146 °C), IR (KBr, cm−1), 3300 (–NH stretching), 3000–2931 (Aromatic CH stretching), 2875–2767 (Aliphatic CH stretching), 1643 (–CO amide); 1H NMR (400 MHz, Chloroform-d), δ 8.01–7.92 (m, 2H), 7.71 (d, J = 8.3 Hz, 1H), 7.03–6.94 (m, 4H), 3.99 (t, J = 6.1 Hz, 2H), 2.61 (t, J = 6.4 Hz, 2H), 2.57–2.51 (m, 8H), 2.32 (s, 3H), 2.24–2.17 (m, 6H), 1.81 (p, J = 6.2 Hz, 2H); 13C-NMR (100 MHz, CDCl3): δ = 164.7, 161.6, 143.3, 134.8, 131.6, 130.9, 128.2, 128.3, 125.8, 121.9, 118.5, 114.5, 114.9, 73.1, 56.2, 57.8, 57.9, 55.4, 55.4, 46.9, 28.6, 21.6, 17.6; ESI MS: m/z = 382.18 (M + 1 H)+.
A-5: N-(3,4-dimethylphenyl)-4-(3-(4-methylpiperazin-1-yl)propoxy)benzamide: White solid, 58%, (mp: 138–140 °C), IR (KBr, cm−1), 3300 (–NH stretching), 3010–2931 (Aromatic CH stretching), 2875–2767 (aliphatic CH stretching), 1645 (–CO amide); 1H NMR (400 MHz, Chloroform-d) δ 8.04–7.98 (m, 2H), 7.55–7.45 (m, 2H), 7.06 (dq, J = 8.4, 1.0 Hz, 1H), 7.00–6.94 (m, 2H), 3.99 (t, J = 6.1 Hz, 2H), 2.61 (t, J = 6.4 Hz, 2H), 2.57–2.50 (m, 8H), 2.32 (s, 3H), 2.21–2.17 (m, 6H), 1.86 (p, J = 6.2 Hz, 2H).
13C-NMR (100 MHz, CDCl3): δ = 13C-NMR (100 MHz, CDCl3): δ = 164.7, 162.8, 143.3, 134.6, 135.6, 130.9, 129.2, 129.3, 125.8, 121.9, 117.5, 114.5, 114.9, 73.1, 56.2, 57.8, 57.9, 55.4, 55.4, 46.9, 27.7, 21.2, 17.6; ESI MS: m/z = 382.19 (M + 1 H)+.
A-6: N-(2,5-dimethylphenyl)-4-(3-(4-methylpiperazin-1-yl)propoxy)benzamide: Off-white solid, 52%, (mp: 130–132 °C), IR (KBr, cm−1), 3300.20 (–NH stretching), 3020–2931 (Aromatic CH stretching), 2875–2767 (Aliphatic CH stretching), 1644 (–CO amide); 1H NMR (400 MHz, Chloroform-d) δ 8.02–7.98 (m, 2H), 7.51 (d, J = 2.1 Hz, 2H), 7.00–6.94 (m, 2H), 6.90–6.85 (m, 1H), 3.99 (t, J = 6.1 Hz, 2H), 2.61 (t, J = 6.4 Hz, 2H), 2.57–2.50 (m, 8H), 2.31 (s, 3H), 2.24 (s, 6H), 1.80 (p, J = 6.2 Hz, 2H); 13C-NMR (100 MHz, CDCl3): δ = 164.7, 161.6, 143.3, 134.8, 131.6, 130.9, 129.2, 129.3, 125.8, 121.9, 118.5, 114.5, 114.9, 73.1, 56.2, 57.8, 57.9, 55.4, 55.4, 46.9, 27.6, 23.0, 21.6; ESI MS: m/z = 382.2 (M + 1 H)+.
A-7: N-(4-Methoxyphenyl)-4-(3-(4-methylpiperazin-1-yl)propoxy)benzamide: White solid, 59%, (mp: 140–142 °C), IR (KBr, cm−1), 3304 (–NH stretching), 3000–2931 (Aromatic CH stretching), 2866–2781 (Aliphatic CH stretching), 1643 (–CO amide); 1H NMR (400 MHz, Chloroform-d) δ 8.04–7.96 (m, 2H), 7.74–7.69 (m, 2H), 7.42–7.35 (m, 2H), 7.00–6.94 (m, 2H), 3.99 (t, J = 6.1 Hz, 2H), 2.61 (t, J = 6.4 Hz, 2H), 2.54 (d, J = 1.1 Hz, 8H), 2.32 (s, 3H), 1.83 (p, J = 6.2 Hz, 2H); 13C-NMR (100 MHz, CDCl3): δ = 164.7, 162.6, 158, 143.3, 134.8, 131.6, 130.9, 129.2, 129.3, 125.8, 121.9, 118.5, 114.5, 114.9, 73.1, 56.2, 55.8, 57.4, 55.4, 55.4, 46.6, 27.7; ESI MS: m/z = 384.1 (M + 1 H)+.
A-8: N-(4-Chlorophenyl)-4-(3-(4-methylpiperazin-1-yl)propoxy)benzamide: Brown solid, 57%, (mp: 146–148 °C), IR (KBr, cm−1), 3300 (–NH stretching) 3012–2937 (Aromatic CH stretching) 2875–2791 (Aliphatic Ch stretching), 1633 (–CO amide); 1H NMR (400 MHz, Chloroform-d) δ, 8.04–7.98 (m, 2H), 7.75–7.69 (m, 2H), 7.42–7.36 (m, 2H), 7.00–6.94 (m, 2H), 3.99 (t, J = 6.1 Hz, 2H), 2.61 (t, J = 6.4 Hz, 2H), 2.50 (d, J = 1.1 Hz, 8H), 2.32 (s, 3H), 1.89 (p, J = 6.2 Hz, 2H); 13C-NMR (100 MHz, CDCl3): δ = 164.7, 162.8, 136.8, 134.6, 130.9, 128.2, 128.3, 125.8, 121.9, 121.6, 118.5, 114.5, 114.9, 73.1, 56.2, 55.8, 57.4, 55.4, 55.3, 46.6, 27.6; ESI MS: m/z = 388.1 (M + 1 H)+.
A-9: N-(4-Bromophenyl)-4-(3-(4-methylpiperazin-1-yl)propoxy)benzamide: Brown solid, 57%, (mp: 156–158 °C), IR (KBr, cm−1), 3317 (–NH stretching), 3016–2937 (Aromatic CH stretching), 2855–2769 (Aliphatic CH stretching), 1645 (–CO amide); 1H NMR (400 MHz, Chloroform-d) δ 8.04–7.98 (m, 2H), 7.69–7.63 (m, 2H), 7.53–7.47 (m, 2H), 7.00–6.92 (m, 2H), 3.99 (t, J = 6.1 Hz, 2H), 2.63 (t, J = 6.4 Hz, 2H), 2.53 (d, J = 1.1 Hz, 8H), 2.32 (s, 3H), 1.79 (p, J = 6.2 Hz, 2H); 13C-NMR (100 MHz, Chloroform-d): δ = 164.7, 162.6, 162.2, 136.5, 131.5, 131.5, 126.3, 126.4, 125.8, 115.3, 115.6, 114.5, 114.9, 73.1, 58.2, 55.8, 57.4, 55.4, 55.4, 46.6, 27.7; ESI MS: m/z = 432.1 (M + 1 H)+.
A-10: N-(4-Fluorophenyl)-4-(3-(4-methylpiperazin-1-yl)propoxy)benzamide: Off white solid, 52%, (mp: 142–144 °C), IR (KBr, cm−1), 3300 (–NH stretching) 3000–2975 (Aromatic CH stretching) 2873–2791 (Aliphatic CH stretching), 1656 (–CO amide); 1H NMR (400 MHz, Chloroform-d) δ, 8.04–7.93 (m, 2H), 7.62–7.54 (m, 2H), 7.17–7.09 (m, 2H), 7.01–6.94 (m, 2H), 3.99 (t, J = 6.1 Hz, 2H), 2.64 (t, J = 6.4 Hz, 2H), 2.54 (d, J = 1.1 Hz, 8H), 2.32 (s, 3H), 1.81 (p, J = 6.2 Hz, 2H); 13C-NMR (100 MHz, Chloroform-d): δ = 164.7, 162.6, 162.2, 133.5, 128.5, 128.5, 126.3, 126.4, 125.8, 115.3, 115.6, 114.5, 114.9, 73.1, 58.2, 55.8, 57.4, 55.4, 55.4, 46.6, 27.7; ESI MS: m/z = 372.1 (M + 1 H)+.
A-11: (4-(3-Methoxyphenyl)piperazin-1-yl)(4-(3-(4-methylpiperazin-1-yl)propoxy)phenyl) methanone: Brown solid, 45%, (mp: 166–168 °C), IR (KBr, cm−1), 3050–2956.52 (Aromatic CH stretching), 2858–2762 (Aliphatic CH stretching), 1633 (–CO amide); 1H NMR (400 MHz, Chloroform-d) δ 7.85–7.78 (m, 2H), 7.12 (t, J = 7.8 Hz, 1H), 7.00–6.94 (m, 2H), 6.45 (ddd, J = 7.9, 2.0, 1.3 Hz, 1H), 6.27 (ddd, J = 7.9, 1.9, 1.2 Hz, 1H), 6.20 (t, J = 1.9 Hz, 1H), 3.98 (t, J = 6.1 Hz, 2H), 3.78 (s, 3H), 3.61(t, J = 5.3 Hz, 4H), 3.36–3.17 (m, 4H), 2.65 (t, J = 6.4 Hz, 2H), 2.57–2.50 (m, 8H), 2.30 (s, 3H), 1.82 (p, J = 6.2 Hz, 2H); 13C-NMR (100 MHz, Chloroform-d): δ = 168.9, 160.2, 161.2, 150.2, 130.2, 127.1, 127.6, 126.2, 114.1, 114.1, 110.5, 106.2, 97.5, 73.1,58.2, 57.6, 57.6, 55.4, 55.4, 55.8, 53.5, 53.0, 50.1, 50.2,46.6, 27.3; ESI MS: m/z = 453.2 (M + 1 H)+.
A-12: (4-(2-Methoxyphenyl)piperazin-1-yl)(4-(3-(4-methylpiperazin-1-yl)propoxy)phenyl) methanone: White solid, 45%, (mp: 160–162 °C), IR (KBr, cm−1) 3012–2958 (Aromatic CH stretching), 2868–2762 (Aliphatic CH stretching), 1633 (–CO amide); 1H NMR (400 MHz, Chloroform-d) δ 7.89–7.77 (m, 1H), 7.07–7.01 (m, 1H), 7.00–6.94 (m, 1H), 6.89–6.83 (m, 1H), 3.97 (t, J = 6.1 Hz, 2H), 3.78 (s, 2H), 3.60 (t, J = 5.3 Hz, 2H), 3.28–3.15 (m, 2H), 2.61 (t, J = 6.3 Hz, 1H), 2.54 (d, J = 1.1 Hz, 4H), 2.31 (s, 2H), 1.82 (p, J = 6.2 Hz, 1H); 13C-NMR (100 MHz, Chloroform-d): δ = 168.9, 160.4, 161.2, 150.2, 130.2, 127.8, 127.8, 123.2, 113.1, 114.1, 110.5, 106.2, 97.5, 73.1, 58.2, 57.6, 57.6, 55.8, 55.4, 55.7, 53.5, 53.0, 50.1, 50.2, 46.6, 27.7; ESI MS: m/z = 453.2 (M + 1 H)+.
A-13: (4-(2-chlorophenyl)piperazin-1-yl)(4-(3-(4-methylpiperazin-1-yl)propoxy)phenyl) methanone: Off white solid, 49%, (mp: 168–170 °C), IR (KBr, cm−1), 3010–2954 (Aromatic CH stretching), 2900–2810 ( Aliphatic CH stretching), 1631 (–CO amide); 1H NMR (400 MHz, Chloroform-d) δ 7.85–7.78 (m, 2H), 7.27 (dd, J = 7.8, 1.5 Hz, 1H), 7.04 (td, J = 7.6, 1.5 Hz, 1H), 7.00–6.93 (m, 3H), 6.77 (td, J = 7.6, 1.6 Hz, 1H), 3.96 (t, J = 6.1 Hz, 2H), 3.60 (t, J = 5.3 Hz, 4H), 3.27 (dt, J = 11.9, 5.3 Hz, 2H), 3.18 (dt, J = 11.9, 5.3 Hz, 2H), 2.60 (t, J = 6.4 Hz, 2H), 2.57–2.50 (m, 8H), 2.36 (s, 3H), 1.83 (p, J = 6.2 Hz, 2H); 13C-NMR (100 MHz, Chloroform-d): δ = 168.9, 160.4, 161.2, 150.8, 130.1, 129.1, 127.8, 124.2, 113.1, 114.1, 110.5, 106.2, 97.5, 73.1, 58.2, 57.6, 57.6, 55.8, 55.4, 55.7, 53.5, 52.5, 50.1, 50.2, 46.6, 27.7; ESI MS: m/z = 458.2 (M + 1 H)+.
A-14: [4-(4-chlorophenyl)piperazin-1-yl][(4-(3-(4-methylpiperazin-1-yl)propoxy)phenyl] methanone: Off white solid, 46%, (mp: 174–176 °C), IR (KBr, cm−1) 3000–2954 (Aromatic CH stretching), 2900–2810 (Aliphatic CH stretching), 1643 (–CO amide); 1H NMR (400 MHz, Chloroform-d) δ 7.86–7.78 (m, 1H), 7.26–7.20 (m, 1H), 7.00–6.94 (m, 1H), 6.77–6.73 (m, 1H), 3.97 (t, J = 6.1 Hz, 2H), 3.60 (t, J = 5.3 Hz, 2H), 3.27–3.15 (m, 2H), 2.61 (t, J = 6.4 Hz, 1H), 2.54 (d, J = 1.1 Hz, 4H), 2.32 (s, 2H), 1.83 (td, J = 6.2 Hz, 1H); 13C-NMR (100 MHz, Chloroform-d): δ = 168.9, 160.4, 149.2, 135.2, 131.8, 127.8, 126.4, 121.2, 118.4, 114.1, 114.5, 110.5, 106.2, 73.1, 58.2, 57.6, 57.6, 55.8, 55.4, 55.7, 53.5, 50.1, 50.2, 46.6, 27.7; ESI MS: m/z = 458.2 (M + 2 H)+.
A-15: (4-(2,3-dichlorophenyl)piperazin-1-yl)(4-(3-(4-methylpiperazin-1-yl)propoxy)phenyl) methanone: Off white solid, 41%, (mp: 156–158 °C), IR (KBr, cm−1), 3061–2904 (Aromatic CH stretching), 2870–2787 (Aliphatic CH stretching), 1643 (–CO amide); 1H NMR (400 MHz, Chloroform-d) δ 7.85–7.78 (m, 2H), 7.23–7.14 (m, 2H), 7.00–6.92 (m, 2H), 6.89 (dd, J = 7.1, 2.0 Hz, 1H), 3.96 (t, J = 6.1 Hz, 2H), 3.61 (t, J = 5.3 Hz, 4H), 3.27 (dt, J = 11.9, 5.3 Hz, 2H), 3.18 (dt, J = 11.7, 5.3 Hz, 2H), 2.60 (t, J = 6.4 Hz, 2H), 2.54–2.50 (m, 8H), 2.32 (s, 3H), 1.81 (p, J = 6.2 Hz, 2H); 13C-NMR (100 MHz, Chloroform-d): δ = 168.9, 160.4, 150.2, 133.2, 129.8, 127.8, 127.8, 127.8, 126.4, 123.2, 117.6, 114.1, 114.5, 73.1, 58.2, 57.6, 57.6, 55.4, 55.4, 52.1, 52.2, 50.1, 50.2, 46.6, 27.7; ESI MS: m/z = 458.2 (M + 2 H)+.
A-16: (4-(3-(4-methylpiperazin-1-yl)propoxy)phenyl)(4-(p-tolyl)piperazin-1-yl) methanone: White solid, 42%, (mp: 196–198 °C), IR (KBr, cm−1) 3000–2950 (Aromatic CH stretching), 2880–2778 Aliphatic CH stretching), 1630 (–CO amide); 1H NMR (400 MHz, Chloroform-d) δ 7.85–7.78 (m, 1H), 7.19–7.12 (m, 1H), 7.00–6.94 (m, 1H), 6.82–6.76 (m, 1H), 3.97 (t, J = 6.1 Hz, 2H), 3.60 (t, J = 5.3 Hz, 2H), 3.27–3.14 (m, 2H), 2.61 (t, J = 6.4 Hz, 1H), 2.57–2.50 (m, 4H), 2.32 (d, J = 6.0 Hz, 3H), 1.83 (td, J = 6.2 Hz, 1H); 13C-NMR (100 MHz, Chloroform-d): δ = 168.9, 160.4, 149.2, 134.2, 134.1, 127.8, 127.8, 126.4, 114.6, 114.1, 93.5, 73.1, 58.2, 57.6, 57.6, 55.4, 55.4, 53.7, 53.7, 50.9, 50.9, 46.6, 34.2, 32.4, 27.7; ESI MS: m/z = 436.1 (M + 1 H)+.
Cell viability/MTT assay
In adenocarcinomic human alveolar basal epithelial cell line (Lung cancer, A-549) gefitinib showed IC50 value of 16.56 µM, in the human colon carcinoma cell line (HCT-116 cell line), it showed an IC50 value of 10.51 µM, whereas in human pancreatic carcinoma MIAPaCa-2 cell line, the IC50 value was 49.50 µM. Results of MTT assay for all the compounds are given in Table 1.
In silico studies (docking and physiochemical properties)
The series was prepared keeping similar features to that of piperidine salicylanilide, having EGFR inhibitory activity. Therefore, it was thought to study the in silico docking of active compounds with EGFR to suggest the possible mechanism of action. Glide score and physicochemical propoerties of active compounds are given in Table 2 [20,21,22,23].
Discussion
Cell viability/MTT assay
In the A-549 lung cancer cell line, electron-donating substitution on the aniline portion showed better inhibition. Compound A6 (2,5 di-CH3) with IC50 7.74 µM showed better inhibition as compared to compound A4 (2,4 di-CH3) and A5 (3,4 di-CH3). Similarly, among scaffold-II compounds, A11 (3-OCH3) and A12 (4-OCH3) showed the IC50 value of 5.71 µM, 13.16 µM and A16 (4-CH3)14.28 µM, respectively. In this cell line, meta substitution resulted in favorable activity and compounds with electron withdrawing groups (EWGs) such as, A8 (4-Cl), A9 (4-Br), A10 (4-F) were weakly active.
In the HCT-116 colon cancer line, three compounds showed better activity than gefitinib. Compounds A1 (-H) and A4 (2,4 di-CH3) displayed IC50 value of 6.54 µM and 10.54 µM, respectively while compound A-11 having meta methoxy substitution was most active with IC50 of 4.26 µM. Again, in this cell line, electron donating substituted compounds exhibited good inhibition and compounds with electron withdrawing groups were inactive.
In the MIAPaCa-2 cell line, dimethyl substituted aniline derivatives showed better results when compared to mono substituted compounds. Compounds A-4 (2,4 di-CH3), A-5 (3,4 di-CH3) and A-6 (2,5 di-CH3) showed IC50 values of 11.54, 6.26 and 14.98 µM respectively. From scaffold-II derivatives, compound A-11 (3-OCH3) and compound A-16 (4-CH3) showed better inhibition than gefitinib.
Overall, compound A-11 was found to be the most active in two of the three cell lines. Also, by observing the IC50 values of all the compounds, it can be said that compounds with electron-donating substituents on the aniline portion exhibited better inhibition as compared to compounds with electron withdrawing groups.
Docking
It was found that the docked ligand superimposed well with reference ligand (gefitinib, co-crystallized ligand) with root mean square deviation value of 0.458 Å. The gefitinib in active site of EGFR displayed hydrogen bonding interaction with MET793 of hinge region, which is essential. Additionally, it shows hydrophobic interactions with CYS797 and LEU792 of hinge region and LEU844 and MET766 in the C-helix with a glide score of − 7.31.
It was observed that compounds with electron-withdrawing substituents did not dock well in the active site and compounds with electron donating substituents fit well. Compound A-10 with p-fluoro substituent on scaffold I was the only compound with electron withdrawing substituent and weak in the MTT assay was found to have least activity glide score. Its glide score was less than the unsubstituted compound. It was observed that it does not take part in any hydrogen bonding interaction.
When all the compounds were docked, it was observed that hydrophobic interactions with CYS797, LEU792, MET793 of hinge region, LEU844 and MET766 in the C-helix were similar for all compounds. However, the compounds showed hydrogen bonding interaction with MET793 and those with better score also showed side chain hydrogen bonding with ASP855 in activation loop. This indicates that these compounds do show necessary interactions in active site and hence may exert anticancer activity by inhibiting as EGFR.
Docking interactions of representative compounds are shown in Fig. 2.
Conclusion
A range of 16 compounds consisting of methyl piperazine-incorporated phenyl benzamide and phenyl methanone derivatives were synthesized and assessed for their potential as anticancer agents against A-549 human lung carcinoma, HCT-116 human colon cancer, and MIAPaCa-2 human pancreatic carcinoma cell lines. It was noted that compounds possessing electron-donating groups exhibited heightened cytotoxicity across the three cell lines. Additionally, a hypothesis was put forward suggesting that the observed anticancer effects may stem from the inhibition of EGFR. Previous literature indicates that hydrogen bond interactions with MET793 have been linked to EGFR inhibition, and the compounds exhibiting superior anticancer activity were found to interact with MET793 within the EGFR binding site. The results suggest that these derivatives function as anticancer agents; nevertheless, further investigations are necessary to ascertain their specificity as EGFR inhibitors.
Availability of data and materials
Data and supplementary material will be provided on demand.
Abbreviations
- EGFR:
-
Epidermal growth factor receptor
- HCT:
-
Human colon Carcinoma
- NSCLC:
-
Non small cell lung cancer
- CYS:
-
Cysteine
- LEU:
-
Leucine
- MET:
-
Methionine
- TK:
-
Tyrosine kinase
- HOBt:
-
Hydroxy-O-benztriazole
- EDC.HCl:
-
N-(3-Dimethylaminopropyl)-N’-ethylcarbidiimide hydrochloride
- MTT:
-
3-(4, 5-Dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide
- CDCl3 :
-
Deuteriated chloroform
- DMSO:
-
Dimethyl sulfoxide
- TMS:
-
Trimethyl silane
References
Yu H, Jove R (2004) The STATs of cancer-new molecular targets come of age. Nat Rev Cancer 4(2):97–105
American Cancer Society (2016) Cancer facts & figures. Cancer Facts Fig 2016:1–9
Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A (2016) Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 66(4):271–289
Prabhakar CN (2015) Epidermal growth factor receptor in non-small cell lung cancer. Transl Lung Cancer Res 4:110–118
Shagufta S, Ahmad I (2017) An insight into the therapeutic potential of quinazoline derivatives as anticancer agents. Med Chem Commun 8(5):871–885
Cheng H, Nair SK, Murray BW, Almaden C, Bailey S, Baxi S, Behenna D, Cho-Schultz S, Dalvie D, Dinh DM, Edwards MP, Feng JL, Ferre RA, Gajiwala KS, Hemkens MD, Jackson-Fisher A, Jalaie M, Johnson TO, Kania RS, Kephart S, Lafontaine J, Lunney B, Liu KKC, Liu Z, Matthews J, Nagata A, Niessen S, Ornelas MA, Orr STM, Pairish M, Planken S, Ren S, Richter D, Ryan K, Sach N, Shen H, Smeal T, Solowiej J, Sutton S, Tran K, Tseng E, Vernier W, Walls M, Wang S, Weinrich SL, Xin S, Xu H, Yin MJ, Zientek M, Zhou R, Kath JC (2016) Discovery of 1-{(3R,4R)-3-[({5-Chloro-2-[(1-Methyl-1H-Pyrazol-4-Yl)amino]-7H-pyrrolo[2,3-D]pyrimidin-4-Yl}oxy)methyl]-4-Methoxypyrrolidin-1-Yl}prop-2-En-1-One (PF-06459988), a Potent, WT Sparing, Irreversible Inhibitor of T790M-Containing EGFR Mutants. J Med Chem 59(5):2005–2024
Chopra A, Anderson A, Giardina C (2014) Novel piperazine-based compounds inhibit microtubule dynamics and sensitize colon cancer cells to tumor necrosis factor-induced apoptosis. J Biol Chem 289(5):2978–2991
Gurdal EE, Durmaz I, Cetin-Atalay R, Yarim M (2015) Cytotoxic activities of some benzothiazole-piperazine derivatives. J Enzyme Inhib Med Chem 30(4):649–654
Lin HH, Wu WY, Cao SL, Liao J, Ma L, Gao M, Li ZF, Xu X (2013) Synthesis and antiproliferative evaluation of piperazine-1-carbothiohydrazide derivatives of indolin-2-one. Bioorganic Med Chem Lett 23(11):3304–3307
Riganas S, Papanastasiou I, Foscolos GB, Tsotinis A, Bourguignon JJ, Serin G, Mirjolet JF, Dimas K, Kourafalos VN, Eleutheriades A, Moutsos VI, Khan H, Georgakopoulou S, Zaniou A, Prassa M, Theodoropoulou M, Pondiki S, Vamvakides A (2012) Synthesis, σ 1, σ 2-receptors binding affinity and antiproliferative action of new C1-substituted adamantanes. Bioorganic Med Chem 20(10):3323–3331
Yurttaş L, Demirayak Ş, Ilgin S, Atli Ö (2014) In vitro antitumor activity evaluation of some 1,2,4-triazine derivatives bearing piperazine amide moiety against breast cancer cells. Bioorganic Med Chem 22(22):6313–6323
Cheepsattayakorn A, Cheepsattayakorn R (2014) Lung cancer chemotherapy, new treatment and related patents. Recent Pat Anticancer Drug Discov 9(3):372–381
Ma Y, Zheng X, Gao H, Wan C, Rao G, Mao Z (2016) Design, synthesis, and biological evaluation of novel benzofuran derivatives bearing N-aryl piperazine moiety. Molecules 21(12):1684
Al-Harthy T, Zoghaib WM, Pflüger M, Schöpel M, Önder K, Reitsammer M, Hundsberger H, Stoll R, Abdel-Jalil R (2016) Design, synthesis, and cytotoxicity of 5-fluoro-2-methyl-6-(4-aryl-piperazin-1-Yl) benzoxazoles. Molecules 21(10):1290
Al-Ghorbani M, Bushra Begum A, Zabiulla Z, Mamatha SV, Khanum SA (2015) Piperazine and morpholine: synthetic preview and pharmaceutical applications. Res J Pharm Technol 8:611–628
Hu M, Ye W, Li J, Zhong G, He G, Xu Q, Zhang Y (2014) Synthesis and evaluation of salicylanilide derivatives as potential epidermal growth factor receptor inhibitors. Chem Biol Drug Des 85:280–289
Halgren TA, Murphy RB, Friesner RA, Beard HS, Frye LL, Pollard WT, Banks JL (2004) Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J. Med. Chem. 47(7):1750–1759
Ertl, P. Polar surface area. Mol Drug Prop 111–126 (2007)
Gerlier D, Thomasset N (1986) Use of MTT colorimetric assay to measure cell activation. J Immunol Methods 94(1–2):57–63
Wenlock MC, Barton P (2013) In silico physicochemical parameter predictions. Mol Pharm 10(4):1224–1235
Yun CH, Boggon TJ, Li Y, Woo MS, Greulich H, Meyerson M, Eck MJ (2007) Structures of lung cancer-derived EGFR mutants and inhibitor complexes: mechanism of activation and insights into differential inhibitor sensitivity. Cancer Cell 11(3):217–227
Kamal A, Tamboli JR, Vishnuvardhan MVPS, Adil SF, Nayak VL, Ramakrishna S (2013) Synthesis and anticancer activity of heteroaromatic linked 4β-amido podophyllotoxins as apoptotic inducing agents. Bioorganic Med Chem Lett 23(1):273–280
Sussman JL, Lin D, Jiang J, Manning NO, Prilusky J, Ritter O, Abola EE (1998) Protein Data Bank (PDB): database of three-dimensional structural information of biological macromolecules. Acta Crystallogr Sect D Biol Crystallogr 54:1078–1084
Acknowledgements
Authors acknowledge BITS pilani for support facilities.
Author information
Studies involving plants must include a statement specifying the local, national or international guidelines and legislation and the required or appropriate permissions and/or licences for the study: NA.
Funding
No governing body is involved in funding of work it was institutional funding.
Author information
Authors and Affiliations
Contributions
The contributions of all authors to the manuscript are as follows: MS: design, synthesis, and characterization of the compounds. HRJ: Outlined the study and provided overall guidance. AC: Conducted the anticancer activity assays. PW: Performed the molecular docking studies and prepared the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Author declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Singh, M., Jadhav, H.R., Choudhary, A. et al. Design, synthesis and evaluation of new methyl piperazine derivatives as anticancer agents. Futur J Pharm Sci 10, 88 (2024). https://doi.org/10.1186/s43094-024-00663-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-024-00663-9