Abstract
Background
Acinetobacter baumannii (A. baumannii) is an opportunistic pathogen that poses dangerous health threat. It is a main cause of biofilm-associated infections that are mostly resistant to antibiotic therapy. Because of its capacity to form biofilm on biotic and abiotic surfaces, it has been linked to most nosocomial infections such as ventilator-associated pneumonia, urinary tract infections, bacteremia, meningitis, wound infections, soft tissue infections, and peritonitis.
Main body of the abstract
The biofilm refers to an organized group of microbial cells that are embedded in an exopolymeric substance made of protein, extracellular DNA, and polysaccharide. Bacterial cells in biofilms are resistant to chemicals, phagocytosis, and other elements of the body’s innate and acquired immune systems posing treatment challenges. Biofilm formation in A. baumannii is a complicated process that is influenced by a variety of factors such as outer membrane protein A, poly-β-(1,6)-N acetyl glucosamine (PAGE), biofilm-associated protein, two-component system (Bfm/S BfmR), chaperone–usher (Csu) pilus assembly system of pili, BlaPER-1 belonging to β-lactamase family, extracellular polymeric substance, and the quorum sensing system. Several biofilm-associated genes influence antimicrobial susceptibility, implying a link between biofilm formation and antimicrobial resistance.
Short conclusion
This review describes the complex biofilm system of A. baumannii, which gives it a survival advantage and increases its colonization ability. Also, it demonstrates various extrinsic and intrinsic factors that function and regulate the biofilm machinery of A. baumannii. Furthermore, this study considers prospective ways for preventing biofilm development on relevant medical equipment, as well as potential therapeutic strategies for eradicating mature biofilms, which can aid in the treatment of biofilm-associated A. baumannii infection.
Similar content being viewed by others
Background
Acinetobacter baumannii is a gram-negative, non-fastidious, non-fermentative, non-motile, catalase-positive, and oxidative-negative coccobacillus [1]. It is a member of Eubacteria class Proteobacteria family Moraxellaceae and genus Acinetobacter. In addition, it is also considered one of the ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus (S. aureus), Klebsiella pneumoniae (K. pneumoniae), A. baumannii, Pseudomonas aeruginosa (P. aeruginosa), and Enterobacter species) that are linked to alarming multidrug-resistant hospital-acquired infections [2, 3]. An estimate of 1 million cases of A. baumannii infections is reported per year worldwide with death rates ranging from 20 to 80% [4, 5].
A. baumannii has an ability to form biofilm on biotic (host mucosal tissue) and abiotic surfaces (e.g., catheters) which plays a critical role in causing nosocomial infections [1, 6]. The most prevalent nosocomial illnesses caused by Acinetobacter are urinary tract infections (UTI), meningitis, pneumonia, wound, bacteremia, burn, endocarditis, as well as skin and soft tissue infections [7, 8]. A. baumannii bloodstream infections and ventilator-associated pneumonia have been connected to substantial death rates of up to 35% [9]. However, community-acquired pneumonia is more dangerous than nosocomial pneumonia and has a 60% mortality rate [10].
A. baumannii infections are frequently associated with multidrug resistance; and recently, it shows resistance to all antibiotics including carbapenem and colistin [9]. There are different resistance mechanisms owned by A. baumannii such as modifications of target sites, permeability deficiencies, multidrug efflux pumps, and enzymatic drug degradation, for example, β-lactamases and aminoglycoside-modifying enzymes [10,11,12]. Currently, a serious threat to public health is posed by the rapid expansion of antibiotic resistance in A. baumannii. A. baumannii's capacity to colonize and create biofilm on biotic and abiotic surfaces contributes to long-lasting infections, antibiotic resistance, as well as survival and transmission in hospital settings [13]. As a result, the biofilm matrix, that surrounds the bacteria, enables them to tolerate harsh conditions and resist antibiotic treatments. Consequently, current treatments for infections caused by biofilm-forming A. baumannii are most probably unsuccessful [14]. Therefore, there is an urgent need for alternative medications and/or therapies to prevent the establishment of biofilms and inactivate Acinetobacter adherence to biotic or abiotic surfaces. Numerous efficient innovative anti-biofilm treatments have been developed, including antibiotic therapy, quorum sensing inhibitors, natural products/essential oils, antimicrobial peptides, efflux pump inhibitors, nanoparticles, and phage therapy. Additionally, photodynamic treatment (PDT) offers a potential strategy to tackle antibiotic resistance [15].
Main text
A. baumannii pathogenesis and biofilm formation
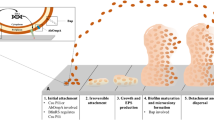
Biofilm formation and development is a complex process in which microorganism cells change their growth mode from planktonic to sessile. It is fueled by a variety of physical, chemical, and biological processes [16, 17]. The common steps in the formation of a biofilm are summarized in Fig. 1 [18, 19].
The common steps of biofilm development. Step 1: The planktonic cells adhere to the biotic or abiotic surface. Step 2: Bacterial cells aggregate and form microcolonies, secrete EPS, and the attachment becomes irreversible. Step 3: A biofilm is formed and matures, and cells form multi-layered clusters. Step 4: The growth of three-dimensional and further maturation of the biofilm, providing protection against the external environment. Step 5: The biofilm reaches a critical mass, and planktonic bacteria disperse to colonize other surfaces
The pathophysiology of A. baumannii infections is still inadequately understood, despite the fact that they are a serious clinical practice concern as well as a worldwide health threat. Evidence showed that phospholipases, extracellular polysaccharides, a siderophore-mediated iron acquisition system, outer membrane protein A (OmpA), phospholipids, and the K1 capsule are virulence factors that play a significant part in bacterial pathogenicity [20,21,22,23,24]. Biofilms are responsible for a number of subacute or chronic infections that are difficult to cure [22, 25]. Furthermore, the ability of A. baumannii to form biofilms (due to fimbriae and pili) is a significant factor that exacerbates the disease process. In comparison with other species, A. baumannii has a biofilm formation rate of nearly 80–91%, whereas the other species has a rate of approximately 5–24% [21, 22, 24]. The assembly of pili and the production of the biofilm-associated protein (bap), a surface adhesion protein, are crucial for the development and maturation of biofilms once A. baumannii adheres to abiotic surfaces [26]. The ability of cells to adhere to abiotic surfaces such as medical devices and environmental surfaces, as well as the level of expression of outer membrane proteins, mRNAs, is virulence factors for A. baumannii pathogenesis [8, 22,23,24]. Additionally, OmpA interacts with fibronectin on the host cell surface to facilitate bacterial attachment to lung epithelial cells and helps in the formation of biofilms on biotic surfaces like epithelial cells. It may also induce apoptosis in human epithelial cells [24, 27].
A. baumannii biofilm formation and antimicrobial resistance
A. baumannii has been classified as a “red-alert” human pathogen because it can develop resistance to all antimicrobial agents that are currently available in the market [23].
The phrase “biofilm resistance” refers to the ability of cells to persist for extended periods of time while embedded in biofilm when antimicrobials are present. Biofilms are better suited to evading antimicrobials than planktonic cells because they are not easily killed by antimicrobials.
There are different factors that cause biofilm resistance and explain the reasons why biofilm cells survive longer than planktonic cells in the presence of antimicrobials and they are demonstrated in Fig. 2 [28]. However, the relationship between the development of biofilms and the phenotypes of antibiotic resistance is still up for debate [29]. According to some studies, the development of biofilm and multidrug resistance is positively correlated [29, 30]. One study revealed that biofilm formers showed greater resistance to ampicillin–sulbactam, amikacin, ciprofloxacin, and ceftazidime as compared to imipenem and piperacillin [31]. Another study found that A. baumannii clinical isolates exhibit a significant propensity for the development of biofilms, and biofilms have a correlation to multiple drug resistance. It was also revealed that biofilm producers had a greater rate of antibiotic resistance than isolates who did not build biofilms [32]. A similar investigation on the relationship between biofilm generation and drug susceptibility was done on A. baumannii strains obtained from hospital-acquired illnesses, where ceftazidime-sensitive bacteria were observed to create less biofilm than ceftazidime-resistant strains, but tobramycin- and amikacin-sensitive strains produced more biofilm than antibiotic-resistant ones. On the other hand, powerful biofilm producers from intensive care units (ICUs) are frequently more vulnerable to antibiotics because bacteria protected in biofilm do not need the resistance mechanisms required by planktonic cells [33]. According to other research, non-biofilm-forming isolates were frequently more resistant to imipenem and ciprofloxacin than biofilm-forming isolates [34]. Similarly, A. baumannii isolates that were resistant to meropenem were less able to generate biofilms than isolates that were sensitive to meropenem [35]. Acinetobacter strains with multidrug-resistant (MDR) and biofilm production often continue to pose a substantial concern in the hospital setting because they quickly gain drug resistance to routinely used drugs, and their potential to produce biofilm is statistically significant with imipenem resistance [36].
Diagram depicting the various processes of antibiotic resistance in biofilm ecosystems. Biofilm cells are adhered to the biotic or abiotic surface. In the stress zone where the nutrient and oxygen availabilities are low, the persister cells and less active deep layer cells develop. The figure depicts the following resistance: Antibiotic penetration is slowed by matrix of exopolysaccharides (EPS); extracellular DNA (eDNA); multidrug efflux pumps; outer membrane protein; antibiotic degrading enzymes; target alterations; drug neutralization; quorum sensing; and stress responses (SOS response and stringent response (SR))
Therefore, bacteria are able to tolerate harsh conditions and resist antibiotic treatments owing to the biofilm matrix that surrounds them. Consequently, current treatments for infections caused by A. baumannii biofilms are unsuccessful [14]. Many successful innovative anti-biofilm treatments have been developed [28].
Factors influencing A. baumannii biofilm formation
Biofilm formation is triggered by three different methods of interaction. Firstly, the interaction between the microbial cells. Secondly, bacterial adherence to the surface of human tissues or other objects, and thirdly, information transfer that takes place in the environment through the acylation of serine lactones. These mechanisms have been impacted by several intrinsic and extrinsic factors, including physicochemical and microbiological determinants, such as the aggregation of substances, adherence of collagen, expression of pili, capsular polysaccharides, and resistance determinants. There are additional elements that contribute to the biofilm's formation, including macromolecule secretion, cell interaction, and surface-regulated attachment [37].
Intrinsic factors associated with A. baumannii biofilm formation
A. baumannii biofilm development is influenced by a number of virulence factors. Genes or proteins that provide pathogenicity, cellular architectures, and phenotypic or genotypic traits are all included. Table 1 summarizes these factors that are related to the development and control of biofilms in A. baumannii.
Outer membrane proteins (Omps)
Omps are considered as one of the most important porins that regulate cellular permeability and play a significant role in adaptability, environmental communication, and microbial pathogenicity through the use of drug exclusion mechanisms across outer membrane channels [27]. Numerous OMPs, including CarO, Omp33 OprD-like, and PstS, are present in Acinetobacter species and are associated with pathogenicity and the emergence of antibiotic resistance. OmpA is the primary outer membrane protein in A. baumannii which is considered as a beta barrel-shaped monomeric integral outer membrane protein with 8–26 antiparallel strands joined by four loops on the outer membrane surface and three short twists on the periplasmic side [38]. It is also a well-characterized virulence factor because of the numerous essential roles it plays in A. baumannii's survival and pathogenesis, including maintaining cell membrane integrity, mediating drug resistance, altering host immune response, starting the formation of biofilms, invading host epithelial cells, and inducing host cell apoptosis [39]. A study demonstrated that A. baumannii cells quickly attached to a 96-well plate coated with fibronectin as a result of OmpA's interaction with fibronectin, indicating the beginning of the interaction between A. baumannii biofilm formation on biotic surfaces [53]. OmpA-deficient A. baumannii mutants were less virulent than wild-type cells, with reduced adhesion to human airway epithelial cells and decreased biofilm formation [39].
Bap
Bap is a higher molecular cell surface protein that is found on the surface of bacteria with a molecular weight of about 854-kDa and 8620 amino acids. The protein encoded by the bap gene is important for intercellular cell adhesion, bacterial cell aggregation, maintenance, biofilm development, and maturation on different surfaces such as polypropylene, polystyrene, and titanium [40]. Bap generates a multidimensional pattern of mature biofilm and creates water pathways between them. A study found that bap plays an important role in biofilm maturation rather than the initial stage of adherence since wild-type A. baumannii strains produced typical biofilm phenotypes, whereas bap mutants failed to produce mature biofilm and remained in a single layer [54, 55]. Furthermore, Bap protein increased A. baumannii adhesion to both normal human neonatal keratinocytes and bronchial epithelial cells [55]. Several investigations have shown that bap is present in A. baumannii strains and that it is associated with significant biofilm formation. Also, A. baumannii bap is a type I secretion system that primarily targets carbohydrates in host cells [56, 57].
Csu pilus assembly system of pili
A. baumannii has been considered as non-motile due to the lack of flagella [58, 59]. However, research reveals that this organism, by using its twitching motility, is able to survive after infection and to spread on hospital surfaces [60]. Type IV pili are used to push cells in media through extension and retraction movements [61]. These pili play a critical role in gene transfer, biofilm formation, and organism adherence to host cells [62]. A. baumannii has a clustered gene known as the Csu operon that produces confined structures resembling pilus. It is regarded as the most important virulence factor in facilitating the irreversible adhesion of cells to an abiotic surface. The Csu pilus is a polycistronic adhesive surface organelle that is produced by the ancient chaperone–usher (CU) pathway from four protein subunits, CsuA/B, CsuA, CsuB, and CsuE [41]. There are another two subunits such as CsuC and CsuD which act as transporter proteins. Biofilm formation was affected by gene deletion because CsuA/B and CsuE mutants completely lost their pilus structure, whereas CsuA and CsuB mutants produced small number of abnormal fibers. The deletion mutants failed to establish a biofilm on the plastic surface, demonstrating that all four subunits are required for a functioning pilus [63].
PER-1 β-lactamase
The bla PER1 gene is classified as a class A extended spectrum beta-lactamase. Additionally, this gene's presence and expression help A. baumanni adhere to both biotic and abiotic surfaces, which promotes the formation of biofilm [32]. Several studies have found that strains with the bla PER1 gene have higher cell adhesiveness and biofilm formation than those without this genetic trait [32, 64, 65]. However, Bardbari et al. discovered no relationship between the production of PER1 beta-lactamase and the development of biofilms [66]. Therefore, the presence of bla PER1 probably improves the ability of cells that express this gene to adhere, but it does not always aid in the formation of biofilms.
EPS
EPS are organic polymers of microbial origin involved in bacterial cells’ interactions with their environment [42]. EPS is made up of polysaccharides, proteins, eDNA, and lipids. Bacterial cells are stimulated to produce EPS, which form the biofilm matrix after irreversible cell attachment to the surface and considered the most important component of biofilm matrix. Bacterial cells use EPS such as glycolipids, glycoproteins, alginate, poly-β-(1,6)-N acetyl glucosamine (PNAG), DNA, and bap to form a stable three-dimensional structure of mature biofilm. The development and pathogenicity of biofilms are significantly influenced by EPS. The main components of EPS are alginate and antibiotics hydrolytic enzymes immobilized on biofilm, and they work by preventing antibacterial chemicals from reaching the target and reducing antibacterial action [67]. The negatively charged amino acid side chains of a polypeptide chain, which constitute the majority of EPS, have a proclivity to attract positively charged amino acid side chains. This attraction prevents hydrophilic antibiotics from entering cell bodies and significantly reduces bactericidal activity, making it more difficult to eradicate cells after biofilm formation. EPS has a significant role in the O-glycosylation system and capsule formation of the MDR A. baumannii strain [68].
Quorum sensing (QS) system
QS is a cell-to-cell communication process that is influenced by the density of the bacterial population. Several small diffusible signaling molecules known as autoinducers (AI) are involved. These are hormone-like substances, such as acyl homoserine lactones (AHLs), which stimulate the expression of genes that regulate a variety of biological processes, including biofilm formation, motility, pathogenicity, bioluminescence, and sporulation [43, 44]. A. baumannii often produces 3-hydroxy-C12-homoserine lactone as an AHL [69]. Additionally, it contains a two-component system called AbaI/AbaR that manages the QS system. This system is similar to the typical LuxI/LuxR system found in gram-negative bacteria. AHL is synthesized by autoinducer synthases, which are catalyzed by the gene AbaI and are receptor proteins for AHL, which are encoded by the protein AbaR [70]. According to a study, the interaction of AHL with the AbaR receptor causes enhanced expression of the csu pili and the development of biofilms [71]. Another gene termed abaM, an uncharacterized member of the RsaM protein family located between AbaR and AbaI, plays a crucial role in the regulation of A. baumannii QS, virulence, surface motility, and biofilm formation. abaM has recently been found to regulate both QS-dependent and QS-independent genes in A. baumannii. Increased levels of N-(3-hydroxydodecanoyl)-l-HSL positively trigger the expression of abaM, a negative auto-regulator that inhibits the expression of AbaI and AbaR and hence adversely controls the generation of AHL. QS deficiency results in thinner biofilm formation and lower EPS production, making bacteria more susceptible to antibiotics. The QS gene, which controls the production of virulence genes and the creation of biofilms, is more highly expressed when there is an iron deficiency [72]. Therefore, developing QS cascade inhibitors or quenchers to treat persistent A. baumannii biofilm infections would be a useful strategy [73].
Efflux pumps
Efflux pumps are membrane proteins that can expel a wide variety of compounds including antibiotics, detergents, dyes, poisons, and waste metabolites. The efflux system families identified in A. baumannii are major facilitator superfamily (MFS), multidrug and toxin compound extrusion (MATE), AbeM, resistance nodulation division (RND), small multidrug resistance (SMR), ATP-binding cassette (ABC), and MacB [74]. Numerous studies show that efflux pumps are essential for the growth and maturation of biofilms through a variety of mechanisms, including the indirect regulation of biofilm-forming genes, the efflux of antibiotics or metabolic intermediates, the efflux of quorum quenching (QQ) molecules, and the efflux of EPSs and quorum-suppressing molecules [37, 45]. There are three types of RND efflux pumps associated with A. baumannii: AdeABC, AdeFGH, and AdeIJK. Yoon et al. discovered that mutant AdeABC, AdeFGH, and AdeIJK efflux pumps generate considerably less biofilm than wild-type AdeABC, AdeFGH, and AdeIJK efflux pumps. Thus, in order to initiate and maintain biofilm formation in A. baumannii, efflux pump genes must be expressed [75]. Another study discovered that mutations in the AdeABC and AdeIJK efflux genes were associated with reduced expression of many pilus system-encoding proteins, including CsuA/B, CsuC, and FimA. These proteins are required for A. baumannii adhesion, surface colonization, and biofilm formation [76]. According to Richmond et al., when AdeABC efflux pumps were inhibited in A. baumannii mutant strain, biofilm production on mucosal tissue was dramatically reduced compared to the wild-type strain [77]. A. baumannii's pilus gene expression, biofilm formation, and altered membrane composition are regulated by the overexpression of the AdeABC and AdeIJK efflux pumps. Additionally, the production and transfer of autoinducer molecules during the development of biofilm in A. baumannii are connected to the overexpression of the efflux pump AdeFGH, which confersMDR [76, 78].
PNAG
PNAG is a polysaccharide that is necessary for biofilm development in both gram-positive and gram-negative bacteria [79]. It is encoded by a set of four genes called pgaA, pgaB, pgaC, and pgaD, which are related to those in Yersinia pestis and Escherichia coli (E. coli) [46, 80]. A. baumannii’s pgaA gene encodes a predicted transmembrane protein with a porin-like domain, suggesting that it may be involved in the movement of PNAG across the outer membrane. The production of biofilms was significantly reduced after the pgaABCD locus was deleted. Numerous studies have, therefore, demonstrated that PNAG is essential for preserving the integrity of A. baumannii biofilms in a more dynamic and demanding environment [47, 80].
eDNA
eDNA plays a significant role in cell adhesion, biofilm development, and maintenance. It was first reported by Whitchurch et al. in P. aeruginosa [48]. Later, it was shown that the presence of eDNA in bacteria serves a crucial role in providing structural stability to the biofilm, increasing the creation of extracellular polymeric compounds, and transforming additional neighboring competent bacterial cells [81]. The role of eDNA in the production of A. baumannii biofilms is not well understood. A study using an A. baumannii clinical isolate showed that the release of eDNA was promoted either in free form or trapped in outer membrane vesicles, and it did not necessitate cell lysis at an early stage. This suggests that eDNA may assist other adhesion proteins that may later play a role in the earliest stages of adhesion in the formation of biofilm, promoting the adhesion that occurs in the first stages of the biofilm [82]. Furthermore, DNaseI treatment of a 24-h-old prepared biofilm resulted in its depletion by about 60%, showing the importance of eDNA in biofilm preservation [82, 83].
Alginate
Alginate C (algC) is a biofilm-associated gene that controls the synthesis of phosphomannomutase/phosphoglucomutase, a bi-functional enzyme that belongs to the alpha-d-hexomutase superfamily and is necessary for the production of exopolysaccharide alginate and lipopolysaccharide core when Mg2+ and the activator glucose 1,6-bisphosphate are present during biofilm formation [84,85,86].
Alginate lyase is a glycoside hydrolase that degrades alginate, allowing bacteria to escape biofilms and improving the effectiveness of drugs against the A. baumannii biofilm. It was discovered that the MDR strain of A. baumannii expressed more algC in a biofilm mode of development than in a planktonic phase. Biofilm cells showed an 81.59-fold expression compared to planktonic cells, which reach 3.24-fold, according to real-time PCR, which was utilized to characterize the quantitative gene expression pattern [87].
Amyloidogenic proteins
Curli fibers are an amyloid protein involved in matrix formation that is encoded by the curli-specific gene (csg) operon. Amyloids have been found in bacteria as well as fungi. Amyloids are known to facilitate bacterial cell–host adhesion and biofilm formation. The amyloid protein is made up of a major subunit encoded by the gene csgA. Curli fibers contribute to host cell invasion and adhesion as well as to the host's inflammatory response [50]. The management of bacterial infections caused by biofilms may be aided by focusing on amyloid formations [88].
Second messenger signaling pathway
In the bacterial population, several nucleotide messenger signaling pathways have been found, including cyclic di-guanosine monophosphate (c-di-GMP), c-AMP, (p)pp-G-pp, c-di-AMP, and c-AMP-GMP, which regulate a wide range of physiological features [89]. All bacterial populations share a high degree of conservation for cyclic di-guanosine monophosphate (c-di-GMP) second messenger signaling, which controls physiology including cell motility, cell division, and differentiation as well as harmful features such as virulence and biofilm formation [51, 52]. They are known to act as a universal positive regulator in the production of biofilms in gram-negative bacteria [90]. A. baumannii controls the transition of planktonic to biofilm bacteria by using the secondary messenger cdiGMP. The development of the biofilm matrix components is induced by an elevated amount of cdiGMP, which also controls the formation of the biofilm. The production of biofilm matrix was downregulated, and the distribution of biofilm development was spread due to the decreased level of cdiGMP [90, 91]. Because of its known function in regulating biofilm development and because it is largely conserved in bacteria, targeting c-di-GMP signaling may be effective in inhibiting biofilms [90].
Extrinsic factors associated with A. baumannii biofilm formation
Environmental factors that influence biofilm structure and function include physical parameters that control these ecosystems, chemical factors, and biological factors. Light penetration, temperature, and water are examples of the physical features, while pH, nutrient availability, and toxicant effects are chemical factors that influence biofilm formation. Biological factors are community composition (bacteria, algae, and fungi), relative contribution of autotrophs and heterotrophs, biomass thickness, and grazing [92, 93].
Surface property
A. baumannii develops biofilms three times faster than the other Acinetobacter species at the solid–liquid interface. Clinical strains are more capable of developing biofilms than environmental strains, and the formation of biofilm on surfaces with potential medical importance affects the strains' ability to tolerate the availability of nutrients, desiccation, stress, and antibiotic therapy [29]. A. baumannii adhesion to abiotic surfaces and biofilm formation are influenced by a variety of factors, including surface roughness, physicochemical properties, and the presence of biological components [94]. Also, pH, ionic content, and biomaterial of protein adsorption have all been connected to A. baumannii’s capacity to develop mature biofilms on polypropylene, polystyrene, titanium, and other medical-related devices [95]. Blood, sweat, tears, urine, saliva, interstitial fluid, wound cultures, and respiratory secretions are examples of biomaterials that have an impact on how well bacteria adhere to their surfaces and promote the growth of biofilms [96]. Surfaces made of polycarbonate are known to statistically create more biofilm mass than surfaces made of glass, rubber, porcelain, and polypropylene [94]. Latex catheters, despite being less expensive and more elastic, are more susceptible to bacterial adhesion and biofilm formation. Therefore, latex catheters are so favored than silicone catheters [97].
Growth temperature
Temperature has an impact on the development of biofilms. The optimal temperature for bacterial growth is dependent on the rise in the nutrient intake [96]. Also, it is well understood that the nutrient metabolism depends on the presence of enzymes and reaction rates that regulate the development of many physiological and biochemical systems in bacteria. As a result, ideal temperature encourages bacterial growth, resulting in biofilm development [98, 99]. In contrast, when the temperature falls below the optimum range, bacterial growth can be slowed down as a result of a decline in response rates, which may have an effect on the development of biofilms. In addition to enzymes, environmental temperature can influence the physical properties of substances inside and around cells [16]. Temperature can also have an impact on the characteristics of bacterial EPS, such as the viscosity of the polysaccharides. It was discovered that as the temperature of EPS rises, a gel-like substance is produced. After reaching a critical degree, the gel turns into a solution [100]. Thus, lower temperatures may result in the polysaccharides' characteristics being more consistent, which frequently raises the likelihood of bacterial biofilm adherence [16]. On the other hand, it was discovered that some bacteria grew more adhered to the surface when exposed to high temperatures [101]. A. baumannii survived effectively at temperatures between 20 and 44 °C [102]. However, other researches have shown that different optimal temperatures for biofilm growth in A. baumannii, including 30 °C, at pH 7, in a medium containing sodium chloride (NaCl), or 25 °C [7, 103]. Another study found that the overexpression of specific baps, Csu pili, and iron-uptake proteins led to a high level of biofilm production in A. baumannii on plastic surfaces at 28 °C [95].
Growth media
The growth medium is another element that influences the development of biofilms. The availability of oxygen may play a role in bacterial adherence to submerged surfaces and subsequent biofilm formation [104]. Additionally, the availability of oxygen can affect how much bacteria produce energy, which may have an impact on the development of biofilms. For instance, a lack of oxygen might reduce the metabolic activity of bacteria in biofilms [100]. Therefore, it is thought that the availability of oxygen is a crucial environmental component that might affect the structure and growth of biofilm [105, 106]. Lower oxygen availability typically causes active dispersal, which is essential for the biofilm life cycle [107]. For instance, it was discovered that bacterial cells near the base of a biofilm received less oxygen than those at the surface, which facilitated their separation from the biofilm's deeper layers [108]. Nutrients can have an impact on the transition between planktonic and biofilm bacterial lifestyles because the bacterial response to develop biofilm or to remain suspended depends on the nutritional condition [98]. A higher concentration of nutrients speeds up the rate of microbial attachment since it has been discovered that the presence of nutrients abundantly in the surrounding media aids bacterial adhesion to surfaces [108]. Furthermore, nutrient levels can influence biofilm development and cell dispersal from biofilms [109, 110]. Additionally, it has been demonstrated that variations in the essential nutrient availability have an effect on the physiology of bacteria in developing biofilms [111]. Numerous methods have been used to investigate how nutrient concentrations affect the formation of bacterial biofilms. For instance, the number of cells in biofilms increase when nutrients were present in high concentrations in drinking water distribution networks [112]. Additionally, the incidence and the amount of Pseudomonas putida (P. putida) biofilm forming in a paper mill water stream increased with rising nutrient levels [113]. Also, it has been discovered that adding glucose as a carbon source to the medium promotes the development of biofilm in several bacteria, such as E. coli and P. putida [111, 113]. On the other hand, adding glucose to different media prevented the development of biofilms in a number of Enterobacteriaceae family species, including K. pneumoniae, Citrobacter freundii, and Salmonella enterica [114]. According to a research by Rochex and Lebeault, the rate and amount of P. putida biofilm formation increased when nitrogen concentration decreased from a carbon/nitrogen ratio of 90–20. On the other hand, the absence of nitrogen caused the Pseudomonas fluorescens biofilm to actively detach, as was the case when the amount of glucose was limited [115]. However, variations in yeast extract and peptone concentrations, which are both effective sources of nitrogen, had no discernible effects on the development of E. coli biofilms [116]. Increased NaCl concentrations prevented the development of biofilms in other bacteria, including Salmonella species, Sinorhizobium meliloti, S. aureus, Enterococcus faecalis, and P. aeruginosa [117,118,119]. According to a research, A. baumannii biofilm formation is influenced more by a static environment with high nutrient-containing medium (tryptic soy broth (TSB) or brain–heart infusion broth (BHI)) and components of carbon, glucose, and cation sources (sodium, calcium, and ferric ion) than by hydrodynamic environment. Also, these amendment characteristics of the medium and supplementary sources have an impact on the structural and mechanical characteristics of A. baumannii biofilms by reducing stiffness and increasing adhesiveness [120].
Iron concentration
The production of biofilm is hampered by the sources and concentration of iron. Bap is typically increased by the scarcity of iron. A higher iron concentration can make some selective antibiotics more difficult to employ by producing signal or reacting directly with the antibiotics. To scavenge the available iron in the environment, the majority of bacteria produce potent iron-chelating molecules known as siderophores. When siderophores come into contact with iron, an iron–siderophores complex eventually is formed. It binds to the particular outer membrane receptors, which makes it easier for molecules to flow through the outer membrane. To the contrary, when the iron is present in larger concentrations, siderophores are unable to develop complexes and cannot create passageways in the outer membrane, which prevents the antibiotics from dispersing to the outside membrane [121]. Clinical isolates of A. baumannii showed a significant decrease in adhesiveness and biofilm development when present on abiotic surfaces with ethanol and an iron-chelating agent [122, 123].
pH
The pH of the environment has a considerable impact on the development of biofilms [124]. It has been shown that this element has an impact on the process of microbial adhesion to surfaces, which is the first step in the formation of biofilms [125, 126]. Additionally, it has been demonstrated that the pH of the medium influences the development of bacterial biofilm, which may affect enzyme function because each enzyme has a preferred pH [127]. Bacterial biofilms are more resistant to pH fluctuations than planktonic cells [128]. For instance, the gel-like structure of the bacterial biofilm can reduce the rapid transport of ions and enable the creation of a pH gradient within the extracellular matrix in severely acidic conditions [129]. However, under alkaline conditions, poorly structured and very thin biofilms have been observed as a result of impaired biofilm maturation, as well as adhesion inhibition for some bacteria such as S. aureus and Staphylococcus epidermidis [130]. The ideal pH for A. baumannii to develop biofilms in a solution containing NaCl is pH 7 [131].
Strategies for prevention and control A. baumannii biofilm
It is difficult to control A. baumannii biofilm infections due to increasing rate of biofilms’ resistance to antibiotics and limited antibiotics penetration in the matrix. Furthermore, it is crucial to stop these organisms from creating biofilms. So, numerous potent new anti-biofilm treatments have been created to prevent Acinetobacter adherence to biotic as well as abiotic surfaces and to prevent biofilm generation. Three fundamental strategies have been investigated: inhibition of bacterial attachment to both abiotic and/or biotic surfaces, disrupting targets of biofilms during the maturation steps, and interference with signals or QQ [132]. Properties of biomaterials, chemical and physical, are modified to inhibit initial attachment of biofilm, while different chemicals such as matrix-degrading enzymes, amino acids, surfactants, nitric acid donors, and free fatty acids, in addition to physical forces, can be utilized to remove biofilm. Additionally, inhibition of biofilm via QQ method works by degrading signals of QS, opposing signaling molecules, and inhibiting generation, transduction, and transport of signal [29, 132].
Quorum sensing quenchers
Inhibition of QS signaling pathways reduces the creation of biofilms since QS contributes to the production of biofilms, making it a potential new treatment approach [133]. Enoyl-acyl carrier protein reductase, a crucial enzyme for AHL acyl chains production, may be inhibited by a small amount of the chemical agent having antibacterial qualities triclosan [134]. Vanillin, allin, and patulin/clavacin were discovered to interact with receptors of AHL and prevent QS signals transmission [135,136,137]. Additionally, AHL analogs, AbaR antagonists (like streptomycin), anoR antagonists (like virstain), as well as antagonists for the enzyme di-guanylate cyclase, which produces cyclic di-GMP, were also discovered to block QS, and hence, development of biofilm is prevented in A. baumannii and Acinetobacter noscomialis [138,139,140,141,142]. It was discovered that A. baumannii’s EPS generation, swarming motility, and biofilm formation were inhibited by siphonocholin, a marine steroid with anti-QS activity [143]. AHL lactonase and MomL are two examples of genetically modified QQ enzymes that can effectively suppress QS signal and cause biofilm structure disintegration [144,145,146].
Natural products/essential oils (EOs)
A. baumannii infections can be successfully reduced by natural materials as animal, microbial, and plant derivatives. Bacterial metabolites were demonstrated to be efficient against the biofilm of A. baumannii [147]. A study revealed that crude cell-free supernatants of several bacteria such as Mycobacterium mucogenicum, Burkholderia cepacia, Sphingomonas capsulate, Staphylococcus spp., and Methylobacterium spp. were found to inhibit Acinetobacter calcoaceticus [148]. A. baumannii biofilm development is prevented on medical supplies such as silicone catheters and endotracheal tubes (polyvinyl chloride) by maipomycin A, an iron chelator obtained from actinomycete metabolites [149]. Several bacterial biofilms, including A. baumannii, were inhibited by many sets of marine sponge-derived chemicals using non-microbicidal methods [150]. The 5-episinuleptolide, a soft coral compound obtained from Sinularia leptoclados, demonstrated activity against biofilms of A. baumannii ATCC R 19606TM and MDR A. baumannii strains through the reduction of the pgaABCD locus expression that encodes the PNAG of biofilm structure [151]. Myrtenol, a bicyclic monoterpene derived from diverse plants, has potent anti-biofilm activities against A. baumannii clinical strains. Myrtenol particularly decreased biofilm thickness, interfered with the mature biofilm, inhibited the virulence factors associated with the biofilm, and made strains more susceptible to conventional antibiotics. Myrtenol therapy resulted in the reduction of biofilm-related genes such as ompA, bfmR, bap, csuA/B, pgaA, katE, and pgaC [152]. Natural products such as EOs and secondary metabolites of plants may have broad-spectrum antibacterial activity through the disruption of integrity of bacterial membrane and inhibition of ATP synthesis, leading to metabolite/ion leakage [153]. A. baumannii’s biofilm structure may be severely harmed by various EOs generated from flowering plants such as Ziziphora tenuior L. and Mentha pulegium L. The main constituents of plant extract were found to be pulegone, menthol, isopulegone, D-isomenthone, and piperitenone [154, 155]. The biofilm inhibition characters of four EO constituents (eugenol, vanillin, carvacrol, and thymol) were tested against food surfaces adhering organisms in meat industry. Thymol and carvacrol demonstrated the highest antimicrobial activity against strains of A. baumannii [156]. Additionally, several studies demonstrated that plant-driven EOs that are rich in these constituents (such as cinnamon oil, which contains eugenol, oregano oil, which contains carvacrol and thymol, and eucalyptus camaldulens oil, which contains thymol) were effective against wound infections caused by A. baumannii [157, 158]. Strong anti-biofilm action against A. baumannii was demonstrated by EO-based nano-emulsions made from the plant Thymus daenensis [159]. Shivaprasad et al. demonstrated that the activity of various antibiotics such as cefipime, amikacin, piperacillin/tazobactum, gentamicin, ciprofloxacin, cefoperazone, imipenem, and cotrimoxazole against MDR/extensively drug-resistant (XDR) complexes of A. baumannii was enhanced when combined with EO of lemongrass, which demonstrated 65–79% activity against biofilm [160]. The activity of most EOs against bacteria was tested in vitro; however, few examples were examined in animal or cellular models [161]. According to Ismail et al., the EOs extracted from the leaf of Pimenta dioica showed a higher activity (85% inhibition) against A. baumannii biofilm than EOs extracted from the leaf of Pimenta racemosa (34% inhibition). Both extracts demonstrated bactericidal activity against A. baumannii. Furthermore, A. baumannii microbial density was significantly reduced in the mouse wound infection model [162].
Antimicrobials peptides (AMPs)
AMPs, cationic peptides with a length of 15–30 amino acids, are alternatives to antibiotics. They are released by the innate immune response and attack the bacterial cell membrane which harbors negative charge [163]. A. baumannii biofilm was found to be inhibited by a variety of AMPs with biological sources. Human AMP LL37 is one of the AMPs with activity against A. baumannii biofilm [164]. Lactoferrin extracted from human milk and considered an AMP that chelates iron, was less effective for preventing the formation of the A. baumannii biofilm than lactoferrin extracted from bovine milk [165]. A derivative (D-RR4) of the short chain synthetic peptide RR, with a length of 12 amino acids, demonstrated high anti-biofilm as well as antibacterial activities against A. baumannii and P. aeruginosa in both Caenorhabditis elegans model and macrophages cells [166]. A. baumannii biofilm was inhibited by the antimicrobial peptide magainin 2, which is made up of 23 amino acids and was obtained from the skin of Xenopus laevis, an African clawed frog [167]. Many AMPs obtained from flies, including larvae immune peptides derived from Calliphora vicina, and cecropin AMP from Musca domestica, have also been shown to have potent efficacy against A. baumannii biofilm [168, 169]. Jakiewicz et al. examined eight peptides (CAMEL, LL-37, r-omiganan, temporin A, aurein 1.2, citropin 1.1, omiganan, and pexiganan) from various biological origins for their antibacterial activity against the biofilm of A. baumannii on tracheal tube segments. Two of these peptides; CAMEL and pexiganan; showed potent anti-biofilm action [170]. Recently, four artificially created chimeric AMPs were demonstrated to exhibit inhibitory actions against the biofilm of MDR A. baumannii. Those AMPs inhibited A. baumannii biofilm in combination with ciprofloxacin, cefotaxime, or erythromycin [171]. Combining the antibiotics imipenem and tobramycin with WLBU2, a cationic 24-residue synthetic peptide with antimicrobial activity, demonstrated encouraging results against the biofilm as well as the planktonic cells of MDR A. baumannii [172]. P. aeruginosa and A. baumannii persisted cells in the biofilm were destroyed by the synthetic cyclic peptide ZY4, which is 17 amino acids long and exhibits biofilm eradication activity [173]. Some AMPs were used as ointments on the medical devices’ surfaces and to prevent superficial tissue infections [167]. Temporin-L demonstrated an anti-biofilm impact in an in vitro trial without causing cell damage, indicating its tremendous potential for clinical application. AMPs have excellent therapeutic treatment potential because they remove biofilms efficiently [174].
Efflux pump inhibitors (EPI)
Many studies have demonstrated that the efflux pumps play different roles in the production of biofilms in ESKAPE infections; as a result, preventing their activity with EPI could also prevent the development of biofilms. When a substance has a wide range of substrate specificities and low off-target toxicity, it can be referred to as a powerful EPI [175]. Phenylalanine–arginine–beta-naphthylamide, one of the most widely applied EPIs against A. baumannii, has the ability to prevent the growth of biofilms on the pathogen [176]. It was discovered that the novel serum-associated EPIs, such as ABEPI1 and ABEPI2, enhanced the antibacterial effects of A. baumannii cultured in human serum. These substances showed comparable antibiotic synergistic patterns for ciprofloxacin and minocycline as well as reduced pump activity [177]. On the other hand, another study produced a group of substances, named pharmacophores, made of 2-substituted benzothiazoles that significantly inhibited AdeABC efflux pumps when applied in conjunction with ciprofloxacin [178]. The effectiveness of two microbicides, including chlorhexidine and cetrimide, which negatively affected operation and expression of the AdeABC efflux pump in the A. baumannii biofilm, was evaluated by Krishnamoorthy et al. Additionally, they demonstrated that the A. baumannii negative charge of the cell membrane was reduced through these antimicrobials, resulting in efflux pump inhibition and eventually cell death [179].
Nanoparticles (NPs)
NPs have a very large surface area, a very small size (100 nm), and are exceedingly reactive. They have broad-spectrum antimicrobial activity against gram-positive as well as gram-negative bacteria, and they have occasionally been chosen in place of antibacterial drugs. They have the ability to disrupt the integrity of biofilm through reactive oxygen species (ROS) generation, penetration of bacterial cell membrane, interaction with eDNA, lipids, proteins, and polysaccharides, and ATP depletion [180]. Numerous investigations were conducted to comprehend the function of NPs in inhibiting biofilm of A. baumannii. Nitric oxide releasing NPs were used in an in vivo study for the treatment of wound infections associated with A. baumannii biofilm in mouse models [181]. According to a different investigation, exposure to a nano-emulsion of the quaternary ammonium salt cetylpyridinium chloride disrupted the A. baumannii biofilm [182]. An inhibitory action of NPs against biofilm and planktonic cells was demonstrated when these particles are combined with metals or extracts of natural product. A specific investigation demonstrated the effectiveness of gold NPs, silver NPs (AgNPs), and silver–gold bimetallic NPs against biofilms of A. baumannii with 88% inhibition [183]. Many studies showed the inhibitory action of gNPs against biofilms of Actinobacter, which could be due to the ease of penetration of these NPs through the dense biofilms’ EPS. The interaction between negatively charged eDNA and positively charged AgNPs is an important factor in the inhibition of biofilms [184, 185]. Additionally, selenium, curcumin, and aluminum oxide NPs were all found to inhibit the growth of A. baumannii biofilms [186,187,188]. NPs have also been noted that NPs have strong anti-biofilm activity when combined with antibiotics. Since imipenem lyses the bacterial cell wall, it was discovered that AgNPs work in concert with it to promote AgNPs' penetration of bacterial cells [189].
Phage therapy
Bacteriophages are considered another strategy for removing and controlling the biofilms. Different lytic bacteriophages, including vB AbaMIME-AB2 and AB7-IBB2 (family of Podoviridae), were reported to inhibit biofilms of A. baumannii (10–8 CFU/well) on both biotic and abiotic surfaces with 60–80% inhibition [190,191,192]. According to Lood et al., 13 different A. baumannii strains caused induction of 21 distinct lysins (prophages). PlyF307 was the most active of these lysins, which resulted in an in vivo and in vitro significant reduction of A. baumannii biofilm [193]. Thandar et al. demonstrated that the C-terminal amino acids of P307, a phage lysin, have a high ability (> 3-logs) to kill A. baumannii, while an enhanced killing activity (> 5-logs) was observed for its engineered product (P307SQ-8C) when combined with Polymyxin B [194]. A. baumannii biofilms were discovered to be inhibited by B AbaM ISTD and vB AbaM NOVI, two distinct phages obtained from Belgrade wastewaters [195]. When antibiotics commonly used to treat UTIs, such as trimethoprim/sulfamethoxazole, ciprofloxacin, tobramycin, gentamicin, meropenem, and imipenem, and were combined with an environmental phage cocktail, A. baumannii biofilm biomass was decreased. While the phage cocktail in combination with levofloxacin and amikacin did not have a synergistic effect [196]. Ran et al. used photodynamic bacteriophages with Nile blue photosensitizers to create an effective way of preventing A. baumannii biofilm. ROS production was demonstrated by NB photosensitizers containing sulfur atoms. It was demonstrated, through in vitro and in vivo studies, that NB-phage bioconjugate has the ability to bind to biofilms’ main constituents and causes a reduction in drug resistance produced by these biofilms [197].
Photodynamic treatment (PDT) surface modification and physical therapy
Antibiotics are often used for biofilm infections associated with medical devices; however, it is crucial to use new strategies when antibiotics fail to eradicate these biofilms. Physical therapy as well as surface modifications are examples of these strategies that could eradicate and prevent microbial adherence to medical devices. PDT is a powerful physical technique used to prevent biofilm formation and microbial adherence. PDT produces ROS, which prevent the synthesis of several toxic substances that impair bacterial adhesion and biofilm matrix formation, and combat biofilm infections [198, 199]. Also, this method is also used to control infections produced by other implant-related biofilms, such as infections of prosthetic joints and infections brought on by biofilms linked with ventilator-associated pneumonia [200]. By increasing the antibacterial agents’ activity, low-intensity ultrasound used at an acceptable level was proven to be effective in biofilm removal [201]. Another efficient physical technique to remove biofilms attached to implants’ surfaces was the application of water jets. They can remove biofilms through the mechanical action of pressure and pulse [202]. Surface engineering advances have resulted in the production of antibacterial or anti-adhesion agents that have the ability to coat the surfaces of medical devices and subsequently inhibit microorganisms’ growth.
Other biofilm inhibitors
A. baumannii biofilms have also been found to be inhibited by certain additional chemicals or substances that do not precisely belong to the aforementioned classes. Hydrogen peroxide and its formulations were examined to see how well biocides worked to get rid of MDR A. baumanni biofilms. In comparison with single-species Acinetobacter biofilm cells, mixed culture biofilms were resistant to various biocides, such as sulfathiazole and hydrogen peroxide. Compared to non-oxidizing biocides such as glutaraldehyde and sulfathiazole, it was found that oxidizing biocides (sodium hypochlorite and hydrogen peroxide) have an increased ability to remove and destroy biofilms [203, 204]. An inhibition of A. baumannii biofilms by more than 95% was observed for 2-aminoimidazole compounds when applied at a concentration of 100 M. These compounds could enhance the permeability of many conventional antibiotics through bacterial cell membrane, so they can be utilized in a variety of systems as a novel “drug delivery” approach [205]. The disinfectant octenidine dihydrochloride was found to be efficient in preventing A. baumannii biofilms, which were found to accumulate on polystyrene and stainless steel catheters [206]. Orthophthalaldehyde, peracetic acid, sodium hypochlorite, chlorhexidine, and peracetic acid were investigated for their biocidal activities against A. baumanni. Also, biofilm-producing A. baumannii was sensitive to all studied antiseptics and disinfectants in about 78% of cases [207]. It was found that replacing 6-position on N2,N4-dibenzylquinazoline-2,4-diamines, inhibitors of dihydrofolate reductase enzyme, with an alkyl or a halide substituent, resulted in a 90% activity against A. baumannii biofilms [208]. According to a clinical investigation, reduction in bacterial colonization was observed after using silver alloy, which, in turn, decreased UTIs [209]. Additionally, it was discovered in many clinical trials that nitrofurazone-coated silicon catheters had the ability to minimize UTI during brief use (30 days) [210].
Future prospects
Failure to respond to treatment is brought about by a build-up of resistance via numerous secretory chemicals and cell markers. Situations are getting worse all over the world due to the growth of MDR microbes found in biofilms, which makes this problem a new public health threat. We have covered topics such as how virulence factors of biofilms are increased, the roles of different components involved for biofilm formation, chronic illnesses linked to biofilms, and novel, potentially effective treatments for pathogenic biofilms and biofilm-related infections. In addition, other alternative approaches such as vaccination approach might be future options to protect against the MDR biofilms and prevent the spread of A. baumannii infections. In order to immunize against A. baumannii, a number of biofilm-associated components, including Bap, PNAG, OmpA, outer membrane vesicles, and Ata, were investigated as prospective candidates for vaccine development [211,212,213,214,215,216].
Conclusion
A. baumannii is the causative agent of a variety of biofilm-associated and MDR infections both in hospitals and community. For both researchers and clinicians, the genus Acinetobacter is difficult bacteria, because it has the ability to acquire genes responsible for antibiotic resistance and can form biofilms. A. baumannii forms biofilms through a multifactorial process that includes both intrinsic and extrinsic variables and is controlled by different regulatory mechanisms that control bacterial cell release from the biofilm, bacterial adhesion, and biofilm maturation. The genes implicated in the development of biofilms were found in both strains that produced biofilms and those that did not; however, these genes were highly expressed in biofilm producers compared to non-producers. Antibiotics are ineffective in treatment due to the development of antibiotic resistance as well as the intricate biofilm matrix structure. To increase the pipeline for antimicrobial drugs, the current situation urgently calls for innovation, discovery, or repurposing medicinal molecules. Chronic infections produced by preformed biofilms could be managed by exposing the bacterial cells to antibiotics and focusing on matrix components to break down the biofilm's structure. Controlling A. baumannii infections linked to biofilms may be possible by interfering with regulatory processes as QS, two-component systems, and nucleotide signaling. Due to the global spread of antibiotic resistance, phage, photodynamic, and nanoparticle therapies have lately attracted attention and demonstrated promising outcomes. To completely minimize the risk associated with these novel techniques, additional in vitro and in vivo researches utilizing them are needed. Due to the fact that biofilms may become polymicrobial and the existing non-invasive in vivo diagnostic techniques have difficulty identifying the etiological bacterium in biofilm-related infections, the management of these infections must include the development of broad-spectrum drugs for enhanced outcomes.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Abbreviations
- A. baumannii :
-
Acinetobacter baumannii
- P. aeruginosa :
-
Pseudomonas aeruginosa
- P. putida :
-
Pseudomonas putida
- K. pneumoniae :
-
Klebsiella pneumoniae
- S. aureus :
-
Staphylococcus aureus
- E. coli :
-
Escherichia coli
- ABC:
-
ATP-binding cassette
- AgNPs:
-
Silver nanoparticles
- AHLs:
-
Acyl homoserine lactones
- AI:
-
Autoinducers
- NaCl:
-
Sodium chloride
- AlgC:
-
Alginate C
- AMPs:
-
Antimicrobials peptides
- Bap:
-
Biofilm-associated protein
- Bla PER1:
-
Beta-lactamase PER-1
- C-di-GMP:
-
Cyclic di-guanosine monophosphate
- Csg:
-
Curli-specific gene
- Csu:
-
Chaperon–usher
- eDNA:
-
Extracellular DNA
- EO:
-
Essential oil
- EPI:
-
Efflux pump inhibitors
- EPS:
-
Extracellular polymeric substance
- ICUs:
-
Intensive care units
- MATE:
-
Multidrug and toxin compound extrusion
- MDR:
-
Multidrug-resistant
- MFS:
-
Major facilitator superfamily
- NPs:
-
Nanoparticles
- IN:
-
Inner membrane
- OM:
-
Outer membrane
- OmpA:
-
Outer membrane protein A
- PCR:
-
Polymerase chain reaction
- PDT:
-
Photodynamic treatment
- PNAG:
-
Poly-β-(1,6)-N-acetyl glucosamine
- QQ:
-
Quorum quenching
- QS:
-
Quorum sensing
- RND:
-
Resistance nodulation division
- ROS:
-
Reactive oxygen species
- SMR:
-
Small multidrug resistance
- UTI:
-
Urinary tract infections
- XDR:
-
Extensively drug-resistant
- TSB:
-
Tryptic soy broth
- BHI:
-
Brain–heart infusion broth
References
Lin M-F, Lan C-Y (2014) Antimicrobial resistance in Acinetobacter baumannii: from bench to bedside. World J Clin Cases WJCC 2(12):787. https://doi.org/10.12998/WJCC.V2.I12.787
Mulani MS, Kamble EE, Kumkar SN, Tawre MS, Pardesi KR (2019) Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: a review. Front Microbiol 10(APR):539. https://doi.org/10.3389/FMICB.2019.00539/BIBTEX
Joly-Guillou ML (2005) Clinical impact and pathogenicity of Acinetobacter. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis 11(11):868–873. https://doi.org/10.1111/J.1469-0691.2005.01227.X
Piperaki ET, Tzouvelekis LS, Miriagou V, Daikos GL (2019) Carbapenem-resistant Acinetobacter baumannii: in pursuit of an effective treatment. Clin Microbiol Infect 25(8):951–957. https://doi.org/10.1016/J.CMI.2019.03.014
Ma C, McClean S (2021) Mapping global prevalence of Acinetobacter baumannii and recent vaccine development to tackle it. Vaccines. https://doi.org/10.3390/VACCINES9060570
Peleg AY, Seifert H, Paterson DL (2008) Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21(3):538–582. https://doi.org/10.1128/CMR.00058-07
Pour NK, Dusane DH, Dhakephalkar PK, Zamin FR, Zinjarde SS, Chopade BA (2011) Biofilm formation by Acinetobacter baumannii strains isolated from urinary tract infection and urinary catheters. FEMS Immunol Med Microbiol 62(3):328–338. https://doi.org/10.1111/J.1574-695X.2011.00818.X
McConnell MJ, Actis L, Pachón J (2013) Acinetobacter baumannii: human infections, factors contributing to pathogenesis and animal models. FEMS Microbiol Rev 37(2):130–155. https://doi.org/10.1111/J.1574-6976.2012.00344.X
Antunes LCS, Visca P, Towner KJ (2014) Acinetobacter baumannii: evolution of a global pathogen. Pathogens Dis 71(3):292–301. https://doi.org/10.1111/2049-632X.12125
Lin MF, Land CY (2014) Antimicrobial resistance in Acinetobacter baumannii: from bench to bedside. World J Clin Cases 2(12):787. https://doi.org/10.12998/WJCC.V2.I12.787
Gordon NC, Wareham DW (2010) Multidrug-resistant Acinetobacter baumannii: mechanisms of virulence and resistance. Int J Antimicrob Agents 35(3):219–226. https://doi.org/10.1016/J.IJANTIMICAG.2009.10.024
Kim YJ, Kim SI, Kim YR, Hong KW, Wie SH, Park YJ, Kang MW (2012) Carbapenem-resistant Acinetobacter baumannii: diversity of resistant mechanisms and risk factors for infection. Epidemiol Infect 140(1):137–145. https://doi.org/10.1017/S0950268811000744
Yang CH, Su PW, Moi SH, Chuang LY (2019) Biofilm formation in Acinetobacter baumannii: genotype-phenotype correlation. Molecules (Basel, Switzerland). https://doi.org/10.3390/MOLECULES24101849
Roy R, Tiwari M, Donelli G, Tiwari V (2018) Strategies for combating bacterial biofilms: a focus on anti-biofilm agents and their mechanisms of action. Virulence 9(1):522. https://doi.org/10.1080/21505594.2017.1313372
Murugaiyan J, Anand Kumar P, Rao GS, Iskandar K, Hawser S, Hays JP, van Dongen MBM (2022) Progress in alternative strategies to combat antimicrobial resistance: focus on antibiotics. Antibiotics 11(2):200. https://doi.org/10.3390/ANTIBIOTICS11020200
Garrett TR, Bhakoo M, Zhang Z (2008) Bacterial adhesion and biofilms on surfaces. Prog Nat Sci 18(9):1049–1056. https://doi.org/10.1016/J.PNSC.2008.04.001
Qureshi N, Annous BA, Ezeji TC, Karcher P, Maddox IS (2005) Biofilm reactors for industrial bioconversion process: employing potential of enhanced reaction rates. Microb Cell Fact 4(1):1–21. https://doi.org/10.1186/1475-2859-4-24/FIGURES/6
Jamal M, Tasneem U, Hussain T, Andleeb S (2015) Bacterial biofilm: its composition, formation and role in human infections. undefined
Stoodley P, Sauer K, Davies DG, Costerton JW (2002) Biofilms as complex differentiated communities. Annu Rev Microbiol 56:187–209. https://doi.org/10.1146/ANNUREV.MICRO.56.012302.160705
Evans BA, Hamouda A, Amyes GBS (2013) The rise of carbapenem-resistant Acinetobacter baumannii. Curr Pharm 19(2):223–238
Antunes LCS, Imperi F, Carattoli A, Visca P (2011) Deciphering the multifactorial nature of Acinetobacter baumannii pathogenicity. PLoS ONE 6(8):e22674. https://doi.org/10.1371/JOURNAL.PONE.0022674
Richards AM, Abu Kwaik Y, Lamont RJ (2015) Code blue: Acinetobacter baumannii, a nosocomial pathogen with a role in the oral cavity. Mol Oral Microbiol 30(1):2–15. https://doi.org/10.1111/OMI.12072
Cerqueira GM, Peleg AY (2011) Insights into Acinetobacter baumannii pathogenicity. IUBMB Life 63(12):1055–1060. https://doi.org/10.1002/IUB.533
Sato Y, Unno Y, Kawakami S, Ubagai T, Ono Y (2017) Virulence characteristics of Acinetobacter baumannii clinical isolates vary with the expression levels of omps. J Med Microbiol 66(2):203–212. https://doi.org/10.1099/JMM.0.000394
Greene C, Vadlamudi G, Newton D, Foxman B, Xi C (2016) The influence of biofilm formation and multidrug resistance on environmental survival of clinical and environmental isolates of Acinetobacter baumannii. Am J Infect Control 44(5):e65–e71. https://doi.org/10.1016/J.AJIC.2015.12.012
Gaddy JA, Actis LA (2009) Regulation of Acinetobacter baumannii biofilm formation. Future Microbiol 4(3):273–278. https://doi.org/10.2217/FMB.09.5
Gaddy JA, Tomaras AP, Actis LA (2009) The Acinetobacter baumannii 19606 OmpA protein plays a role in biofilm formation on abiotic surfaces and in the interaction of this pathogen with eukaryotic cells. Infect Immun 77(8):3150–3160. https://doi.org/10.1128/IAI.00096-09
Roy S, Chowdhury G, Mukhopadhyay AK, Dutta S, Basu S (2022) Convergence of biofilm formation and antibiotic resistance in Acinetobacter baumannii Infection. Front Med 9:364. https://doi.org/10.3389/FMED.2022.793615/BIBTEX
Elkheloui R, Laktib A, Mimouni R, Aitalla A, Hassi M, Elboulani A, Hamadi F (2020) Acinetobacter baumannii biofilm: intervening factors, persistence, drug resistance, and strategies of treatment. Mediter J Infect Microbes Antimicrob. https://doi.org/10.4274/MJIMA.GALENOS.2020.2020.7
Badave GK, Dhananjay K (2015) Biofilm producing multidrug resistant Acinetobacter baumannii: an emerging challenge. J Clin Diagn Res JCDR 9(1):DC08-DC10. https://doi.org/10.7860/JCDR/2015/11014.5398
Badave GK, Dhananjay K (2015) Biofilm producing multidrug resistant Acinetobacter baumannii: an emerging challenge. J Clin Diagn Res JCDR 9(1):08. https://doi.org/10.7860/JCDR/2015/11014.5398
Rao RS, Karthika RU, Singh S, Shashikala P, Kanungo R, Jayachandran S, Prashanth K (2008) Correlation between biofilm production and multiple drug resistance in imipenem resistant clinical isolates of Acinetobacter baumannii. Indian J Med Microbiol 26(4):333–337. https://doi.org/10.1016/S0255-0857(21)01809-0
Krzyściak P, Chmielarczyk A, Pobiega M, Romaniszyn D, Wójkowska-Mach J (2017) Acinetobacter baumannii isolated from hospital-acquired infection: biofilm production and drug susceptibility. APMIS Acta Pathologica Microbiologica Immunologica Scandinavica 125(11):1017–1026. https://doi.org/10.1111/APM.12739
Rodríguez-Baño J, Martí S, Soto S, Fernández-Cuenca F, Cisneros JM, Pachón J, Vila J (2008) Biofilm formation in Acinetobacter baumannii: associated features and clinical implications. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis 14(3):276–278. https://doi.org/10.1111/J.1469-0691.2007.01916.X
Rodrigues Perez LR (2014) Acinetobacter baumannii displays inverse relationship between meropenem resistance and biofilm production. J Chemother (Florence, Italy) 27(1):13–16. https://doi.org/10.1179/1973947813Y.0000000159
Gedefie A, Demsis W, Ashagrie M, Kassa Y, Tesfaye M, Tilahun M, Sahle Z (2021) Acinetobacter baumannii biofilm formation and its role in disease pathogenesis: a review. Infect Drug Resist 14:3711. https://doi.org/10.2147/IDR.S332051
Rumbo-Feal S, Gómez MJ, Gayoso C, Álvarez-Fraga L, Cabral MP, Aransay AM, Poza M (2013) Whole transcriptome analysis of Acinetobacter baumannii assessed by RNA-sequencing reveals different mRNA expression profiles in biofilm compared to planktonic cells. PLoS ONE. https://doi.org/10.1371/JOURNAL.PONE.0072968
Confer AW, Ayalew S (2013) The OmpA family of proteins: roles in bacterial pathogenesis and immunity. Vet Microbiol 163(3–4):207–222. https://doi.org/10.1016/J.VETMIC.2012.08.019
Nie D, Hu Y, Chen Z, Li M, Hou Z, Luo X, Xue X (2020) Outer membrane protein A (OmpA) as a potential therapeutic target for Acinetobacter baumannii infection. J Biomed Sci 27(1):1–8. https://doi.org/10.1186/S12929-020-0617-7/FIGURES/1
Ghasemi E, Ghalavand Z, Goudarzi H, Yeganeh F, Hashemi A, Dabiri H, Foroumand M (2018) Phenotypic and genotypic investigation of biofilm formation in clinical and environmental isolates of Acinetobacter baumannii. Arch Clin Infect Dis 13(4):12914. https://doi.org/10.5812/ARCHCID.12914
Busch A, Waksman G (2012) Chaperone–usher pathways: diversity and pilus assembly mechanism. Philos Trans R Soc B Biol Sci 367(1592):1112. https://doi.org/10.1098/RSTB.2011.0206
Flemming HC (2016) EPS-then and now. Microorganisms. https://doi.org/10.3390/MICROORGANISMS4040041
Bhargava N, Sharma P, Capalash N (2010) Quorum sensing in Acinetobacter: an emerging pathogen. Crit Rev Microbiol 36(4):349–360. https://doi.org/10.3109/1040841X.2010.512269
Ng WL, Bassler BL (2009) Bacterial quorum-sensing network architectures. Annu Rev Genet 43:197–222. https://doi.org/10.1146/ANNUREV-GENET-102108-134304
Alav I, Sutton JM, Rahman KM (2018) Role of bacterial efflux pumps in biofilm formation. J Antimicrob Chemother 73(8):2003–2020. https://doi.org/10.1093/JAC/DKY042
Flannery A, Le Berre M, Pier GB, O’gara JP, Kilcoyne M (2020) Glycomics microarrays reveal differential in situ presentation of the biofilm polysaccharide poly-N-acetylglucosamine on Acinetobacter baumannii and Staphylococcus aureus cell surfaces. Int J Mol Sci. https://doi.org/10.3390/IJMS21072465
Itoh Y, Rice JD, Goller C, Pannuri A, Taylor J, Meisner J, Romeo T (2008) Roles of pgaABCD genes in synthesis, modification, and export of the Escherichia coli biofilm adhesin poly-beta-1,6-N-acetyl-D-glucosamine. J Bacteriol 190(10):3670–3680. https://doi.org/10.1128/JB.01920-07
Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS (2002) Extracellular DNA required for bacterial biofilm formation. Science (New York, N.Y.) 295(5559):1487. https://doi.org/10.1126/SCIENCE.295.5559.1487
Rasamiravaka T, Labtani Q, Duez P, El Jaziri M (2015) The formation of biofilms by Pseudomonas aeruginosa: a review of the natural and synthetic compounds interfering with control mechanisms. BioMed Res Int. https://doi.org/10.1155/2015/759348
Barnhart MM, Chapman MR (2006) Curli biogenesis and function. Annu Rev Microbiol 60:131. https://doi.org/10.1146/ANNUREV.MICRO.60.080805.142106
Vansteenkiste M, Ryan RM (2013) On psychological growth and vulnerability: basic psychological need satisfaction and need frustration as a unifying principle. J Psychother Integr 23(3):263–280. https://doi.org/10.1037/A0032359
Beitelshees M, Hill A, Jones CH, Pfeifer BA (2018) Phenotypic variation during biofilm formation: implications for anti-biofilm therapeutic design. Materials 11(7):1086. https://doi.org/10.3390/MA11071086
Smani Y, McConnell MJ, Pachón J (2012) Role of fibronectin in the adhesion of Acinetobacter baumannii to host cells. PLoS ONE 7(4):33073. https://doi.org/10.1371/JOURNAL.PONE.0033073
Brossard KA, Campagnari AA (2012) The Acinetobacter baumannii biofilm-associated protein plays a role in adherence to human epithelial cells. Infect Immun 80(1):228–233. https://doi.org/10.1128/IAI.05913-11
Loehfelm TW, Luke NR, Campagnari AA (2008) Identification and characterization of an Acinetobacter baumannii biofilm-associated protein. J Bacteriol 190(3):1036–1044. https://doi.org/10.1128/JB.01416-07
De Gregorio E, Del Franco M, Martinucci M, Roscetto E, Zarrilli R, Di Nocera PP (2015) Biofilm-associated proteins: news from Acinetobacter. BMC Genomics 16(1):1–14. https://doi.org/10.1186/S12864-015-2136-6/FIGURES/9
Molecular analysis and expression of bap gene in biofilm-forming multi-drug-resistant Acinetobacter baumannii. (n.d.). ncbi.nlm.nih.gov. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5214686/
Skiebe E, de Berardinis V, Morczinek P, Kerrinnes T, Faber F, Lepka D, Wilharm G (2012) Surface-associated motility, a common trait of clinical isolates of Acinetobacter baumannii, depends on 1,3-diaminopropane. Int J Med Microbiol IJMM 302(3):117–128. https://doi.org/10.1016/J.IJMM.2012.03.003
Vijayakumar S, Rajenderan S, Laishram S, Anandan S, Balaji V, Biswas I (2016) Biofilm formation and motility depend on the nature of the Acinetobacter baumannii clinical isolates. Front Public Health. https://doi.org/10.3389/FPUBH.2016.00105
Harding CM, Tracy EN, Carruthers MD, Rather PN, Actis LA, Munson RS (2013) Acinetobacter baumannii strain M2 produces type IV pili which play a role in natural transformation and twitching motility but not surface-associated motility. MBio. https://doi.org/10.1128/MBIO.00360-13
Wilharm G, Piesker J, Laue M, Skiebe E (2013) DNA uptake by the nosocomial pathogen Acinetobacter baumannii occurs during movement along wet surfaces. J Bacteriol 195(18):4146. https://doi.org/10.1128/JB.00754-13
Burrows LL (2012) Pseudomonas aeruginosa twitching motility: type IV pili in action. Annu Rev Microbiol 66:493–520. https://doi.org/10.1146/ANNUREV-MICRO-092611-150055
Upmanyu K, Haq QMR, Singh R (2022) Factors mediating Acinetobacter baumannii biofilm formation: opportunities for developing therapeutics. Curr Res Microb Sci 3:100131. https://doi.org/10.1016/J.CRMICR.2022.100131
Lee HW, Koh YM, Kim J, Lee JC, Lee YC, Seol SY, Cho DT (2008) Capacity of multidrug-resistant clinical isolates of Acinetobacter baumannii to form biofilm and adhere to epithelial cell surfaces. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis 14(1):49–54. https://doi.org/10.1111/J.1469-0691.2007.01842.X
Khamari B, Lama M, Pachi Pulusu C, Biswal AP, Lingamallu SM, Mukkirla BS, Bulagonda EP (2020) Molecular analyses of biofilm-producing clinical Acinetobacter baumannii isolates from a South Indian Tertiary Care Hospital. Med Princ Pract Int J Kuwait Univ Health Sci Centre 29(6):580–587. https://doi.org/10.1159/000508461
Bardbari AM, Arabestani MR, Karami M, Keramat F, Alikhani MY, Bagheri KP (2017) Correlation between ability of biofilm formation with their responsible genes and MDR patterns in clinical and environmental Acinetobacter baumannii isolates. Microb Pathog 108:122–128. https://doi.org/10.1016/J.MICPATH.2017.04.039
Lewis K (2008) Multidrug tolerance of biofilms and persister cells. Curr Top Microbiol Immunol 322:107–131. https://doi.org/10.1007/978-3-540-75418-3_6/COVER
Cuccui J, Wren BW (2013) Bacteria like sharing their sweets. Mol Microbiol 89(5):811–815. https://doi.org/10.1111/MMI.12328
Dou Y, Song F, Guo F, Zhou Z, Zhu C, Xiang J, Huan J (2017) Acinetobacter baumannii quorum-sensing signalling molecule induces the expression of drug-resistance genes. Mol Med Rep 15(6):4061. https://doi.org/10.3892/MMR.2017.6528
Oh MH, Han K (2020) AbaR is a LuxR type regulator essential for motility and the formation of biofilm and pellicle in Acinetobacter baumannii. Genes Genomics 42(11):1339–1346. https://doi.org/10.1007/S13258-020-01005-8/FIGURES/5
Luo LM, Wu LJ, Xiao YL, Zhao D, Chen ZX, Kang M, Xie Y (2015) Enhancing pili assembly and biofilm formation in Acinetobacter baumannii ATCC19606 using non-native acyl-homoserine lactones. BMC Microbiol. https://doi.org/10.1186/S12866-015-0397-5/TABLES/1
Hwang Kim I, Wen Y, Son JS, Lee KH, Kim KS (2013) The fur-iron complex modulates expression of the quorum-sensing master regulator, SmcR, to control expression of virulence factors in Vibrio vulnificus. Infect Immun 81(8):2888. https://doi.org/10.1128/IAI.00375-13
Zhou L, Zhang Y, Ge Y, Zhu X, Pan J (2020) Regulatory mechanisms and promising applications of quorum sensing-inhibiting agents in control of bacterial biofilm formation. Front Microbiol 11:2558. https://doi.org/10.3389/FMICB.2020.589640/BIBTEX
Elekhnawy E, Sonbol F, Abdelaziz A, Elbanna T (2020) Potential impact of biocide adaptation on selection of antibiotic resistance in bacterial isolates. Future J Pharm Sci 6(1):1–10. https://doi.org/10.1186/S43094-020-00119-W
Yoon EJ, Courvalin P, Grillot-Courvalin C (2013) RND-type efflux pumps in multidrug-resistant clinical isolates of Acinetobacter baumannii: major role for AdeABC overexpression and AdeRS mutations. Antimicrob Agents Chemother 57(7):2989–2995. https://doi.org/10.1128/AAC.02556-12
He X, Lu F, Yuan F, Jiang D, Zhao P, Zhu J, Lu G (2015) Biofilm formation caused by clinical Acinetobacter baumannii isolates is associated with overexpression of the AdeFGH efflux pump. Antimicrob Agents Chemother 59(8):4817–4825. https://doi.org/10.1128/AAC.00877-15
Richmond GE, Evans LP, Anderson MJ, Wand ME, Bonney LC, Ivens A, Piddock LJV (2016) The Acinetobacter baumannii two-component system AdeRS regulates genes required for multidrug efflux, biofilm formation, and virulence in a strain-specific manner. MBio. https://doi.org/10.1128/MBIO.00430-16
Roca I, Espinal P, Martí S, Vila J (2011) First identification and characterization of an AdeABC-like efflux pump in Acinetobacter Genomospecies 13TU. Antimicrob Agents Chemother 55(3):1285. https://doi.org/10.1128/AAC.01142-10
Itoh Y, Rice JD, Goller C, Pannuri A, Taylor J, Meisner J, Romeo T (2008) Roles of pgaABCD genes in synthesis, modification, and export of the Escherichia coli biofilm adhesin poly-β-1,6-N-acetyl-d-glucosamine. J Bacteriol 190(10):3670. https://doi.org/10.1128/JB.01920-07
Choi AHK, Slamti L, Avci FY, Pier GB, Maira-Litrán T (2009) The pgaABCD locus of Acinetobacter baumannii encodes the production of poly-beta-1-6-N-acetylglucosamine, which is critical for biofilm formation. J Bacteriol 191(19):5953–5963. https://doi.org/10.1128/JB.00647-09
Ibáñez de Aldecoa AL, Zafra O, González-Pastor JE (2017) Mechanisms and regulation of extracellular DNA release and its biological roles in microbial communities. Front Microbiol. https://doi.org/10.3389/FMICB.2017.01390
Sahu R, Kaushik S, Clement CC, Cannizzo ES, Scharf B, Follenzi A, Santambrogio L (2011) Microautophagy of cytosolic proteins by late endosomes. Dev Cell 20(1):131–139. https://doi.org/10.1016/J.DEVCEL.2010.12.003
Tetz GV, Artemenko NK, Tetz VV (2009) Effect of DNase and antibiotics on biofilm characteristics. Antimicrob Agents Chemother 53(3):1204–1209. https://doi.org/10.1128/AAC.00471-08
Coyne MJ, Russell KS, Coyle CL, Goldberg JB (1994) The Pseudomonas aeruginosa algC gene encodes phosphoglucomutase, required for the synthesis of a complete lipopolysaccharide core. J Bacteriol 176(12):3500–3507. https://doi.org/10.1128/JB.176.12.3500-3507.1994
Goldberg JB, Hatano K, Pier GB (1993) Synthesis of lipopolysaccharide O side chains by Pseudomonas aeruginosa PAO1 requires the enzyme phosphomannomutase. J Bacteriol 175(6):1605. https://doi.org/10.1128/JB.175.6.1605-1611.1993
Lu M, Kleckner N (1994) Molecular cloning and characterization of the pgm gene encoding phosphoglucomutase of Escherichia coli. J Bacteriol 176(18):5847. https://doi.org/10.1128/JB.176.18.5847-5851.1994
Sahu PK, Iyer PS, Barage SH, Sonawane KD, Chopade BA (2014) Characterization of the algC gene expression pattern in the multidrug resistant Acinetobacter baumannii AIIMS 7 and correlation with biofilm development on abiotic surface. Sci World J. https://doi.org/10.1155/2014/593546
Iswarya Jaisankar A, Smiline Girija AS, Shoba G, Vijayashree Priyadharsini J (2020) Molecular characterisation of csgA gene among ESBL strains of A. baumannii and targeting with essential oil compounds from Azadirachta indica. J King Saud Univ Sci 32(8):3380–3387. https://doi.org/10.1016/J.JKSUS.2020.09.025
Hengge R, Gründling A, Jenal U, Ryan R, Yildiz F (2016) Bacterial signal transduction by cyclic di-GMP and other nucleotide second messengers. J Bacteriol 198(1):15. https://doi.org/10.1128/JB.00331-15
Qvortrup K, Hultqvist LD, Nilsson M, Jakobsen TH, Jansen CU, Uhd J, Tolker-Nielsen T (2019) Small molecule anti-biofilm agents developed on the basis of mechanistic understanding of biofilm formation. Front Chem 7:742. https://doi.org/10.3389/FCHEM.2019.00742/BIBTEX
Beitelshees M, Hill A, Jones CH, Pfeifer BA (2018) Phenotypic variation during biofilm formation: implications for anti-biofilm therapeutic design. Materials. https://doi.org/10.3390/MA11071086
Sabater S, Guasch H, Romaní A, Muñoz I (2002) The effect of biological factors on the efficiency of river biofilms in improving water quality. Hydrobiologia 469:149–156. https://doi.org/10.1023/A:1015549404082
Besemer K (2015) Biodiversity, community structure and function of biofilms in stream ecosystems. Res Microbiol 166(10):774–781. https://doi.org/10.1016/J.RESMIC.2015.05.006
Greene C, Wu J, Rickard AH, Xi C (2016) Evaluation of the ability of Acinetobacter baumannii to form biofilms on six different biomedical relevant surfaces. Lett Appl Microbiol 63(4):233–239. https://doi.org/10.1111/LAM.12627
Eze EC, El Zowalaty ME (2019) Combined effects of low incubation temperature, minimal growth medium, and low hydrodynamics optimize Acinetobacter baumannii biofilm formation. Infect Drug Resist 12:3523. https://doi.org/10.2147/IDR.S203919
Khatoon Z, McTiernan CD, Suuronen EJ, Mah TF, Alarcon EI (2018) Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon. https://doi.org/10.1016/J.HELIYON.2018.E01067
Gorman SP, Jones DS (2005) Medical device composition and biological secretion influences on biofilm formation. Biofilms Infect Antimicrob Therapy. https://doi.org/10.1201/9781420028232-8/MEDICAL-DEVICE-COMPOSITION-BIOLOGICAL-SECRETION-INFLUENCES-BIOFILM-FORMATION-SEAN-GORMAN-DAVID-JONES
Tilahun A, Haddis S, Teshale A, Hadush T (2016) Review on biofilm and microbial adhesion. Int J Microbiol Res 7(3):63–73. https://doi.org/10.5829/idosi.ijmr.2016.63.73
Adetunji VO, Odetokun IA (2012) Biofilm formation in human and tropical foodborne isolates of Listeria strains. Am J Food Technol 7(9):517–531. https://doi.org/10.3923/AJFT.2012.517.531
García-Gonzalo D, Pagán R (2015) Influence of environmental factors on bacterial biofilm formation in the food industry: a review. J Postdr Res 3(6). Retrieved from www.postdocjournal.com
Marion-Ferey K, Pasmore M, Stoodley P, Wilson S, Husson GP, Costerton JW (2003) Biofilm removal from silicone tubing: an assessment of the efficacy of dialysis machine decontamination procedures using an in vitro model. J Hosp Infect 53(1):64–71. https://doi.org/10.1053/jhin.2002.1320
Dekic S, Hrenovic J, Ivankovic T, Van Wilpe E (2018) Survival of ESKAPE pathogen Acinetobacter baumannii in water of different temperatures and pH. Water Sci Technol 78(6):1370–1376. https://doi.org/10.2166/WST.2018.409
Malaka De Silva P, Chong P, Fernando DM, Westmacott G, Kumara A (2017) Effect of incubation temperature on antibiotic resistance and virulence factors of Acinetobacter baumannii ATCC 17978. Antimicrob Agents Chemother. https://doi.org/10.1128/AAC.01514-17
Chang YW, Fragkopoulos AA, Marquez SM, Kim HD, Angelini TE, Fernández-Nieves A (2015) Biofilm formation in geometries with different surface curvature and oxygen availability. New J Phys 17(3):033017. https://doi.org/10.1088/1367-2630/17/3/033017
Ahn SJ, Burne RA (2007) Effects of oxygen on biofilm formation and the AtlA autolysin of Streptococcus mutans. J Bacteriol 189(17):6293–6302. https://doi.org/10.1128/JB.00546-07
Keleştemur S, Avci E, Çulha M (2018) Raman and surface-enhanced Raman scattering for biofilm characterization. Chemosensors 6(1):5. https://doi.org/10.3390/CHEMOSENSORS6010005
Toyofuku M, Inaba T, Kiyokawa T, Obana N, Yawata Y, Nomura N (2016) Environmental factors that shape biofilm formation. Biosci Biotechnol Biochem 80(1):7–12. https://doi.org/10.1080/09168451.2015.1058701
Kumar CG, Anand SK (1998) Significance of microbial biofilms in food industry: a review. Int J Food Microbiol 42(1–2):9–27. https://doi.org/10.1016/S0168-1605(98)00060-9
Zhao X, Zhao F, Wang J, Zhong N (2017) Biofilm formation and control strategies of foodborne pathogens: food safety perspectives. RSC Adv 7(58):36670–36683. https://doi.org/10.1039/C7RA02497E
Gupta A (2015) Biofilm quantification and comparative analysis of MIC (minimum inhibitory concentration) & MBIC (minimum biofilm inhibitory concentration) value for different antibiotics against E. coli. Int J Curr Microbiol Appl Sci 4(2):198–224
Bühler T, Ballestero S, Desai M, Brown MRW (1998) Generation of a reproducible nutrient-depleted biofilm of Escherichia coli and Burkholderia cepacia. J Appl Microbiol 85(3):457–462. https://doi.org/10.1046/J.1365-2672.1998.853501.X
Volk CJ, LeChevallier MW (1999) Impacts of the reduction of nutrient levels on bacterial water quality in distribution systems. Appl Environ Microbiol 65(11):4957. https://doi.org/10.1128/AEM.65.11.4957-4966.1999
Rochex A, Lebeault JM (2007) Effects of nutrients on biofilm formation and detachment of a Pseudomonas putida strain isolated from a paper machine. Water Res 41(13):2885–2892. https://doi.org/10.1016/J.WATRES.2007.03.041
Jackson DW, Simecka JW, Romeo T (2002) Catabolite repression of Escherichia coli biofilm formation. J Bacteriol 184(12):3406–3410. https://doi.org/10.1128/JB.184.12.3406-3410.2002
Delaquis PJ, Caldwell DE, Lawrence JR, McCurdy AR (1989) Detachment ofPseudomonas fluorescens from biofilms on glass surfaces in response to nutrient stress. Microb Ecol 18(3):199–210. https://doi.org/10.1007/BF02075808
Gomes LC, Moreira JMR, Teodósio JS, Araújo JDP, Miranda JM, Simões M, Mergulhão FJ (2014) 96-well microtiter plates for biofouling simulation in biomedical settings. Biofouling 30(5):535–546. https://doi.org/10.1080/08927014.2014.890713
Rinaudi L, Fujishige NA, Hirsch AM, Banchio E, Zorreguieta A, Giordano W (2006) Effects of nutritional and environmental conditions on Sinorhizobium meliloti biofilm formation. Res Microbiol 157(9):867–875. https://doi.org/10.1016/J.RESMIC.2006.06.002
Johnson M, Cockayne A, Williams PH, Morrissey JA (2005) Iron-responsive regulation of biofilm formation in Staphylococcus aureus involves fur-dependent and fur-independent mechanisms. J Bacteriol 187(23):8211–8215. https://doi.org/10.1128/JB.187.23.8211-8215.2005
Falghoush A, Beyenal H, Besser TE, Omsland A, Call DR (2017) Osmotic compounds enhance antibiotic efficacy against Acinetobacter baumannii biofilm communities. Appl Environ Microbiol. https://doi.org/10.1128/AEM.01297-17
Martinez LR, Casadevall A (2007) Cryptococcus neoformans biofilm formation depends on surface support and carbon source and reduces fungal cell susceptibility to heat, cold, and UV light. Appl Environ Microbiol 73(14):4592–4601. https://doi.org/10.1128/AEM.02506-06
Jacobs AC, Blanchard CE, Catherman SC, Dunman PM, Murata Y (2014) An ribonuclease T2 family protein modulates Acinetobacter baumannii abiotic surface colonization. PLoS ONE. https://doi.org/10.1371/JOURNAL.PONE.0085729
Lee HW, Koh YM, Kim J, Lee JC, Lee YC, Seol SY, Cho DT (2008) Capacity of multidrug-resistant clinical isolates of Acinetobacter baumannii to form biofilm and adhere to epithelial cell surfaces. Clin Microbiol Infect 14(1):49–54. https://doi.org/10.1111/j.1469-0691.2007.01842.x
Nwugo CC, Arivett BA, Zimbler DL, Gaddy JA, Richards AM, Actis LA (2012) Effect of ethanol on differential protein production and expression of potential virulence functions in the opportunistic pathogen Acinetobacter baumannii. PLoS ONE 7(12):e51936. https://doi.org/10.1371/JOURNAL.PONE.0051936
Agarwal RK, Singh S, Bhilegaonkar KN, Singh VP (2011) Optimization of microtitre plate assay for the testing of biofilm formation ability in different Salmonella serotypes. Int Food Res J 18(4):1493–1498
Oder M, Fink R, Bohinc K, Torkar KG (2017) The influence of shear stress on the adhesion capacity of Legionella pneumophila. Arh Hig Rada Toksikol 68(2):109–115. https://doi.org/10.1515/AIHT-2017-68-2904
Pompilio A, Piccolomini R, Picciani C, D’Antonio D, Savini V, Di Bonaventura G (2008) Factors associated with adherence to and biofilm formation on polystyrene by Stenotrophomonas maltophilia: the role of cell surface hydrophobicity and motility. FEMS Microbiol Lett 287(1):41–47. https://doi.org/10.1111/J.1574-6968.2008.01292.X
Chaieb K, Chehab O, Zmantar T, Rouabhia M, Mahdouani K, Bakhrouf A (2007) In vitro effect of pH and ethanol on biofilm formation by clinical ica-positive Staphylococcus epidermidis strains. Ann Microbiol 57(3):431–437. https://doi.org/10.1007/BF03175085/METRICS
D’Urzo N, Martinelli M, Pezzicoli A, De Cesare V, Pinto V, Margarit I, Efstratiou A (2014) Acidic pH strongly enhances in vitro biofilm formation by a subset of hypervirulent ST-17 Streptococcus agalactiae strains. Appl Environ Microbiol 80(7):2176–2185. https://doi.org/10.1128/AEM.03627-13
Nicolau Korres AM, Aquije GMDFV, Buss DS, Ventura JA, Fernandes PMB, Fernandes AAR (2013) Comparison of biofilm and attachment mechanisms of a phytopathological and clinical isolate of Klebsiella pneumoniae Subsp. pneumoniae. Sci World J. https://doi.org/10.1155/2013/925375
Nostro A, Cellini L, Di Giulio M, D’Arrigo M, Marino A, Blanco AR, Bisignano G (2012) Effect of alkaline pH on staphylococcal biofilm formation. APMIS Acta Pathologica Microbiologica Immunologica Scandinavica 120(9):733–742. https://doi.org/10.1111/J.1600-0463.2012.02900.X
MalakaDeSilva P, Chong P, Fernando DM, Westmacott G, Kumara A (2018) Effect of incubation temperature on antibiotic resistance and virulence factors of Acinetobacter baumannii ATCC 17978. Antimicrob Agents Chemother. https://doi.org/10.1128/AAC.01514-17
Subhadra B, Kim DH, Woo K, Surendran S, Choi CH (2018) Control of biofilm formation in healthcare: recent advances exploiting quorum-sensing interference strategies and multidrug efflux pump inhibitors. Materials (Basel, Switzerland). https://doi.org/10.3390/MA11091676
Sperandio V (2007) Novel approaches to bacterial infection therapy by interfering with bacteria-to-bacteria signaling. Expert Rev Anti Infect Ther 5(2):271. https://doi.org/10.1586/14787210.5.2.271
Hoang TT, Schweizer HP (1999) Characterization of Pseudomonas aeruginosa enoyl-acyl carrier protein reductase (FabI): a target for the antimicrobial triclosan and its role in acylated homoserine lactone synthesis. J Bacteriol 181(17):5489–5497. https://doi.org/10.1128/JB.181.17.5489-5497.1999
Choo JH, Rukayadi Y, Hwang JK (2006) Inhibition of bacterial quorum sensing by vanilla extract. Lett Appl Microbiol 42(6):637–641. https://doi.org/10.1111/J.1472-765X.2006.01928.X
Rasmussen TB, Bjarnsholt T, Skindersoe ME, Hentzer M, Kristoffersen P, Köte M, Givskov M (2005) Screening for quorum-sensing inhibitors (QSI) by use of a novel genetic system, the QSI selector. J Bacteriol 187(5):1799–1814. https://doi.org/10.1128/JB.187.5.1799-1814.2005/ASSET/3E19DEAC-918D-4D39-90CE-60AA2B60421A/ASSETS/GRAPHIC/ZJB0050545040006.JPEG
Cady NC, McKean KA, Behnke J, Kubec R, Mosier AP, Kasper SH, Musah RA (2012) Inhibition of biofilm formation, quorum sensing and infection in Pseudomonas aeruginosa by natural products-inspired organosulfur compounds. PLoS ONE 7(6):e38492. https://doi.org/10.1371/JOURNAL.PONE.0038492
Stacy DM, Welsh MA, Rather PN, Blackwell HE (2012) Attenuation of quorum sensing in the pathogen acinetobacter baumannii using non-native N-acyl homoserine lactones. ACS Chem Biol 7(10):1719–1728. https://doi.org/10.1021/CB300351X/SUPPL_FILE/CB300351X_SI_001.PDF
Sambanthamoorthy K, Luo C, Pattabiraman N, Feng X, Koestler B, Waters CM, Palys TJ (2014) Identification of small molecules inhibiting diguanylate cyclases to control bacterial biofilm development. Biofouling 30(1):17–28. https://doi.org/10.1080/08927014.2013.832224
Young DM, Parke D, Ornston LN (2005) Opportunities for genetic investigation afforded by Acinetobacter baylyi, a nutritionally versatile bacterial species that is highly competent for natural transformation. Annu Rev Microbiol 59:519–551. https://doi.org/10.1146/ANNUREV.MICRO.59.051905.105823
Nait Chabane Y, Mlouka MB, Alexandre S, Nicol M, Marti S, Pestel-Caron M, Dé E (2014) Virstatin inhibits biofilm formation and motility of Acinetobacter baumannii. BMC Microbiol 14(1):1–7. https://doi.org/10.1186/1471-2180-14-62/FIGURES/3
Oh MH, Choi CH (2015) Role of LuxIR homologue AnoIR in Acinetobacter nosocomialis and the effect of virstatin on the expression of anoR gene. J Microbiol Biotechnol 25(8):1390–1400. https://doi.org/10.4014/JMB.1504.04069
Jiao Y, Tay FR, Niu LN, Chen JH (2019) Advancing antimicrobial strategies for managing oral biofilm infections. Int J Oral Sci 11(3):1–11. https://doi.org/10.1038/s41368-019-0062-1
Chow JY, Yang Y, Tay SB, Chua KL, Yew WS (2014) Disruption of biofilm formation by the human pathogen Acinetobacter baumannii using engineered quorum-quenching lactonases. Antimicrob Agents Chemother 58(3):1802. https://doi.org/10.1128/AAC.02410-13
Zhang Y, Brackman G, Coenye T (2017) Pitfalls associated with evaluating enzymatic quorum quenching activity: the case of MomL and its effect on Pseudomonas aeruginosa and Acinetobacter baumannii biofilms. PeerJ. https://doi.org/10.7717/PEERJ.3251/SUPP-3
Paluch E, Rewak-Soroczyńska J, Jędrusik I, Mazurkiewicz E, Jermakow K (2020) Prevention of biofilm formation by quorum quenching. Appl Microbiol Biotechnol 104(5):1871–1881. https://doi.org/10.1007/S00253-020-10349-W/TABLES/1
Seo H, Kim J, Jung J, Jin HM, Jeon CO, Park W (2012) Complexity of cell–cell interactions between Pseudomonas sp. AS1 and Acinetobacter oleivorans DR1: metabolic commensalism, biofilm formation and quorum quenching. Res Microbiol 163(3):173–181. https://doi.org/10.1016/J.RESMIC.2011.12.003
Simões LC, Simões M, Vieira MJ (2008) Intergeneric coaggregation among drinking water bacteria: evidence of a role for Acinetobacter calcoaceticus as a bridging bacterium. Appl Environ Microbiol 74(4):1259–1263. https://doi.org/10.1128/AEM.01747-07/ASSET/4BCCD3FC-4FA2-4579-AAA2-E7A304B7DF14/ASSETS/GRAPHIC/ZAM0040885870002.JPEG
Zhang J, Liang X, Zhang S, Song Z, Wang C, Xu Y (2021) Maipomycin A, a novel natural compound with promising anti-biofilm activity against gram-negative pathogenic bacteria. Front Microbiol 11:3480. https://doi.org/10.3389/FMICB.2020.598024/BIBTEX
Stowe SD, Richards JJ, Tucker AT, Thompson R, Melander C, Cavanagh J (2011) Anti-biofilm compounds derived from marine sponges. Mar Drugs 9(10):2010–2035. https://doi.org/10.3390/MD9102010
Tseng SP, Hung WC, Huang CY, Lin YS, Chan MY, Lu PL, Sheu JH (2016) 5-Episinuleptolide decreases the expression of the extracellular matrix in early biofilm formation of multi-drug resistant Acinetobacter baumannii. Mar Drugs 14(8):143. https://doi.org/10.3390/MD14080143
Selvaraj A, Valliammai A, Sivasankar C, Suba M, Sakthivel G, Pandian SK (2020) Antibiofilm and antivirulence efficacy of myrtenol enhances the antibiotic susceptibility of Acinetobacter baumannii. Sci Rep 10(1):1–14. https://doi.org/10.1038/s41598-020-79128-x
Nazzaro F, Fratianni F, De Martino L, Coppola R, De Feo V (2013) Effect of essential oils on pathogenic bacteria. Pharmaceuticals 6(12):1451–1474. https://doi.org/10.3390/PH6121451
Tutar U, Çelik C, Karaman İ, Ataş M, Hepokur C (2016) Anti-biofilm and antimicrobial activity of Mentha pulegium L essential oil against multidrug-resistant Acinetobacter baumannii. Trop J Pharm Res 15(5):1039–1046. https://doi.org/10.4314/tjpr.v15i5
Celik C, Tutar U, Karaman I, Hepokur C, Atas M (2015) Evaluation of the antibiofilm and antimicrobial properties of Ziziphora tenuior L. essential oil against multidrug-resistant Acinetobacter baumannii. Int J Pharmacol 12(1):28–35. https://doi.org/10.3923/IJP.2016.28.35
Orhan-Yanıkan E, da Silva-Janeiro S, Ruiz-Rico M, Jiménez-Belenguer AI, Ayhan K, Barat JM (2019) Essential oils compounds as antimicrobial and antibiofilm agents against strains present in the meat industry. Food Control 101:29–38. https://doi.org/10.1016/J.FOODCONT.2019.02.035
Mayaud L, Carricajo A, Zhiri A, Aubert G (2008) Comparison of bacteriostatic and bactericidal activity of 13 essential oils against strains with varying sensitivity to antibiotics. Lett Appl Microbiol 47(3):167–173. https://doi.org/10.1111/J.1472-765X.2008.02406.X
Knezevic P, Aleksic V, Simin N, Svircev E, Petrovic A, Mimica-Dukic N (2016) Antimicrobial activity of Eucalyptus camaldulensis essential oils and their interactions with conventional antimicrobial agents against multi-drug resistant Acinetobacter baumannii. J Ethnopharmacol 178:125–136. https://doi.org/10.1016/J.JEP.2015.12.008
Moghimi R, Aliahmadi A, Rafati H, Abtahi HR, Amini S, Feizabadi MM (2018) Antibacterial and anti-biofilm activity of nanoemulsion of Thymus daenensis oil against multi-drug resistant Acinetobacter baumannii. J Mol Liq 265:765–770. https://doi.org/10.1016/J.MOLLIQ.2018.07.023
Aparna S, Beena A, Rekha B (2019) Antibiofilm activity and the synergistic effect of lemon grass essential oil with antibiotics against A. baumannii complex—a preliminary report. EJPMR 6:505–510
Li XH, Lee JH (2017) Antibiofilm agents: a new perspective for antimicrobial strategy. J Microbiol (Seoul, Korea) 55(10):753–766. https://doi.org/10.1007/S12275-017-7274-X
Ismail MM, Samir R, Saber FR, Ahmed SR, Farag MA (2020) Pimenta oil as a potential treatment for Acinetobacter baumannii wound infection: in vitro and in vivo bioassays in relation to its chemical composition. Antibiotics 9(10):679. https://doi.org/10.3390/ANTIBIOTICS9100679
Fjell CD, Hiss JA, Hancock REW, Schneider G (2011) Designing antimicrobial peptides: form follows function. Nat Rev Drug Discov 11(1):37–51. https://doi.org/10.1038/nrd3591
Feng X, Sambanthamoorthy K, Palys T, Paranavitana C (2013) The human antimicrobial peptide LL-37 and its fragments possess both antimicrobial and antibiofilm activities against multidrug-resistant Acinetobacter baumannii. Peptides 49:131–137. https://doi.org/10.1016/J.PEPTIDES.2013.09.007
Avery TM, Boone RL, Lu J, Spicer SK, Guevara MA, Moore RE, Gaddy JA (2021) Analysis of antimicrobial and antibiofilm activity of human milk lactoferrin compared to bovine lactoferrin against multidrug resistant and susceptible Acinetobacter baumannii clinical isolates. ACS Infect Dis 7(8):2116–2126. https://doi.org/10.1021/ACSINFECDIS.1C00087/SUPPL_FILE/ID1C00087_SI_001.PDF
Mohamed MF, Brezden A, Mohammad H, Chmielewski J, Seleem MN (2017) A short D-enantiomeric antimicrobial peptide with potent immunomodulatory and antibiofilm activity against multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Sci Rep 7(1):1–13. https://doi.org/10.1038/s41598-017-07440-0
Kim MK, Kang N, Ko SJ, Park J, Park E, Shin DW, Park Y (2018) Antibacterial and antibiofilm activity and mode of action of magainin 2 against drug-resistant Acinetobacter baumannii. Int J Mol Sci 19(10):3041. https://doi.org/10.3390/IJMS19103041
Peng J, Long H, Liu W, Wu Z, Wang T, Zeng Z, Wu J (2019) Antibacterial mechanism of peptide Cec4 against Acinetobacter baumannii. Infect Drug Resist 12:2417–2428. https://doi.org/10.2147/IDR.S214057
Gordya N, Yakovlev A, Kruglikova A, Tulin D, Potolitsina E, Suborova T, Chernysh S (2017) Natural antimicrobial peptide complexes in the fighting of antibiotic resistant biofilms: Calliphora vicina medicinal maggots. PLoS ONE 12(3):e0173559. https://doi.org/10.1371/JOURNAL.PONE.0173559
Jaśkiewicz M, Neubauer D, Kazor K, Bartoszewska S, Kamysz W (2019) Antimicrobial activity of selected antimicrobial peptides against planktonic culture and biofilm of Acinetobacter baumannii. Probiotics Antimicrob Proteins 11(1):317–324. https://doi.org/10.1007/S12602-018-9444-5/TABLES/3
Gopal R, Kim YG, Lee JH, Lee SK, Chae JD, Son BK, Park Y (2014) Synergistic effects and antibiofilm properties of chimeric peptides against multidrug-resistant acinetobacter baumannii strains. Antimicrob Agents Chemother 58(3):1622–1629. https://doi.org/10.1128/AAC.02473-13/ASSET/2C7F0F1D-5DB7-4149-8302-BA76CA447AFB/ASSETS/GRAPHIC/ZAC0031426620003.JPEG
Swedan S, Shubair Z, Almaaytah A (2019) Synergism of cationic antimicrobial peptide WLBU2 with antibacterial agents against biofilms of multi-drug resistant Acinetobacter baumannii and Klebsiella pneumoniae. Infect Drug Resist 12:2019–2030. https://doi.org/10.2147/IDR.S215084
Mwangi J, Yin Y, Wang G, Yang M, Li Y, Zhang Z, Lai R (2019) The antimicrobial peptide ZY4 combats multidrugresistant Pseudomonas aeruginosa and Acinetobacter baumannii infection. Proc Natl Acad Sci USA 116(52):26516–26522. https://doi.org/10.1073/PNAS.1909585117/SUPPL_FILE/PNAS.1909585117.SAPP.PDF
Somma AD, Recupido F, Cirillo A, Romano A, Romanelli A, Caserta S, Duilio A (2020) Antibiofilm properties of temporin-L on Pseudomonas fluorescens in static and in-flow conditions. Int J Mol Sci 21(22):8526. https://doi.org/10.3390/IJMS21228526
Reza A, Mark Sutton J, Rahman KM (2019) Effectiveness of efflux pump inhibitors as biofilm disruptors and resistance breakers in gram-negative (ESKAPEE) bacteria. Antibiotics 8(4):229. https://doi.org/10.3390/ANTIBIOTICS8040229
Chen L, Li H, Wen H, Zhao B, Niu Y, Mo Q, Wu Y (2020) Biofilm formation in Acinetobacter baumannii was inhibited by PAβN while it had no association with antibiotic resistance. Microbiol 9(9):e1063. https://doi.org/10.1002/MBO3.1063
Blanchard C, Barnett P, Perlmutter J, Dunman PM (2014) Identification of acinetobacter baumannii serum-associated antibiotic efflux pump inhibitors. Antimicrob Agents Chemother 58(11):6360–6370. https://doi.org/10.1128/AAC.03535-14/SUPPL_FILE/ZAC010143342SD2.XLSX
Yilmaz S, Altinkanat-Gelmez G, Bolelli K, Guneser-Merdan D, Over-Hasdemir MU, Yildiz I, Yalcin I (2014) Pharmacophore generation of 2-substituted benzothiazoles as AdeABC efflux pump inhibitors in A. baumannii. SAR QSAR Environ Res 25(7):551–563. https://doi.org/10.1080/1062936X.2014.919357
Krishnamoorthy S, Shah BP, Lee HH, Martinez LR (2016) Microbicides alter the expression and function of RND-type efflux pump AdeABC in biofilm-associated cells of Acinetobacter baumannii clinical isolates. Antimicrob Agents Chemother 60(1):57–63. https://doi.org/10.1128/AAC.01045-15/ASSET/2039B9F5-0983-4B71-A17B-1ECBC8D1A9E7/ASSETS/GRAPHIC/ZAC0011646760003.JPEG
Wang L, Hu C, Shao L (2017) The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int J Nanomed 12:1227–1249. https://doi.org/10.2147/IJN.S121956
Mihu MR, Sandkovsky U, Han G, Friedman JM, Nosanchuk JD, Martinez LR (2010) The use of nitric oxide releasing nanoparticles as a treatment against Acinetobacter baumannii in wound infections. Virulence 1(2):62–67. https://doi.org/10.4161/VIRU.1.2.10038
Hwang YY, Ramalingam K, Bienek DR, Lee V, You T, Alvarez R (2013) Antimicrobial activity of nanoemulsion in combination with cetylpyridinium chloride in multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 57(8):3568–3575. https://doi.org/10.1128/AAC.02109-12
Salunke GR, Ghosh S, Santosh Kumar RJ, Khade S, Vashisth P, Kale T, Chopade BA (2014) Rapid efficient synthesis and characterization of silver, gold, and bimetallic nanoparticles from the medicinal plant Plumbago zeylanica and their application in biofilm control. Int J Nanomed 9(1):2635–2653. https://doi.org/10.2147/IJN.S59834
Gaidhani S, Singh R, Singh D, Patel U, Shevade K, Yeshvekar R, Ananda Chopade B (2013) Biofilm disruption activity of silver nanoparticles synthesized by Acinetobacter calcoaceticus PUCM 1005. Mater Lett 108:324–327. https://doi.org/10.1016/J.MATLET.2013.07.023
Martinez-Gutierrez F, Boegli L, Agostinho A, Sánchez EM, Bach H, Ruiz F, James G (2013) Anti-biofilm activity of silver nanoparticles against different microorganisms. Biofouling 29(6):651–660. https://doi.org/10.1080/08927014.2013.794225
Ramya S, Shanmugasundaram T, Balagurunathan R (2015) Biomedical potential of actinobacterially synthesized selenium nanoparticles with special reference to anti-biofilm, anti-oxidant, wound healing, cytotoxic and anti-viral activities. J Trace Elem Med Biol Organ Soc Miner Trace Elem (GMS) 32:30–39. https://doi.org/10.1016/J.JTEMB.2015.05.005
Hosseini A, Nejadsattari T, Zargar M (2019) In vitro anti-biofilm activity of curcumin nanoparticles in Acinetobacter baumannii: a culture-based and molecular approach. Arch Clin Infect Dis 14(4):83263. https://doi.org/10.5812/ARCHCID.83263
Muzammil S, Khurshid M, Nawaz I, Siddique MH, Zubair M, Nisar MA, Hayat S (2020) Aluminium oxide nanoparticles inhibit EPS production, adhesion and biofilm formation by multidrug resistant Acinetobacter baumannii. Biofouling 36(4):492–504. https://doi.org/10.1080/08927014.2020.1776856
Hendiani S, Abdi-Ali A, Mohammadi P, Kharrazi S (2015) Synthesis of silver nanoparticles and its synergistic effects in combination with imipenem and two biocides against biofilm producing Acinetobacter baumannii. Nanomed J 2(4):291–298. https://doi.org/10.7508/NMJ.2015.04.007
Thawal ND, Yele AB, Sahu PK, Chopade BA (2012) Effect of a novel podophage AB7-IBB2 on Acinetobacter baumannii biofilm. Curr Microbiol 65(1):66–72. https://doi.org/10.1007/S00284-012-0127-2
Yele AB, Thawal ND, Sahu PK, Chopade BA (2012) Novel lytic bacteriophage AB7-IBB1 of Acinetobacter baumannii: Isolation, characterization and its effect on biofilm. Adv Virol 157(8):1441–1450. https://doi.org/10.1007/s00705-012-1320-0
Liu Y, Mi Z, Niu W, An X, Yuan X, Liu H, Bai C (2016) Potential of a lytic bacteriophage to disrupt Acinetobacter baumannii biofilms in vitro. Future Microbiol 11(11):1383–1393. https://doi.org/10.2217/fmb-2016-0104
Lood R, Winer BY, Pelzek AJ, Diez-Martinez R, Thandar M, Euler CW, Fischetti VA (2015) Novel phage Lysin capable of killing the multidrug-resistant gram-negative bacterium Acinetobacter Baumannii in a mouse bacteremia model. Antimicrob Agents Chemother 59(4):1983–1991. https://doi.org/10.1128/AAC.04641-14/SUPPL_FILE/ZAC004153817SO1.PDF
Thandar M, Lood R, Winer BY, Deutsch DR, Euler CW, Fischetti VA (2016) Novel engineered peptides of a phage lysin as effective antimicrobials against multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 60(5):2971–2979. https://doi.org/10.1128/AAC.02972-15/SUPPL_FILE/ZAC004165080SO1.PDF
Vukotic G, Obradovic M, Novovic K, Di Luca M, Jovcic B, Fira D, McAuliffe O (2020) Characterization, antibiofilm, and depolymerizing activity of two phages active on carbapenem-resistant Acinetobacter baumannii. Front Med 7:426. https://doi.org/10.3389/FMED.2020.00426/BIBTEX
Grygorcewicz B, Wojciuk B, Roszak M, Łubowska N, Blstrokaejczak P, Jursa-Kulesza J, Dogowska B (2021) Environmental phage-based cocktail and antibiotic combination effects on Acinetobacter baumannii biofilm in a human urine model. Microb Drug Resist 27(1):25–35. https://doi.org/10.1089/MDR.2020.0083
Ran B, Yuan Y, Xia W, Li M, Yao Q, Wang Z, Peng X (2021) A photo-sensitizable phage for multidrug-resistant Acinetobacter baumannii therapy and biofilm ablation. Chem Sci 12(3):1054–1061. https://doi.org/10.1039/D0SC04889E
Hu X, Huang YY, Wang Y, Wang X, Hamblin MR (2018) Antimicrobial photodynamic therapy to control clinically relevant biofilm infections. Front Microbiol 9(JUN):1299. https://doi.org/10.3389/FMICB.2018.01299/BIBTEX
Ghorbanzadeh R, Assadian H, Chiniforush N, Parker S, Pourakbari B, Ehsani B, Bahador A (2020) Modulation of virulence in Enterococcus faecalis cells surviving antimicrobial photodynamic inactivation with reduced graphene oxide-curcumin: an ex vivo biofilm model. Photodiagn Photodyn Therapy 29:101643. https://doi.org/10.1016/J.PDPDT.2019.101643
Geralde MC, Leite IS, Inada NM, Salina ACG, Medeiros AI, Kuebler WM, Bagnato VS (2017) Pneumonia treatment by photodynamic therapy with extracorporeal illumination—an experimental model. Physiol Rep 5(5):e13190. https://doi.org/10.14814/PHY2.13190
Granick MS, Paribathan C, Shanmugam M, Ramasubbu N (2017) Direct-contact low-frequency ultrasound clearance of biofilm from metallic implant materials
Ng E, Lim LP (2019) An overview of different interdental cleaning aids and their effectiveness. Dent J 7(2):56. https://doi.org/10.3390/DJ7020056
Shakeri S, Kermanshahi RK, Moghaddam MM, Emtiazi G (2007) Assessment of biofilm cell removal and killing and biocide efficacy using the microtiter plate test. Biofouling 23(2):79–86. https://doi.org/10.1080/08927010701190011
Perumal PK, Wand ME, Sutton JM, Bock LJ (2014) Evaluation of the effectiveness of hydrogen-peroxide-based disinfectants on biofilms formed byGram-negative pathogens. J Hosp Infect 87(4):227–233. https://doi.org/10.1016/j.jhin.2014.05.004
Stowe S, Thompson R, Peng L, Su Z, Blackledge M, Draughn G, Cavanagh J (2015) Membrane-permeabilizing activity of reverse-amide 2-aminoimidazole antibiofilm agents against Acinetobacter baumannii. Curr Drug Deliv 12(2):223–230. https://doi.org/10.2174/1567201811666140924125740
Narayanan A, Nair MS, Karumathil DP, Baskaran SA, Venkitanarayanan K, Amalaradjou MAR (2016) Inactivation of acinetobacter baumannii biofilms on polystyrene, stainless steel, and urinary catheters by octenidine dihydrochloride. Front Microbiol 7(JUN):847. https://doi.org/10.3389/FMICB.2016.00847/BIBTEX
Karahasan A, Marmara Üniversitesi Y, Fakültesi T, Ve M, Mikrobiyoloji K, Sebit B, Yağci AK (2016) Biofilm production and biocidal efficacy in multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii isolates. Int J Antisep Disinfect Steril 1(1):7–12. https://doi.org/10.14744/ijads.2016.08208
Fleeman R, Van Horn KS, Barber MM, Burda WN, Flanigan DL, Manetsch R, Shaw LN (2017) Characterizing the antimicrobial activity of N2, N4-disubstituted quinazoline-2,4-diamines toward multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. https://doi.org/10.1128/AAC.00059-17/SUPPL_FILE/ZAC005176184S1.PDF
Schumm K, Lam TB (2008) Types of urethral catheters for management of short-term voiding problems in hospitalized adults: a short version cochrane review. Neurourol Urodyn 27(8):738–746. https://doi.org/10.1002/NAU.20645
Johnson JR, Kuskowski MA, Wilt TJ (2006) Systematic review: antimicrobial urinary catheters to prevent catheter-associated urinary tract infection in hospitalized patients. Ann Intern Med 144(2):116–126. https://doi.org/10.7326/0003-4819-144-2-200601170-00009
Ahmad TA, Tawfik DM, Sheweita SA, Haroun M, El-Sayed LH (2016) Development of immunization trials against Acinetobacter baumannii. Trials Vaccinol 5:53–60. https://doi.org/10.1016/J.TRIVAC.2016.03.001
Bentancor LV, Routray A, Bozkurt-Guzel C, Camacho-Peiro A, Pier GB, Maira-Litrán T (2012) Evaluation of the trimeric autotransporter ata as a vaccine candidate against Acinetobacter baumannii Infections. Infect Immun 80(10):3381. https://doi.org/10.1128/IAI.06096-11
Cai W, Kesavan DK, Cheng J, Vasudevan A, Wang H, Wan J, Xu H (2019) Vesicle-mediated dendritic cell activation in Acinetobacter baumannii clinical isolate, which contributes to Th2 response. J Immunol Res. https://doi.org/10.1155/2019/2835256
Fattahian Y, Rasooli I, Mousavi Gargari SL, Rahbar MR, Darvish Alipour Astaneh S, Amani J (2011) Protection against Acinetobacter baumannii infection via its functional deprivation of biofilm associated protein (Bap). Microb Pathog 51(6):402–406. https://doi.org/10.1016/J.MICPATH.2011.09.004
Jun SH, Lee JH, Kim BR, Kim SI, Park TI, Lee JC, Lee YC (2013) Acinetobacter baumannii outer membrane vesicles elicit a potent innate immune response via membrane proteins. PLoS ONE 8(8):e71751. https://doi.org/10.1371/JOURNAL.PONE.0071751
Luo G, Lin L, Ibrahim AS, Baquir B, Pantapalangkoor P, Bonomo RA, Spellberg B (2012) Active and passive immunization protects against lethal, extreme drug resistant-Acinetobacter baumannii infection. PLoS ONE. https://doi.org/10.1371/JOURNAL.PONE.0029446
Acknowledgements
Not applicable.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
All authors participated in the collection of data, writing, reviewing, and editing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohamed, E.A., Raafat, M.M., Samir Mohamed, R. et al. Acinetobacter baumannii biofilm and its potential therapeutic targets. Futur J Pharm Sci 9, 82 (2023). https://doi.org/10.1186/s43094-023-00525-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-023-00525-w