Abstract
Background
This research aims to study the association of genetic polymorphism in genes coding for CYP2C9 and CYP2C19 in phenytoin-induced dose-related toxicity and to assess if the presence of allele CYP2C9*3 plays a role in phenytoin-induced idiosyncratic adverse effects. Current observational case control study included 142 patients with phenytoin-induced adverse drug reactions (ADRs) and 100 controls. All these patients underwent genotyping to determine the type of CYP2C9 allele [CYP2C9*1, CYP2C9*2 or CYP2C9*3) and CYP2C19 allele (CYP2C19*1, CYP2C19*2 or CYP2C19*3] by real-time polymerase chain reaction (RT-PCR) using Applied Biosystems (ABI) 7500 Real-Time PCR System (USA).
Results
Presence of homozygous status for allele CYP2C9*3 was associated with significantly higher risk of phenytoin-induced dose-dependent ADRs, dose-independent ADRs, gum hyperplasia, and skin rash. Presence of heterozygous status for allele CYP2C9*3 was associated with significantly higher risk of phyenytoin-induced dose-dependent ADRs and dose-independent ADRs. Presence of either heterozygous or homozygous status for CYP2C9*2 and CYP2C19*2 did not have any bearing on dose-related side effects. None of the patients showed CYP2C19*3 allele.
Conclusion
Variant alleles of CYP2C9*3 are significantly overexpressed among patients with phenytoin-induced ADRs, thereby suggesting the role for CYP2C9 genotype testing to predict risk of phenytoin-related ADRs.
Similar content being viewed by others
Background
Antiepileptic drugs (AEDs) like phenytoin (Dilantin, diphenylhydantoin, DPH) form the mainstay of treating epilepsy, which is a serious neurological disorder affecting around 1–2% of global population [1,2,3]. The selection of appropriate AED is based on type of epilepsy syndrome, therapeutic profile of the AED, likelihood of drug-related adverse effects, ease of use, price as well as patient-related factors such as pregnancy and lactation. The most important ones among these factors include therapeutic efficacy and side effect profile [4,5]. For its price and ease of use, phenytoin (PHT) continues to be one of the most commonly prescribed AEDs in developing countries despite a relatively high incidence of side effects affecting 14–15% of patients [6,7].
Several factors predispose a patient to the development of adverse drug reactions (ADRs) including dosage and duration of therapy, environmental factors such as brain insult and prior exposure to the drug, as well as presence of liver or kidney or other systemic disease [8]. Also, genetic factors which determine rate of absorption, distribution, metabolism, and excretion of a drug play an important role in the development of ADRs [9,10].
Recent reports suggest that genetic polymorphisms in genes coding for cytochrome P450 system of isozymes (specifically CYP2C9 and CYP2C19) play a role in the development of PHT-induced ADRs [11,12,13,14]. CYP2C9 is the most abundant cytochrome P450 accounting for 20% of all CYP in liver. Metabolism of phenytoin and their associated genes are shown in Fig. 1. Approximately 15% of all drugs are metabolized through CYP2C9. CYP2C subfamily has close to 60 alleles, but only three CYP2C9*1 (most common wild type), CYP2C9*2 and CYP2C9*3 are commonly found. Presence of CYP2C9*2 and CYP2C9*3 alleles is associated with decrease in enzymatic activity of CYP2C and increased serum levels of PHT [15]. Furthermore, data from Japan [16] and Turkey [17] suggest that genetic polymorphisms in CYP2C19 (which accounts for 10% of metabolism of PHT) especially presence of allele CYP2C19*2 and CYP2C19*3 are also associated with reduced PHT metabolism. An association between these polymorphisms and PHT-induced ADRs has not yet been studied in North Indian population. Thus, we planned this study to detect if genetic polymorphisms in genes encoding for cytochrome enzymes CYP2C9 and CYP2C19 could account for PHT-induced ADRs in North Indian population.
Aims and objectives
The present study was designed to determine (i) the association of genetic polymorphism in genes coding for CYP2C9 and CYP2C19 in phenytoin-induced dosages causing toxicity and (ii) to investigate if the presence of CYP2C9*3 allele plays any role in phenytoin-induced idiosyncratic adverse reactions.
Methods
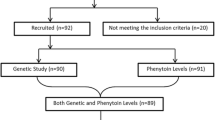
Current study was an observational case control study conducted at a tertiary care institute in North India from July 2012 to January 2017. During this period, we were able to enroll 142 patients on random basis with phenytoin-induced adverse drug reactions (ADRs), which were characterized as idiosyncratic in 95 patients and dose-related in 66 patients. All these patients underwent genotyping to determine the type of CYP2C9 allele (CYP2C9*1, CYP2C9*2 or CYP2C9*3) and CYP2C19 allele (CYP2C19*1, CYP2C19*2 or CYP2C19*3). The presence of CYP2C9*3 was analyzed with respect to both PHT dose-dependent and dose-independent side ADRs, while the presence of CYP2C9*2, CYP2C19*2 and CYP2C19*3 alleles was tested only for PHT dose-dependent ADRs. The results were compared with 100 control patients who did not experience ADRs while being administered PHT. All the patients underwent detailed history, neurological and systemic examinations as well as relevant investigations as per a predesigned proforma. All the patients received standard care for ADRs, as well for epilepsy. PHT was administered to all patients at a dose of 5 mg/Kg daily. The study was approved by Institutional Ethics Committee (histo/14/135 dated 16.1.2014), and written informed consent was obtained from all the patients before inclusion in the study.
Inclusion criteria
Patients more than 18 years of age who presented with epilepsy and willing to give a written consent were included.
Exclusion criteria
Subjects with gross neurological deficits such as mental retardation, motor deficit; patients with severe hepatic, renal disorders and diabetes mellitus; pregnant and lactating women were excluded.
Genotyping
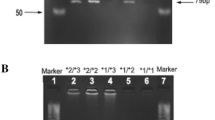
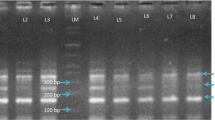
Once enrolled, 2 ml of peripheral venous blood sample was drawn by venipuncture from patients and immediately transferred into 5 ml EDTA-coated vials (to prevent clot formation) for DNA extraction. Genomic DNA was isolated from venous blood using a modified phenol–chloroform-isoamyl alcohol method. The genotyping was done by real-time polymerase chain reaction (RT-PCR) using Applied Biosystems (ABI) 7500 Real-Time PCR System (USA).
Statistical analysis
Statistical analysis was carried out using SPSS 24.0 (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.). The data were described as mean and standard deviation. Chi-square and Fisher’s exact tests were used to compare discrete variables, while Mann–Whitney test was used to compare continuous variables. p < 0.05 was taken as a significant difference.
Results
Current study included 142 patients with DPH-induced ADRs (Group 1). Out of these, 95 patients had dose-independent ADRs [Acute hypersensitivity syndrome (AHS), skin rash, SJS/TEN, gingival hyperplasia, etc.], and 66 patients had dose-dependent ADRs (drowsiness, sedation, ataxia, hirsutism, facial dysmorphism, rickets, and osteomalacia). The overall number of patients (n = 142) is lesser than the number of patients with dose-independent and dose-dependent ADRs combined (95 + 66 = 161), while some patients had both dose-dependent and dose-independent side effects. Group 2 included patients who did not experience ADRs with phenytoin (n = 100).
Demographic profile
The demographic profile of both groups is shown in Table 1. All the demographic variables were comparable in epileptics and controls, excepting for excellent control of seizures which was significantly higher in controls (p = 0.002).
Genotype and allelic frequencies of CYP2C9*1 and *3 in patients on DPH therapy
In the present study, we determined frequencies of different allelic types of CYP2C9. In Group 1, 64.8% of patients were homozygous for the presence of CYP2C9*1, 28.9% were heterozygous for the presence of CYP2C9*3 and 6.3% were homozygous for allele CYP2C9*3. The corresponding frequencies in group 2 were 85%, 15% and 0%, respectively. On comparison, the presence of heterozygous or homozygous status for allele CYP2C9*3 was associated with significantly higher risk of PHT-induced ADRs (both dose-related and dose-independent).
Further on we compared allelic frequencies of CYP2C9 in Group 2 (Controls) and various subgroups of Group 1 (patients), i.e., patients who had only dose-independent (idiosyncratic) ADRs, only dose-related side effects, only gum hyperplasia and only skin rash shown in Fig. 2. On analysis, the presence of homozygous status for allele CYP2C9*3 was associated with significantly higher risk of gum hyperplasia, skin rash, dose-dependent side effects and dose-independent (idiosyncratic) side effects. However, the presence of heterozygous status for allele CYP2C9*3 was associated with significantly higher risk of dose-dependent and dose-independent PHT ADRs, as well as gum hyperplasia, and skin rash was higher in patients who were heterozygous for allele CYP2C9*3 (Table 2).
Genotype and allelic frequencies of CYP2C9*1 and *2, CYP2C19*1, *2 and *3 in patients on DPH therapy
We compared effect of CYP2C9*2 allele in causation of PHT dose-related side effects. The presence of either heterozygous or homozygous status for CYP2C9*2 and CYP2C19*2 did not have any bearing on PHT-induced dose-related side effects (Table 3). None of the patients in current study had allele CYP2C19*3.
Estimation of drug levels
A total of 66 patients were recruited for PHT-induced toxicity. Out of these, 27 samples were sent for evaluation of drug levels. Of these 27 samples, 24 samples showed high PHT levels in their serum (> 20 ng/μL) and three samples showed low PHT levels (< 20 ng/μL).
Discussion
DPH was introduced for antiepileptic therapy nearly 80 years ago by Merritt and Putnam [18]. It continues to be one of the most commonly used AEDs for various types of seizures and status epilepticus. It is metabolized almost exclusively by the hepatic cytochrome P450 system of enzymes (CYP2C9 and CYP2C19) into 5-(4'-hydroxyphenyl)-5-phenylhydantoin (p-HPPH). During this biotransformation, a toxic arene oxide metabolite is produced which plays a major role in DPH-induced idiosyncratic drug reactions [9]. Further metabolic pathways convert arene oxide metabolite into either (a) phenytoin dihydrodiol or (b) hydroxy phenytoin. The hydroxy phenytoin is then either glucuronidated and excreted in urine or converted to phenytoin catechol, while phenytoin dihydrodiol is primarily metabolized to phenytoin catechol. Phenytoin catechol is then further metabolized to phenytoin methylcatechol or phenytoin quinone. The initial hydrolysis of PHT results in the formation of two isomers of HPPH: S and R isomers. The ratio of S relative to R isomer depends upon the primary metabolic pathway: When CYP2C9 is primary metabolizing enzyme, ratio of S to R isomer is 1:1, and when CYP2C19 is the primary metabolizing enzyme, ratio of S to R isomer is 40:1. The S isomer is preferentially glucuronidated by UGT1A1, while R isomer is preferentially glucuronidated by UGT1A9 and UGT2B15. Under normal circumstances, 90% of PHT is metabolized by CYP2C9 and 10% is metabolized by CYP2C19 [9,14,19].
Genetic polymorphisms of CYP2C9 affect metabolism of PHT. Specifically, the presence of CYP2C9*2 (transition C > T at position 432 of exon 3 resulting in replacement of arginine by cysteine at position 144 of CYP2C9 protein) or *3 (transversion A > T transition at position 1077 of exon 7 resulting in replacement of isoleucine by leucine at position 359 of CYP2C9 protein) variants reduces PHT metabolism by 25–50%. This reduction in metabolism is even more important in the case of PHT as its metabolism follows saturation kinetics [9,20], whereby only a minor change in its dosage may cause severe fluctuations in serum drug levels. In the current study, we evaluated the role of allelic variations in CYP2C9 and CYP2C19 in causation of PHT-induced ADRs in North Indian population.
Demographic profile
The mean age was 29.6 ± 12.7 years in patients with DPH-induced ADRs. Thus, most of patients affected by epilepsy were young adults in most productive years of their life. The occurrence of drug-induced ADRs in addition to burden posed by epilepsy itself has significant negative effect not only on their quality of life, but of entire family. Thus, any tool which can predict the occurrence of DPH-related side effects is likely to have significant effect on management of epilepsy. The demographic profile was comparable in cases and controls saved for a significant poor control of seizures in patients with DPH-induced ADRs. This may be due to the fact that patients with ADRs are poor in their compliance and need frequent changes in AED doses resulting in poor seizure control.
DPH toxicity with CYP2C9 gene
The overall allelic frequencies were 62.5% for *1/*1, 7.5% for *1/*2, 27.5% for *1/*3, and 2.5% for *2/*3 in patients with PHT-induced ADRs and 81.3% for *1/*1, 9.1% for *1/*2, 9.6% for *1/*3 in patients without PHT-induced ADRs. In a similar study in South Indian population [21], the genotype frequencies were 82.3% for *1/*1, 4.4% for *1/*2, 12.6% for *1/*3, and 0.7% for *2/*3 in CYP2C9 gene. In another study from Western India [22], the genotype frequencies were 78.38% for *1/*1, 8.88% for *1/*2, 12.36% for *1/*3, and 0.39% for *2/*3 in CYP2C9 gene. The results of our study are more or less consistent with the previous studies from Indian subcontinent.
In the present study, the presence of CYP2C9*3 allele was associated with highly significant risk of PHT-induced ADRs (both dose-dependent and dose-independent). These results are consensus with the findings from two previous studies [21,22] from Indian subcontinent. In the current study, the presence of CYP2C9*2 allele was not associated with any risk of PHT-induced dose-dependent ADRs. Our results were similar to a previous South Indian study [[[[15]]]] where a positive association was found between CYP2C9*3 and PHT-induced ADRs but not between CYP2C9*2 allele and PHT-induced ADRs. Our results were however different from a previous study from Western India where a significant positive association was found between CYP2C9*2 allele and PHT-induced ADRs. Perhaps the different ethnicities of different populations are accountable for these results.
DPH-associated cutaneous drug reactions with CYP2C9*3
In the current study, we also tried to determine the influence of presence of CYP2C9*3 allele on PHT-induced cutaneous drug reactions. When analyzed, we found that the presence of CYP2C9*3 allele was associated with significantly higher risk of PHT-induced cutaneous drug reactions. Our results are similar to the results of a previous study [9], wherein a positive association was found between genotype (*3/*3) of CYP2C9 and PHT-induced cutaneous drug reactions.
DPH-associated gum hyperplasia with CYP2C9*3
In the current study, we also determined if the presence of CYP2C9*3 allele can predict the occurrence of PHT-induced gum hyperplasia. Upon analysis, it was found that the presence of CYP2C9*3 allele is significantly associated with the occurrence of PHT-induced gum hyperplasia. Our results are in contrast to a previous study from Japan [17] who did not find any association between genotype (*1/*3) of CYP2C9 and PHT-induced cutaneous drug reactions. The difference is likely related to the difference in ethnicities of different populations across the globe.
DPH toxicity and CYP2C19 gene
In the present study, the frequency of various alleles was found to be 66.7% for *1/*1, 24.2% for *1/*2, 0% for *1/*3 & *3/*3, 9.1% for *2/*2, and 0% for *2/*3 in cases and 68% for *1/*1, 23% for *1/*2, 0% for *1/*3, *3/*3 & *2/*3 and 9% for *2/*2 in controls. These results were similar to a previous study [12], which reported the allelic frequencies of 51.7% for *1/*1, 43.1% for *1/*2, 1.7% for *1/*3, 29.3% for *2/*2, and 3.4% for *2/*3 in cases and 38.5% for *1/*1, 44.4% for *1/*2, 1% for *1/*3, and 17.1% for *2/*2 in controls. On comparison, we did not find any association between the presence of CYP2C19*2 and *3 alleles with PHT-induced dose-dependent ADRs. These results are similar to the previously published study [12] from Southern India.
Conclusion
In conclusion, variant alleles (both heterozygous and homozygous) of CYP2C9*3 are significantly overrepresented among patients with phenytoin-induced dose-dependent, as well as dose-independent, ADRs. Our results suggest a role for CYP2C9 genotype testing to predict risk of DPH-induced ADRs in future. Main strengths of our study were a relatively large sample size of patients with DPH-induced ADRS, while main limitation was that we have a smaller number of patients with serum drug levels. Future prospective studies employing still larger sample size, targeting entire CYP genome and correlating various genotypes with serum drug levels may better delineate the role of genetic polymorphisms in PHT-induced ADRs.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Abbreviations
- ABI:
-
Applied biosystmes
- ADRs:
-
Adverse drug reactions
- AEDs:
-
Antiepileptic drugs
- AHS:
-
Hypersensitivity syndrome
- CYP:
-
Cytochrome P450
- DNA:
-
Deoxyribonucleic acid
- DPH:
-
Diphenylhydantoin
- EDTA:
-
Ethylene diamine tetraacetic acid
- IDR:
-
Idiosyncratic drug reactions
- PCR:
-
Polymerase chain reaction
- p-HPPH:
-
5-(4ʹ-Hydroxyphenyl)-5-phenylhydantoin
- PHT:
-
Phenytoin
- RT-PCR:
-
Real-time polymerase chain reaction
- SJS:
-
Stevens–Johnson syndrome
- TEN:
-
Toxic epidermal necrolysis
- UGT:
-
Uridine glucuronyl transferases
References
Modi M, Singh R, Goyal MK, Gairolla J, Singh G, Rishi V, Thakur JS, Sehgal RK, Garg VK, Khandelwal N, Kharbanda PS, Prabhakar S, Lal V (2018) Prevalence of epilepsy and its association with exposure to toxocara canis: a community based, case-control study from rural Northern India. Ann Indian Acad Neurol 21:263–269
Modi M, Singh R, Goyal MK, Gairolla J, Singh G, Rishi V, Thakur JS, Sehgal RK, Garg VK, Khandelwal N, Kharbanda PS, Prabhakar S, Lal V (2019) Prevalence of epilepsy and its association with exposure to Toxocara canis: a community-based, case-control study from rural Northern India. Ann Indian Acad Neurol 22:533
Smolarz B, Makowska M, Romanowicz H (2021) Pharmacogenetics of drug-resistant epilepsy (Review of literature). Int J Mol Sci 22:651720
Duncan JS, Sander JW, Sisodiya SM, Walker MC (2006) Adult epilepsy. Lancet (London, England) 367:1087–1100
Zhang C, Lei J, Liu Y, Wang Y, Huang L, Feng Y (2021) Therapeutic drug monitoring and pharmacogenetic testing in Northern China. Front Pharmacol 12:754380
Merwick A, Brien MO’, Delanty N (2021) Complex single gene disorders and epilepsy. Epilepsia 53(Suppl 4):81–91
Fohner AE, Rettie AE, Thai KK, Ranatunga DK, Lawson BL, Liu VX, Schaefer CA (2020) Associations of CYP2C9 and CYP2C19 pharmacogenetic variation with phenytoin-induced cutaneous adverse drug reactions. Clin Transl Sci 13:1004–1009
Pal R, Singh K, Khan SA, Chawla P, Kumar B, Akhtar MJ (2021) Reactive metabolites of the anticonvulsant drugs and approaches to minimize the adverse drug reaction. Eur J Med Chem 226:113890
Silvado CE, Terra VC, Twardowschy CA (2018) CYP2C9 polymorphisms in epilepsy: Influence on phenytoin treatment. Pharmgenom Pers Med 11:51–58
Alomar MJ (2014) Factors affecting the development of adverse drug reactions (Review article). Saudi Pharm J 22:83–94
Argikar UA, Cloyd JC, Birnbaum AK, Leppik IE, Conway J, Kshirsagar S, Oetting WS, Klein EC, Remmel RP (2006) Paradoxical urinary phenytoin metabolite (S)/(R) ratios in CYP2C19*1/*2 patients. Epilepsy Res 71:54–63
Kesavan R, Narayan SK, Adithan C (2010) Influence of CYP2C9 and CYP2C19 genetic polymorphisms on phenytoin-induced neurological toxicity in Indian epileptic patients. Eur J Clin Pharmacol 66:689–696
Rosemary J, Surendiran A, Rajan S, Shashindran CH, Adithan C (2006) Influence of the CYP2C9 & CYP2C19 polymorphisms on phenytoin hydroxylation in healthy individuals from south India. Indian J Med Res 123:665–670
Kanjanasilp J, Sawangjit R, Phanthaisong S, Borihanthanawuth W (2021) A meta-analysis of effects of CYP2C9 and CYP2C19 polymorphisms on phenytoin pharmacokinetic parameters. Pharmacogenomics 22:629–640
Hikino K, Ozeki T, Koido M, Terao C, Kamatani Y, Mizukawa Y, Shiohara T, Tohyama M, Azukizawa H, Aihara M, Nihara H, Morita E, Murakami Y, Kubo M, Mushiroda T (2020) HLA-B*51:01 and CYP2C9*3 are risk factors for phenytoin-induced eruption in the Japanese population: analysis of data from the biobank Japan project. Clin Pharmacol Ther 107:1170–1178
Soga Y, Nishimura F, Ohtsuka Y, Araki H, Iwamoto Y, Naruishi H, Shiomi N, Kobayashi Y, Takashiba S, Shimizu K, Gomita Y, Oka E (2004) CYP2C polymorphisms, phenytoin metabolism and gingival overgrowth in epileptic subjects. Life Sci 74:827–834
Aynacioglu AS, Brockmöller J, Bauer S, Sachse C, Güzelbey P, Öngen Z, Nacak M, Roots I (1999) Frequency of cytochrome P450 CYP2C9 variants in a Turkish population and functional relevance for phenytoin. Br J Clin Pharmacol 48:409–415
Merritt HH (1984) Landmark article Sept 17, 1938: Sodium diphenyl hydantoinate in the treatment of convulsive disorders. By H. Houston Merritt and Tracy. J Putnam J Am Med Assoc 251:1062–1067
Chang WC, Hung SI, Carleton BC, Chung WH (2020) An update on CYP2C9 polymorphisms and phenytoin metabolism: implications for adverse effects. Expert Opin Drug Metab Toxicol 16:723–734
Wang B, Wang J, Huang SQ, Su HH, Zhou SF (2009) Genetic polymorphism of the human cytochrome P450 2C9 gene and its clinical significance. Curr Drug Metab 10:781–834
Adithan C, Gerard N, Vasu S, Balakrishnan R, Shashindran CH, Krishnamoorthy R (2003) Allele and genotype frequency of CYP2C9 in Tamilnadu population. Eur J Clin Pharmacol 59:707–709
Thakkar AN, Bendkhale SR, Taur SR, Gogtay NJ, Thatte UM (2012) Association of CYP2C9 polymorphisms with phenytoin toxicity in Indian patients. Neurol India 60:577–580
Acknowledgements
We would like to acknowledge the help of Department of Neurology, Postgraduate Institute of Medical Education & Research, Chandigarh as well as the Indian Council of Medical Research, New Delhi, India, for the financial support.
Funding
Department of Neurology, Postgraduate Institute of Medical Education & Research, Chandigarh.
Author information
Authors and Affiliations
Contributions
VKG wrote the manuscript; S. proofread the manuscript, RS proofread the manuscript, AP proofread the manuscript, AT proofread the manuscript, MK designed the methodology, BS designed the methodology, BM designed the methodology, MM conceived the concept. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by Institutional Ethics Committee (histo/14/135 dated 16.1.2014), and written informed consent was obtained from all the patients before inclusion in the study.
Consent for publication
The authors declare no conflict of interest.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Garg, V.K., Supriya, Shree, R. et al. Genetic abnormality of cytochrome-P2C9*3 allele predisposes to epilepsy and phenytoin-induced adverse drug reactions: genotyping findings of cytochrome-alleles in the North Indian population. Futur J Pharm Sci 8, 44 (2022). https://doi.org/10.1186/s43094-022-00432-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-022-00432-6