Abstract
Background
Generic antimalarial drugs sold in sub-Saharan Africa require tighter control as counterfeiting has grown more and more out of control. The study aimed to analyze the pharmaceutical quality of quinine sulfate (QS) and Artemether/Lumefantrine(AL) tablets marketed in Bukavu city compared to the current trends in other African cities.
Results
The samples were purchased in community pharmacies or from ambulatory street vendors and analyzed using a set of thirteen simple tests, including visual inspection, UV spectrometry, TLC, and conventional quality control procedures. More than 93% of AL samples had an acceptable global quality score of > 90%. Around 16.6% of QS batches did not satisfy the requirements about hardness, friability, and mass uniformity. Only 33.3% met the disintegration quality; 33.3% did not contain quinine; 8.33% had an active ingredient other than quinine.
Conclusion
The findings strongly alert the circulation of fake antimalarial medicines observed in many countries. Simple TLC procedures may help to detect any low-quality generics to avoid microbial resistance and guarantee the health of the population. Pharmacists and regulatory authorities are alerted to the circulation of low-quality generic quinine preparations in the country.
Similar content being viewed by others
Background
Around 3.2 billion people, or almost half of the world’s population, are exposed to malaria risk [1,2,3,4]. According to the World Malaria Report (WMR), an estimated 228 million malaria cases occurred worldwide in 2018, and 19 countries in sub-Saharan Africa and India carried almost 85% of the global malaria burden [1]. Of an estimated 405,000 deaths from malaria globally, nearly 85% live in 20 countries in the WHO African Region, mainly Nigeria (24%) and the Democratic Republic of Congo (DRC) (11%) [5, 6]. Children aged under 5 years are the most vulnerable group affected by malaria; they accounted for 67% (272,000) of all malaria deaths worldwide in 2018.
Access to adequate and quality antimalarial drugs (AMDs) plays a crucial role in efforts to ensure complete cures and avoid vicious complications [7]. Without treatment, malaria can quickly lead to death from the circulatory disorders it causes. Also, in many parts of the world, malaria parasites have become resistant to several AMDs, due in significant part to fake medications [8,9,10]. Among those counterfeit medicines, some contain sub-therapeutic doses and others not at all [11]. According to the molecular quantitative similarity analysis (MQSA) study, 25–50% of AMDs are of low quality, adulterated, or inferior quality. The figures from the US Food and Drug Administration (USFDA) indicate that up to 25% of all drugs consumed in emerging countries are counterfeit or substandard [12,13,14]. Counterfeiting affects all classes of drugs, but mainly antibiotics and AMDs, for which the demand is high in African countries where malaria is endemic. That demand leads to an informal trade in counterfeit products beyond the control of health authorities.
In the DRC, an estimated 97% of the population lives in zones with stable malaria transmission lasting 8 to 12 months per year; the other 3% is exposed to epidemic malaria in the high mountains of the east of the country [15, 16]. Around 80% of deaths occur at home, which represents millions of cases of death per year. The prevalence of fever in children under 5 years of age is 42%, corresponding to between 6 and 10 malaria episodes per child per year. The combination Artemether/Lumefantrine (CoArtem) and Artesunate/Amodiaquine (AS-AQ) are the most widely used AMDs in the initial treatment of non-severe Falciparum malaria and quinine for severe cases [17]. The circulation of counterfeit or poor-quality products is beyond control. This study aimed to assess the quality of generic quinine sulfate (QS) and Artemether-Lumefantrine (AL) tablets marketed in Bukavu using simple usual analytical techniques.
Methods
Study design
The study took place in Bukavu city, the capital of South-Kivu province in DRC, from May to September 2019. It assessed pharmaceutical technology requirements concerning labeling, physical examination, disintegration time, friability, and mass uniformity. Thin-layer chromatography (TLC), non-aqueous protometry, and UV-spectrophotometry served to identify and assay active ingredients using referral pharmacopeial guidelines and regulations. Critical attributes were labeling quality, general tests on pharmaceutical dosage forms, content and dissolution, identification, and the presence of unidentifiable impurities in formulations.
Equipment, chemicals, and reagents
The equipment included UV spectrophotometer (Pharmacia LKB Ultrrospec Plus, Germany), chromatographic plates (pre-coated TLC sheets Alugram® Xtra Sil G/UV 254 layer: 0.20 mm silica gel 60 with fluorescent indicator UV 254), UV lamp 254 nm (Technology Transfer, Germany), analytical balance (balance digital jewelry scale, capacity 20 g, readability 0.001 g, China), friability device (manufactured by lab-line instruments. Inc. Model n°1641 China), aggregation device (Erweka-apparate bau, Germany), durometer (Schleuniger-2E, Switzerland), hot plate (Protherm Winn Leek, Holland), water bath (Gerhardt Bonn, Germany), rotavapor (Pleuger, Switzerland), centrifuge (IEC Centra-2 centrifuge, USA), and vortex shaker (Vortex-2 genie Bohemia, USA).
The solvents were distilled water; toluene R (Merck KGaA, 64271 Darmstadt, Germany), ethyl acetate R (Merck KGaA, 64271 Darmstadt, Germany), methanol R (Maprochim-Labo, 59, lot 0112, 90% 0.791, DRC), hydrochloric acid R (PANREAC Quimica SAU, E-08211, Spain), glacial acetic acid R (BN: 04H240011, Spain), acetic anhydride R (E. Merck Darmstadt, Germany), acetone R (Merck KGaA 64271 Darmstadt, Germany), perchloric acid R (70%, density 1.76, E. Merck, D-6100 Darmstadt, Germany), chloroform R (Prolabo, Belgium), potassium iodide R (Merck, D-6100 Darmstadt, Germany), and mercury chloride II R (B Zedelgem, Germany).
Method development
Uniformity of mass
It represents the average mass of 20 tablets. The deviation of the individual tablet weight from the mean weight should not exceed a minimum of ± 5.0% and a maximum of ± 10.0% for 18 and 20 tablets, respectively [18].
Friability of tablets
A device manufactured by Lab-Line Instruments Inc. Model n°1641 operating by rotation at 100 revolutions per 4 min served to measure friability, with a total of tablets corresponding, as near as possible, to 6.5 g introduced in the machine. According to the requirement, the maximum acceptable loss of mass (obtained from a single test or the mean of 3 tests) is not greater than 1.0% for the sample [19].
Hardness-resistance of tablets to crushing
A durometer (Schleuniger-2E, Switzerland) served to measure the minimum and maximum force (newton) to crush each of 10 tablets [20].
Disintegration of tablets
We used a 6-tube basket disintegrating device Erweka-apparate, containing HCl 1 N as immersion fluid maintained at 37 ± 2 °C, in triplicate. The basket filled with six tablets was rotated for 30 min and then raised to count the units not completely disintegrated. At least 16 of the 18 dosage units tested should disintegrate in time [21, 22].
Identification of quinine sulfate by Mayer reagent
A tiny amount of crushed tablets powder was mixed with distilled water, filtered, and the filtrate mixed with the Mayer reagent (5 g of potassium iodide (KI), 1.36 g of mercury chloride (HgCl2), and 100 ml of distilled water) in a test tube. The reaction was positive if a yellowish-white precipitate is formed [23].
Identification of quinine sulfate and Artemether-Lumefantrine by TLC
The protocol followed the existing procedures with slight adaptation. For QS samples [24,25,26], the stationary phase was silica gel R6; the mobile phase was methanol/ammonia (20: 0.5 v/v). The final concentration was 1.428 mg/ml. For AL samples [27, 28], the stationary phase was silica gel R6; the mobile phase was toluene/ethyl acetate/anhydrous acetic acid (18:4:2 v/v/v). The final concentration was 1.30 mg/ml of Artemether and 7.80 mg/ml of Lumefantrine. Approximately 2 μl of samples and 2 μl of standards were spotted at 1.5 cm on silica gel R6 plates. After migration, each chromatographic plate was removed, dried, and then examined with ultraviolet light (254 nm) for QS samples. The identification of AL samples required spraying the plate with sulfuric acid R/methanol R mixture (10:190 v/v) and then heating the chromatographic plate to dryness on the heating plate at 50 °C for approximately 10 min. We compared the correspondence in position, appearance, and intensity between the test samples and the standards. The retardation factor error (%Rf error) of Rf-sample and Rf-standard was calculated as follows:
Rule: If Rf error ≤ 5%, the sample is considered valid; if Rferror is between 5 and 10%, the analyte is deemed doubtful; if Rf error ≥ 10%, it is invalid [2].
Quantitative analysis of quinine sulfate in tablets
Aliquots of 20 tablets crushed to a powder, equivalent to 100 mg of quinine sulfate, were gently stirred for 15 min in 40 ml of acetic anhydride R and 40 ml of anhydrous acetic acid. And we titrated with perchloric acid (0.1 mol/L) using violet crystal as the indicator (from violet to blue and apple green). Each milliliter of perchloric acid (0.1 mol/l) is equivalent to 26.10 mg of quinine sulfate [(C20H24N2O2)2, H2SO4.2H2O]. The test requires that the yield be not less than 90.0% and not more than 110.0% of the amount of quinine sulfate [29, 30].
Quantitative analysis of Artemether-Lumefantrine
The principle consists of extracting Artemether and Lumefantrine from TLC spots and measuring the absorbance at 254 nm. For the Artemether (sample and standard), the layer obtained by TLC was carefully scraped, weighed, and diluted with 2 ml of the HCl/ethanol mixture (1 mol/l) in a test tube. After homogenization on the vortex, the test tube was heated in a water bath at 55 °C for 5 h, then cooled to room temperature and centrifuged. The procedure was the same for the blank without Artemether. The resulting solution’s absorbance was measured in a 1-cm layer quartz cell at 254 nm against a solvent cell containing the blank. The dilution was 38.5 for Artemether (C16H26O5) in the sample and standard, using the absorptivity value \( {A}_{1 cm}^{1\%}=385 \) [27]. The percentage content of Artemether (C16H26O5) in the tablets was calculated as follows:
The test met the requirement if the yield is between 95.0 and 105.0% of the amount of Artemether (C16H26O5) and Lumefantrine (C30H32Cl3NO). For the lumefantrine analysis, the TLC layer spots were carefully scraped, weighed, and dissolved in 2 ml of methanol R in a test tube. The mixture was then homogenized on the vortex for 15 min and centrifuged. The absorbance was measured at 380 nm [28] against the blank prepared in the same way without Lumefantrine.
Data analysis
We set the score of the critical quality attributes as 1 or 0 when the test met or not the requirement—the sum of scores allowed calculating the percentages of global quality satisfaction. The quality was excellent (0.90–1.00), good (0.80–0.90), acceptable (0.70–0.80), and low (0.30–0.70), based on a modified psycho-physical Harrington’s scale of quality [31].
The risk of treatment failure, death due to untreated disease, or toxicity was high, moderate, or low concerning the absence or wrong active ingredient, identity, under-dose, over-dose, disintegration, uniformity of mass, and labeling outcomes. MS Excel 2013 was used to calculate the descriptive statistics.
Results
Labeling of medicines
The samples of generic QS and AL tablets analyzed were from the community pharmacies and ambulatory street vendors. As shown in Table 1, QS samples consisted of 12 batches containing 30 tablets each from 5 brands. AL samples consisted of 12 packs containing 24 tablets per blister from two brands. As presented, the visual inspection shows that 4 (30%) batches of QS samples (QSB2.2, QSB2.4, QSB3.5, and QSB4.6) did not meet all the labeling requirements. Some information was inaccurate or missing. The labeling on the packages of AL samples contained all the needed information.
Manufacturing technology quality
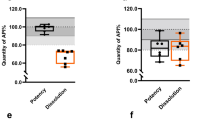
The elements tested were mass uniformity, hardness, friability, and disintegration time. For QS samples, two batches (QSB4.6, QSB5.7) did not meet the mass uniformity criteria because more than three tablets deviated beyond ± 10.0%. The hardness of 2 batches (QSB1.3, QSB3.4) did not lay between 50 and 150 N. The disintegration time of 8 batches (QSB1.1, QSB1.3, QSB1.8, QSB1.9, QSB1.11, QSB2.2, QSB2.4, and QSB3.5) was out of specification limits. For AL samples, two batches (ALB1.4, ALB2.2) did not meet the mass uniformity criteria. All batches satisfied the specification limits of hardness, friability, and disintegration.
Identification of active ingredients
Figure 1 shows the typical TLC chromatograms obtained.
Typical TLC chromatograms of Artemether (A)/Lumefantrine (L) samples at the left and three quinine samples (A, B, C) at right. The TLC of Lumefantrine (L) and Artemether (A) shows that all batches in triplicate spots gave Rf similar to the standards(s), with Rferror < 2% for all the spots. However, the TLC of quinine shows the absence of quinine sulfate in sample spot A(q2); the similarity of B(q1) and B(q2) spots with the standard (s); the presence of other products different from the quinine standard in C(q1) and C(q2)
Identification of QS samples showed that four batches (QSB5.7, QSB5.10, QSB5.12, QSB4.6) did not contain quinine sulfate; QSB4.6 had an active ingredient other than quinine sulfate because the Rferror was > 10%. Four batches of QS samples failed Mayer’s reaction (QSB4.6, QSB5.7, QSB5.10, and QSB5.12).
Content assay of active ingredients
Table 2 shows the results of quantitative assays. For QS samples, the batches QSB4.6, QSB4.7, QSB4.10, and QSB4.12 did not contain quinine (yield = 0%). The qualitative analysis revealed the absence or insufficient amount of active ingredients or the presence of other components than quinine sulfate in some batches.
For AL samples, the yield of Lumefantrine batches ALB1.5, ALB1.7, ALB1.8, and ALB2.3 was < 90%.
Global quality scores
Figure 2 shows the satisfaction of each QS batch to the 14 tests performed, using score 1 (if the requirement is satisfied) or 0 (if not) to evaluate the critical quality attributes.
Quality compliance scores of quinine batches analyzed. Dose (the content strength), no batch (batch number), issue (the issued date), expire (the expiring date), shelf (the shelf life), company (the manufacturer), country (the country of production), uniformity (the uniformity of mass), hardness (the resistance), friability (friction), disintegration (disintegration time), TLC (chromatogram), assay (dosage), and Mayer (identification of alkaloids)
It appears that no quinine batch obtained a 100% score; the maximum score was 92.9%, corresponding to one failure (QSB1.11, QSB1.8, QS1.1), and the minimum was 35.7%, corresponding to 9 fails (QS4.6). For example, batches QSB2.2, QSB2.4, QSB3.5, and QSB4.6 (from brands 2, 3, and 4) did not satisfy all required labeling information; all batches of brands 1, 2, and 3 failed the disintegration test; all samples of brands 4 and 5 did not contain quinine. The most failed tests were disintegration (33.3%) and assay (41.7%).
Figure 3 shows the satisfaction of each AL batch to the 13 tests performed, also using score 1 (if the requirement is satisfied) or 0 (if not) to evaluate the critical quality attributes. All labeling information needed was given. However, only five batches satisfied the assay test (ALB1.1, ALB1.4, ALB1.12, ALB2.2, ALB2.10). The minimum score for AL batches was 92.3%. The most failed test was the assay (41.7%).
Discussion
Counterfeiter medicines are the leading cause of resistance, morbidity, mortality, and dysfunction failure of the public health care system interest [32,33,34,35,36]. Quinine and Artemether/Lumefantrine fixed-dose are on the WHO essential medicines model list for curative malaria [32]. In this study, we assessed the pharmaceutical quality of QS and AL tablets under the following critical quality requirements: labeling quality, general tests on pharmaceutical dosage forms, content and dissolution, identification, and the presence of unidentifiable impurities in formulations. The findings vigorously back the importance of the counterfeiting phenomenon as described in numerous studies.
Drug labeling is a legislative requirement to obtain information on drugs such as brand name, pharmaceutical form, manufacture and expiration dates, administration route, manufacturer, and country manufacturing [37]. Thus, the investigated products might not have a risk of confusion associated with labeling. The visual inspection results on the physical characteristics of tablets and labeling information revealed that QS products did not comply with the WHO guideline on the labeling for pharmaceutical products. All QS samples mentioned the dose; 83.3% mentioned batch number and date of issue and shelf life; only 66.7% mentioned the manufacturer and country. AL samples complied satisfactorily. A tighter inspection of the label can help suspect wrong products. Often, counterfeiters copy the brand’s design or the designation of a product and sometimes the address. In developed countries, anticounterfeit packaging technologies are being developed [38,39,40]. The European trend is towards 2D barcodes (anticounterfeit technologies), and the USA has implemented radio frequency identification (RFID) [41]. The probability of detecting the failure in DRC is low.
According to Good Manufacturing Practice (GMP), uniform mass and friability are also critical attributes of quality [36, 42, 43]. Failure to quickly release the active ingredient for conventional formulations has the risk of therapeutic loss or delayed effect onset, explaining the number of deaths. The analysis of AL brands indicated that all of the samples but one complied with the quality specifications. However, for QS samples, only 3 out of 12 batches fully satisfied the critical technology attributes. Also, mass uniformity is an alternative test for content uniformity of uncoated and film-coated tablets. Here, defaults of two QS batches and one AL batch might not affect the content uniformity of the investigated products’ dose units. Many studies show that most antimalarial solid dosage forms pass the basic tests, such as the uniformity of mass for tablets, the content test, but a few pass in vitro product dissolution test required [36, 42, 43].
TLC and Mayer’s QS identification revealed that 7 out of 12 batches did not contain the active principle. The results of quinine amount indicated that only 41.7% of investigated products did comply with the pharmacopoeial specification limit (90–110%). Contrarily, AL samples showed excellent scores (> 90%) for Artemether, even though the content of Lumefantrine was under-dose in 4 batches. The risk of treatment failure due to bad quality is significantly higher with QS tablets than AL fixed-dose products. Studies carried out in Rwanda and the east of DR Congo had also shown the under-dosing and the absence of the quinine [11, 44]. One study conducted in DRC [45] on 150 AL samples collected from private pharmaceutical outlets in 8 main cities (Goma, Kikwit, Kinshasa, Kisangani, Lubumbashi, Matadi, Mbandaka, and Mbuji-Mayi) found 3 (2%) visual inspection failure and 4 (2.7%) TLC test failure. HPLC assays showed 46 (30.7%) samples had Artemether contents below 90% and 17 (11.3%) above 110% of the content claimed on the label; 32 (21.7%) samples had lumefantrine content below 90% and 8 (5.3%) above 110%. Studies in Kenya [46], Burkina Faso [47], and Ethiopia [48] also detected under-dose artemisinin generic products.
According to the WHO, counterfeit AMDs are often sold at low prices because they do not contain the right ingredients. Oral administration is the most widely accepted route due to its convenience in self-administration and easy manufacturing, which lead to easy counterfeiting. In DRC, pharmacies and free markets are among the potential sellers of inexpensive antimalarial to health facilities and the public. The unemployment rate is high; the average monthly household income remains low [49]. Such poverty leads people to look for cheaper antimalarial products sold in free markets. The situation makes it very hard to detect fake products quickly. Non-pharmacists own most pharmacies, and many are not at all managed by pharmacists. One knows that non-pharmacist health professionals are not qualified to detect the quality characteristics of the drugs they prescribe or administer.
Conclusion
The findings show that antimalarial drugs sold in the DRC pharmaceutical market are imported products from various countries, and not all brands are of good quality. African countries face multiple kinds of counterfeiting favored by poverty, lack of proxy control tools, and inefficacy of regulatory authority. That raises the importance of surveying the quality of all pharmaceutical products imported. Pharmacists should be involved at each step of the medication management system to detect any anomalies to minimize the counterfeiting phenomenon impact. Simple tests may be of significant help.
Availability of data and materials
All data is available upon request.
Abbreviations
- AL:
-
Artemether-Lumefantrine
- ALB:
-
Artemether-Lumefantrine fixed-dose batch
- AMD:
-
Antimalarial drug
- DRC:
-
Democratic Republic of the Congo
- GMP:
-
Good Manufacturing Practice
- MQSA:
-
Molecular quantitative similarity analysis
- QS:
-
Quinine sulfate
- QSB:
-
Quinine sulfate batch
- RFID:
-
Radio-frequency identification
- TLC:
-
Thin-layer chromatography
- WHO:
-
Word Health Organization
- WMR:
-
World Malaria Report
References
WHO Paludism (2018). https://www.who.int/topics/malaria/fr/. Accessed 20 Jan 2020
CDC. Malaria (2015). https://www.cdc.gov/malaria/about/[fr-fr]-faqs.html. Accessed 20 Jan 2020
Trampuz A, Jereb M, Muzlovic I, Prabhu RM (2003) Clinical review: severe malaria. Crit Care 7(4):315–323 https://link.springer.com/content/pdf/10.1186/cc2183.pdf
Brooker SJ, Clarke S, Deepika Fernando CWG, Nankabirwa J, Schellenberg D, Greenwood B (2017) Chapter 14 Malaria in middle childhood and adolescence. In: Child and Adolescent Health and Development, 3rd edn, Washington (DC) https://www.ncbi.nlm.nih.gov/books/NBK525246/
WHO (2010) World malaria report, Geneva https://apps.who.int/iris/bitstream/handle/10665/330011/9789241565721-eng.pdf. Accessed 20 Jan 2019
WHO Malaria (2020). https://www.who.int/news-room/fact-sheets/detail/malaria. Accessed 20 Jan 2020
Medicines for Malaria Venture (MMV) Developing antimalarials to save lives. https://www.mmv.org/malaria-medicines/malaria-facts-figures. Accessed 25 Aug 2020.
Basco L (2004) Molecular epidemiology of malaria in Cameroon-XIX. Quality of antimalarial drugs used for self-medication. Am J Trop Med Hyg 70(3):245–250. https://doi.org/10.4269/ajtmh.2004.70.245
Winstanley P, Ward S, Snow R, Breckenridge A (2004) Therapy of falciparum malaria in sub-Saharan Africa: from molecule to policy. Clin Microbiol Rev 17(3):612–637. https://doi.org/10.1128/CMR.17.3.612-637.2004
Takata J, Sondo P, Humphreys GS, Burrow R, Maguire B, Hossain MS, Das D, Commons RJ, Price RN, Guerin PJ (2020) The WorldWide Antimalarial Resistance Network Clinical Trials Publication Library: a live, open-access database of Plasmodium treatment efficacy trials. Am J Trop Med Hyg 103(1):359–368. https://doi.org/10.4269/ajtmh.19-0706
Habyalimana V, Mbinze JK, Yemoa AL, Kadima NJL, Hubert P, Roland Marini RD (2017) Simple LC isocratic methods development, validation, and application in the analysis of poor quality antimalarial medicines. Am J Anal Chem 8(9):582–603. https://doi.org/10.4236/ajac.2017.89042
OMS (2012) Médicaments essentiels frauduleux depuis l’Asie du Sud et de l’Est vers l’Afrique de l’Ouest. https://www.unodc.org/documents/toc/Reports/TOCTAWestAfrica/West_Africa_TOC_FRAUD_MEDICINES_FR.pdf
Frankish H (2003) WHO steps up campaign on counterfeit drugs. Lancet 362:1730. https://doi.org/10.1016/S0140-6736(03)14891-X
Kelesidis T, Falagas ME (2015) Substandard/counterfeit antimicrobial drugs. Clin Microbiol Rev 28(2):443–464. https://doi.org/10.1128/CMR.00072-14
Ministere de la santé publique (MSP) (2011) Rapport narratif. Profil pharmaceutique de la République Démocratique du Congo
National Malaria Control Program (2018) Evaluation of the impact of malaria control interventions on all-cause mortality in children under five in the Democratic Republic of the Congo from 2005 to 2015, Kinshasa https://www.pmi.gov/docs/default-source/default-document-library/pmi-reports/drc-malaria-impact-evaluation-group-executive-summary-french.pdf?sfvrsn=3. Accessed 15 Aug 2019
WHO (2019) Artemisinin resistance and artemisinin-based combination therapy efficacy. Global Malaria https://www.who.int/malaria/areas/treatment/drug_efficacy/en/. Accessed 15 Aug 2020
WHO (2019) Uniformity of mass for single-dose preparations, 9th edn. The International Pharmacopoeia (Ph.Int) https://apps.who.int/phint/pdf/b/7.5.3.5.2-Uniformity-of-mass-for-single-dose-preparations.pdf. Accessed 15 Aug 2020
EDQM. 2.9.7 (2008) Friability of uncoated tablets. In: European Pharmacopoeia (Ph Eur)Ph Eur, 6th edn. European Directorate for the Quality of Medicines Strasburg, p 278 http://www.uspbpep.com/ep60/2.9.7
EDQM. 2.9.8 (2008) Resistance to crushing of tablets. In: European Pharmacopoeia (Ph Eur), 6th edn. European Directorate for the Quality of Medicines Strasburg, p 279 http://www.uspbpep.com/ep60/2.9.8
EDQM. 2.9.1 (2008) Disintegration of tablets and capsules. In: European Pharmacopoeia (Ph. Eur), 6th edn. European Directorate for the Quality of Medicines Strasburg, p 263 http://www.uspbpep.com/ep60/2.9.1
World Health Organization (2019) Disintegration test for tablets and capsules. 9th. The International pharmacopoeia (Ph.Int). https://apps.who.int/phint/pdf/b/7.5.4.5.3-Disintegration-test-for-tablets-and-capsules.pdf
World Health Organization (2019) Quinine sulfate tablets (Quinini sulfas compressi), 9th edn. The International Pharmacopoeia https://apps.who.int/phint/2019/index.html#d/b.6.2.2.114
Richard J, Peter P, Peter G, Schuster A (2016) Tests de chromatographie sur Couche Mince. Global Pharma Health Fung(GPHF), Allemagne, p 209
Gaur R, Azizi M, Hansal P, Harper K (2009) Stationery Office on behalf of the Medicines and Healthcare product Regulatory Agency (MHRA). British Pharmacopoeia, London, ISBN 9780 113227990
Rwo J, Dwornik K, Fischer K (2014) Thin layer chromatographic tests. In: A Concise Quality Control Guide on Essential Drugs and other Medicines, 2nd edn. Global Pharma Health Fund (GPHF), Darmstadt https://www.gphf.org/images/downloads/DemoMinilabSupplement2014English.pdf
WHO (2016) Artemether tablets. In: The International Pharmacopoeia (PhInt), 6th edn, Geneva https://apps.who.int/phint/2016/index.html#d/b.6.2.2.17
Karajgi SR, Tanveer AR, Kalyane NV (2020) Simultaneous determination of Artemether and Lumefantrine by area under curve UV spectrophotometric method. J Pharm Sci Res 12(2):258–263
WHO (2018) Quinine sulfate tablets: final text for addition to the International Pharmacopoeia. https://www.who.int/medicines/publications/pharmacopoeia/Quinine-sul-tab_QAS07_219FINAL.pdf
Cholvy S (1960) Practical applications of protometry in anhydrous media for the analytical control of some pharmaceutical preparations. Ann Pharm Fr 18:138–144 https://pubmed.ncbi.nlm.nih.gov/13809987/
Bikbulatov ES, Stepanova IE (2011) Harrington’s desirability function for natural water quality assessment. Russ J Gen Chem 81(13):2694–2704. https://doi.org/10.1134/S1070363211130111
World Health Organization (2019) World Health Organization model list of essential medicines, 21st edn, Geneva, pp 1–60. https://apps.who.int/iris/bitstream/handle/10665/325771/WHO-MVP-EMP-IAU-2019.06-eng.pdf
Newton PN, Green MD, Mildenhall DC, Plançon A, Nettey H, Nyadong L et al (2011) Poor quality vital antimalarials in Africa - an urgent neglected public health priority. Malar J 1:22
Rana GS (2005) Counterfeit defeat brands. SSRN Electron J https://papers.ssrn.com/sol3/Delivery.cfm/SSRN_ID701189_code464609.pdf?abstractid=701189&mirid=1
WHO (2010) Counterfeit medical products, Geneva http://apps.who.int/gb/ebwha/pdf_files/WHA63/A63_23-en.pdf. Accessed 15 Aug 2019
World Health Organization (2009) Does quality of medicines matter? , Geneva http://www.who.int/medicines/services/counterfeit/faqs/QACounterfeit-October2009.pdf
Nayyar GML, Breman JG, Newton PN, Herrington J (2012) Poor-quality antimalarial drugs in Southeast Asia and sub-Saharan Africa. Lancet Infect Dis 12(6):488–496. https://doi.org/10.1016/S1473-3099(12)70064-6
Moore S (2012) Labelling and its role in pharmaceutical packaging. Int Pharm Ind 4(3):114–118 Available from: http://ipimediaworld.com/wp-content/uploads/2012/09/Pages-from-IPI-Volume4Issue3-35.pdf
Bansal D, Malla S, Gudala K, Tiwari P (2013) Anti-counterfeit technologies: a pharmaceutical industry perspective. Sci Pharm 81(1):1–13. https://doi.org/10.3797/scipharm.1202-03
Shah RY, Prajapati PN, Agrawal YK (2010) Anticounterfeit packaging technologies. J Adv Pharm Technol Res 1(4):368–373. https://doi.org/10.4103/0110-5558.76434
Centers for Disease Control and Prevention (CDC) Epi InfoTM: Population Survey or Descriptive Study, 7th edn. Division of Health Informatics & Surveillance, Atlanta, Georgia https://www.cdc.gov/epiinfo/user-guide/statcalc/samplesize.html
Yapar EA (2014) Orally disintegrating tablets: an overview. J Appl Pharm Sci 4(2):118–125. http://www.japsonline.com. https://doi.org/10.7324/JAPS.2014.40219
Sastry SV, Nyshadham JR, Fix JA (2000) Recent technological advances in oral drug delivery - a review. Pharm Sci Technol Today 3(4):138–145. https://doi.org/10.1016/S1461-5347(00)00247-9
Namegabe LM, Kadhesi MT, Hamuli PM, Mahano AO, Brioen PB (2019) Quality control of quinine in pharmaceutical forms tablets found in east of the Democratic Republic of Congo. Am J Anal Chem 10(9):415–422. https://doi.org/10.4236/ajac.2019.109029
Mufusama JP, Ndjoko Loset K, Feineis D, Hoellein L, Holzgrabe U, Bringmann G (2018) Quality of the antimalarial medicine Artemether – Lumefantrine in 8 cities of the Democratic Republic of the Congo. Drug Test Anal 10(10):1599–1606. https://doi.org/10.1002/dta.2420
Atemnkeng MA, De Cock K, Plaizier-Vercammen J (2007) Quality control of active ingredients in artemisinin-derivative antimalarials within Kenya and DR Congo. Tropical Med Int Health 12(1):68–74 https://onlinelibrary.wiley.com/doi/epdf/10.1111/j.1365-3156.2006.01769.x
Tipke M, Diallo S, Coulibaly B, Störzinger D, Hoppe-Tichy T, Sie A, Müller O (2008) Substandard antimalarial drugs in Burkina Faso. Malar J 7(1):95. https://doi.org/10.1186/1475-2875-7-95
Belew S, Suleman S, Mohammed T, Mekonnen Y, Duguma M, Teshome H, Bayisa B, Wynendaele E, D’Hondt M, Duchateau L, De Spiegeleer B (2019) Quality of fixed dose Artemether/Lumefantrine products in Jimma Zone, Ethiopia. Malar J 18(1):236. https://doi.org/10.1186/s12936-019-2872-1
United Nations Development Programme (UNDP) (2009) South-Kivu province: summary profile, poverty and household living conditions. http://www.undp.org/content/dam/dem_rep_congo/docs/povred/UNDP-CD-Profil-PROVINCE-Sud-Kivu.pdf
Acknowledgements
The authors are thankful to the laboratory technicians in the Department of Pharmacy (UOB).
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
AOM and AZM designed the study and literature search. AZM, LMN, and PMH operated the pharmaceutics testing. NHC, FMK, and BBZ conducted the identification tests. AOM and NJK revised the protocols and supervised and wrote the final draft. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mahano, A.O., Mahano, A.Z., Cubaka, N.H. et al. Pharmaceutical quality of antimalarial drugs: quinine sulfate and Artemether/Lumefantrine tablets sold on Bukavu Market. Futur J Pharm Sci 7, 131 (2021). https://doi.org/10.1186/s43094-021-00290-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-021-00290-8