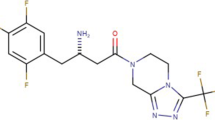
Abstract
Background
Analytical quality by design driven HPLC method has been optimized for simultaneous estimation of dapagliflozin and saxagliptin in pharmaceutical dosage form. Response surface methodology was employed for optimization of experimental conditions using three factors such as organic phase (%), pH of aqueous phase, and flow rate of mobile phase.
Results
Virtuous separation of analytes was achieved with mobile phase consisted of acetonitrile: phosphate buffer, pH 5.8 (26:74% v/v) with flow rate 0.96 mL/min using SPOLAR C18 column (250 × 4.6 mm, 5 μ) with run time 6 min and UV detection at 236 nm. Retention time for dapagliflozin and saxagliptin were found to be 3.5 and 5.0 min, respectively. Method was validated as per ICH guidelines. The plot between peak area vs concentration for dapagliflozin and saxagliptin were rectilinear in the range of 0.2-300 μg/mL and 0.1-150 μg/mL respectively with detection and quantification limits were 0.061 and 0.18 μg/mL for dapagliflozin and 0.014 and 0.043 μg/mL for saxagliptin, respectively.
Conclusion
The proposed method was exploited for assay, in vitro dissolution, and stability studies of target drugs in marketed dosage form.
Similar content being viewed by others
Background
Dapagliflozin and saxagliptin are hypoglycemic agents used in the treatment of type II diabetes mellitus as a fixed-dose combination, and their tablets are available on the market under the FDA-approved brand name QTERN® (2017). Dapagliflozin is an inhibitor of sodium glucose co-transporter-2, which is responsible for glucose reabsorption from the glomerular filtrate in the kidney. Saxagliptin is a competitive and reversible dipeptidyl peptidase-4 enzyme inhibitor that decreases glucagon-like peptide insulinotropic hormone-1 breakdown for improved glycemic regulation in patients with diabetes [1].
Analytical methods with HPLC technique are developed to support drug testing during production and quality release operations as well as during long-term stability studies against requirements. Due to numerous factors such as restricted availability of chromatographic columns, solvents and chemicals and critical physicochemical properties of analytes such as solubility, pKa, and stability, one factor at a time (OFAT) based analytical methods (trial and error based on one variable) experience several difficulties in optimizing robust chromatographic conditions. The model of analytical quality by design (AQbD) is a preferred and recommended approach to be adopted in the development of analytical methods to achieve regulatory versatility [2,3,4,5,6,7]. AQbD approach in chromatographic method development explores greater understanding of the parameters that must be regulated and tracked during the method’s life cycle.
Literature survey revealed that there were few reports published on the simultaneous quantification of dapagliflozin and saxagliptin using UV [8], HPTLC [9], HPLC [10,11,12,13,14,15,16,17,18], and LC-MS [19, 20] techniques in bulk drug and pharmaceutical dosage forms. Few methods were reported for analysis of dapagliflozin and saxagliptin separately [21,22,23,24] and in combination with other drugs [25,26,27,28,29]. Literature methods concerted largely on the development and validation of a RP-HPLC methods using different organic phases at different wavelengths and sensitive spectroscopic methods. The literature HPLC methods were based on OFAT variation conditions, which were time consuming and may not have scientific evidence during development stage. Hence, present project has undertaken with the quality-by-design approach in robust HPLC method development for simultaneous analysis of dapagliflozin and saxagliptin in API and marketed dosage form.
Methods
Instrumentation
The HPLC system (Shimadzu Corporation, Japan) was connected to the LC solution software, consisting of binary pump (LC-20AD), Rheodyne syringe sample injector (20 μL), and an UV detector (SPD-20A). Type-II (paddle) dissolution apparatus (Electro lab TDT-08L), ultra-sonicator (RK 106, Spincotech), millipore (0.45 μm) filters, and digital pH meter (LI-120, Elico) were utilized for this work. Design-Expert version 11.0.5.0 software (Stat-Ease Inc. Minneapolis) was employed for designing of LC experiments and response-modeling to generate design space for optimized robust analytical method.
Chemicals and reagents
The reference standards of saxagliptin and dapagliflozin were obtained from Hetero Laboratories, Hyderabad, India. Marketed formulation (QTERN) with label claim 10 mg of dapagliflozin and 5 mg of saxagliptin per tablet was procured from local pharmacy. All solvents used for mobile phase were of HPLC grade and obtained from Merck, Mumbai, India.
Chromatographic conditions
Chromatographic separation of analytes was achieved on SPOLAR C18 (250 × 4.6 mm, 5μ) column at ambient temperature with isocratic elution. The mobile phase consisting of acetonitrile: phosphate buffer, pH 5.8 (26:74% v/v) at flow rate of 0.96 mL/min. Injection volume was 20 μL and UV detection at 236 nm.
Preparation of standard solution
In order to prepare binary standard solution, dapagliflozin (20 mg), and saxagliptin (10 mg) were weighed, decamped into volumetric flask of 10 mL and dissolved in diluent by sonication. Volume was contrived up to the mark with diluent solution and the flask was shaken well (solution A). Further 0.1 mL of this solution was diluted to 10 mL with diluent so as to get binary working standard solution concentration of 20 μg/mL and 10 μg/mL for dapagliflozin and saxagliptin, respectively.
Method development using AQbD approach
Method optimization was premeditated with 20 experimental runs under (8 factorial points, 6 center points, and 6 axial points with α= 0.6073) central composite design (CCD) to study the interactions of the three critical method parameters (CMPs), namely [S1], % acetonitrile (X1), aqueous phase pH (X2), and flow rate (X3). The chromatographic responses (resolution, capacity factor) were obtained through experimentation on HPLC instrument using working standard solution of dapagliflozin (20 μg/mL) and saxagliptin (10 μg/mL). Statistical analysis was performed together with CCD in the Design-Expert software. The significance of factors was calculated using analysis of variance (ANOVA). Design space was generated by numerical optimization where Derringer’s desirability function was used to attain high method performance criteria.
Method validation
The validation parameters like precision, linearity, accuracy, system suitability, sensitivity, and robustness were premeditated in accordance to ICH Q2(R1) guidelines.
Linearity
Aliquots of 0.002, 0.5, 1.0, 1.5, 2.0, 2.5, and 3.0 mL were withdrawn from mixed standard stock and diluted to 10 mL with mobile phase such that the final concentration of dapagliflozin and saxagliptin and in the range of 0.2-300 μg/mL and 0.1-150 μg/mL was obtained respectively. Calibration curve was generated by plotting the peak area against the concentration of drug.
System suitability
The efficiency of optimized method was monitored by system suitability test. It was carried out via injection of freshly prepared working standard solution into HPLC under optimized conditions for six times. The chromatographic responses (analytical attributes) studied were retention time, resolution, capacity factor, theoretical plate number, peak area, selectivity factor, and tailing factor of analyte peaks.
Precision
Repeatability of proposed method was verified by analyzing 6 replicate injections of freshly prepared working standard solution of dapagliflozin (100 μg/mL) and saxagliptin (50 μg/mL) in mobile phase on the same day. Intermediate precision was performed by analyzing replicates of same concentration solution prepared in three consecutive days. The peak area of the analytes was determined and %RSD was calculated.
Accuracy
Recovery of standard that spiked to target concentration of sample at three levels (80, 100, 120%) was studied for method accuracy. The % recovery of drug was calculated by measurement of its peak area in the chromatogram.
Specificity
Specificity of the method was established by comparing the chromatogram of blank (mobile phase), placebo solution, and matrix (degradants) with test solution (analytes in mobile phase). The placebo solution comprises all the commonly used excipients for manufacturing of tablet dosage form. Stress degraded samples were prepared as mentioned in the forced degradation studies where the drugs were exposed to various stress conditions such as acid, base, peroxide, heat, and light to generate their degradation products.
Sensitivity
Limit of detection (LOD) and limit of quantification (LOQ) were premeditated from the regression analysis data of linearity studies (LOD = 3.3 σ/s, LOQ = 10 σ/s; where σ, standard deviation of response; s, slope of calibration curve).
Robustness
In order to assess the robustness of the method, deliberate changes in method, critical parameters were made within the design space. The variations in the parameters include pH (± 0.1), organic phase (± 1 %), and flow rate (± 0.04 mL/min) of mobile phase. The % RSD of theoretical plate number and retention time of chromatogram obtained for every variation was calculated.
Assay of marketed formulation
Twenty tablets of marketed formulation (QTERN), each containing 10 mg of dapagliflozin and 5 mg of saxagliptin were taken, average weight was measured and the fine powder was crushed. An accurately weighed amount of powder equal to 10 mg of dapagliflozin and 5 mg of saxagliptin was transferred to volumetric flask of 10 mL capacity containing methanol and sonicated for 15 min. The flask was shaken and volume was produced with methanol till the mark and filtered via Whatman filter paper (no: 41). From the filtrate, 0.2 mL was transferred into 10 mL volumetric flask and the volume was leveled to the mark with mobile phase. The amount of saxagliptin and dapagliflozin present in sample solution was determined.
Dissolution studies
Dissolution testing of marketed formulation was carried out in FDA-recommended dissolution media, i.e., acetate buffer pH 4.5 (1000 mL) for dapagliflozin and 0.1 N HCl with pH 1.2 (900 mL) for saxagliptin using paddle apparatus (USP apparatus 2) at 50 rpm and 37 ± 0.5 °C for 45 min. Sampling aliquots of 5 mL were withdrawn at 10, 15, 20, 25, 30, and 45 min interval and replacing the fresh medium with an equal amount. After the end of each test time, sample aliquots were filtered. Filtrate of 0.1 mL was diluted 10 mL with mobile phase (phosphate buffer pH 5.8: acetonitrile, 74:26) and analyzed by the contemplated RP-HPLC method. Amount of drug dissolved (saxagliptin/dapagliflozin) was calculated using their respective calibration curves. The cumulative percentage of drug dissolved was plotted against the time.
Forced degradation studies
Stress studies were executed on saxagliptin and dapagliflozin standards under acid, base, oxidative, thermal, and UV light conditions. Acid degradation was carried out with 5 mL of mixed standard stock solution, to this 5 mL of 1 N hydrochloric acid was added and kept at 60 °C for 60 min, then neutralized with 1 N sodium hydroxide. From this, 0.2 mL was diluted to 10 mL with mobile phase and injected into the HPLC system. Similarly, 1 N sodium hydroxide for base degradation and hydrogen peroxide (6%) for oxidative conditions were used. Dry heat degradation (105 °C, 6 h) and light degradation (UV Chamber, 200-Watt h/m2, 48 h) were performed on selected drugs in solid state. After degradation, the solid samples (20 mg dapagliflozin/saxagliptin 10 mg) were dissolved in 10 mL methanol and 0.1 mL of this solution was diluted to 10 mL with mobile phase. These solutions were injected into the HPLC system and sample stability assessment chromatograms were documented.
Results
Analytical method development
Selection of detection wavelength
The individual standard solutions of dapagliflozin (20 μg/mL) and saxagliptin (10 μg/mL) were prepared in mobile phase and scanned in UV spectrophotometer [S3]. The overlaid UV spectra showed an iso-absorptive point at 236 nm, which was selected as detection wavelength in PDA detector.
Design of experiments
Design of experiments (DoE) tool of RSM was employed to optimize the critical method parameters (CMP). Central composite design runs [S4] were executed on HPLC system with respect to three variables such as % acetonitrile (X1), aqueous phase pH (X2), and flow rate (X3) to acquire resolution (Rs), capacity factor (K1’, K2’) as method control responses. CCD plan and experimental results were illustrated in Table 1. The data generated was analyzed using the statistical software.
Statistical analysis
Statistical analysis of experimental observations was performed to evaluate significant factors that affect the chromatographic response, given in Table 2. Based on ANOVA results, it was found that the process model with X1, X2, and X3 along with interactions is highly significant (p<0.05) at 95% confidence level with curvature effect. The following second-order polynomial (quadratic) equations were generated consistently with models for each critical quality attribute.
Factor–response relationship and probable interaction effects were studied by response surface analysis. Contour (2D) plots and response surface (3D) plots (Fig. 1) were produced as a function of significant variables while the third variable was kept constant at a definite level. Colored regions (blue-green-yellow-red) in Fig. 1 denoted the obtained response values from low to high with respect to variable interactions. Figure 1a signifies the influence of variable interaction on resolution, high resolution values observed at high pH, low % acetonitrile, and minimum flow values. Figure 1b, c signifies the factor interactions on capacity factor of both drugs. Optimum K’ values are accomplished with high pH and high flow rate at optimum % acetonitrile concentration.
Design space
Multiple response optimization of variables for chromatographic separation of target analytes is carried using numerical optimization by setting up responses at desired goals. The one with desirability 1 was selected as an optimized solution out of three different solutions provided by the software. The design space generated through Derringer’s desirability function was portrayed in Fig. 2a, indicated high method performance owing to maximum desirability value (equal to 1). The perusal to Fig. 2a indicated the different regions for desirability values, the region (marked red) showed the maximum value which meets the desired goals, i.e., maximum resolution and capacity factor at target rage (1-5). The overlay plot of responses obtained from graphical optimization as illustrated in Fig. 2b, which exhibited method operable design region (MODR) and location of the optimized solution for the studied design. The perusal to Fig. 2b, indicated a region (yellow), which met the all-desired goals such as high resolution and optimum capacity factor values. The gray region with borders indicated the boundaries of responses against variables. MODR for the proposed method can be defined as organic phase (26-30%); pH (5.2-6.0); and flow rate (0.9-1.0 mL/min).
Optimum chromatographic conditions were derived from the design space as mobile phase consists of acetonitrile: phosphate buffer, pH 5.8 (26:74% v/v) at flow rate of 0.96 mL/min gave efficient chromatographic separation of analytes on SPOLAR C18 (250 × 4.6 mm, 5μ) column with isocratic elution. Injection volume was 20 μL and UV detection at 236 nm. The chromatogram of standard solution of saxagliptin and dapagliflozin under optimized method conditions was illustrated in Fig. 3. Retention time of dapagliflozin was 3.5 min, saxagliptin was 5.0 min and symmetrical peaks were observed. Tailing factor of both the drugs were less than 2.0, resolution was greater than 2, and plate count of both the drugs were greater than 2000.
Method validation
The method was validated according to ICH Q2(R1) guidelines and obeyed all validation parameters. A linear calibration plot [S5] for the method was obeyed over the calibration range [S2, S6] of 0.2-300 μg/mL for dapagliflozin (Y=17890X+111969, r2=0.9991) and 0.1-150 μg/mL for saxagliptin (Y=73126X–75712, r2=0.999). Results of system suitability test and validation were represented in Table 3. System suitability test results indicated that analytical attributes produced with optimized method parameters were analogous within the predicted space. The % RSD values for intra-day and inter-day study were less than 2.0, endorsed the good repeatability of proposed method. Recovery tests confirmed the accuracy of the method and found it to be important within specification limits and afforded recovery 100.1-100.3% and 100.3-100.9% of dapagliflozin and saxagliptin, respectively.
Chromatograms of blank and placebo had lack of peak at the retention time of target analytes. The chromatograms of stressed samples showed well-separated peaks of the analytes and degradation products (resolution>2), without alteration of analyte peak retention times, indicates method specificity. The LOD and LOQ values were found to be 0.061, 0.014 μg/mL, and 0.18, 0.043 μg/mL respectively for dapagliflozin and saxagliptin. Deliberate changes in method critical variables such as pH, flow rate, and organic phase (%) were made within MODR in order to facilitate the robustness of the method and results were represented in Table 4. The % RSD was below 2 for all variables, which point toward the robust method.
Application of contemplated method
The results obtained for assay of saxagliptin and dapagliflozin were compared in the marketed formulation (QTERN) with the corresponding labeled quantities and the chromatogram obtained for sample solution had dearth of additional peaks, indicated no interference of the formulation excipients used in the tablets. The % assay of dapagliflozin and saxagliptin and found to be 99.3% and 99.6%, respectively.
Dissolution testing of saxagliptin and dapagliflozin combined marketed tablet formulation was carried out in FDA-described dissolution media. The amount of drug dissolved was analyzed by the proposed RP-HPLC method without interference due to excipients. The cumulative drug release (%) was plotted against the time, depicted in Fig. 4. The perusal to Fig. 4 indicated that 97 and 94% of dapagliflozin and saxagliptin were released respectively from the marketed formulation (QTERN). The maximum in vitro release of drugs was found within 45 min. Hence, the proposed method can be used for dissolution testing of targeted drugs.
Forced degradation studies have been carried out to establish the stability-indicating property of the method and the peaks of degraded products are well estranged from the peaks of title analytes at different retention times, recorded in Fig. 5, proven method’s specificity. Summary of degradation studies was reported in Table 5. It was observed that saxagliptin was prone oxidative degradation. Significant degradation of dapagliflozin was observed with acidic and alkali hydrolysis condition. Stress degradation study results of both drugs revealed their stability under neutral hydrolysis condition. However, these degradation products do not be (or less) observed in normal course of degradation.
Discussion
Central composite design (CCD) with response surface methodology (RSM) was exploited to optimize experimental conditions for HPLC separation of saxagliptin and dapagliflozin, since RSM in HPLC method development provides an effective, competent approach for studying all critical parameters at the same instance, and at two levels (low, high) with lowest number of runs. Statistical analysis revealed that factor effects and interactions are highly significant (p<0.05) at 95% confidence levels. The highest least squares regression values (r2>0.993) of responses are obtained for selected models (quadratic). Coefficient of variation (CV) found to be less than 10% indicates reproducibility of model. A strong relationship between the experimental results and those of the models fitted was suggested. The model aptness is endorsed by lowest PRESS value, high adjusted R2 value (~1), high adequate precision (>4), non-significant lack of fit (p>0.05), and agreement of predicted R2 with adjusted R2 (difference<0.2). High degree of interaction among the studied CMPs on the method attributes is revealed by curvatures in response surface plots.
Figure 6 showed the contour plots of all responses and design space for optimized method are characterized with respect to critical method variables. It can be used to find out the response for a given set of input variables. The perusal to Fig. 6a showed the two variable interaction effect on resolution. The resolution is evidenced to vary non-linearly descending manner with increase in % acetonitrile. The maximum resolution observed with extremely high levels of buffer pH values. High resolution between analyte peaks is recommended to get good separation, accomplished with high flow rate. Figure 6b represents the influence of variables on capacity factor of dapagliflozin. The % organic phase revealed a curvilinear trend with aqueous buffer pH of mobile phase. The influence of variables on the saxagliptin capacity factor is markedly different, portrayed in Fig. 6c. It showed significant interaction between X1 and X2. Low-capacity factor values were observed with mobile phase containing low % organic component and buffer with high pH value.
The accuracy of predicted conditions within design space is verified by actual experimentation, results obtained are compared with predicted space and observed that the % prediction error (= [(observed−predicted)/predicted]×100) associated with predicted set of conditions is less than ± 10%. This assured the consistency of method performance as per intended use.
Method comparison with literature methods
Present method was found to be cost-effective owing to utilization of low % (26%) organic mobile phase than latest literature [10]. The analytical run duration for the proposed method is short (6 min); hence, this method was rapid and superior than literature methods [8, 11,12,13]. When compared with previous HPLC reports [14,15,16,17,18] for this drug combination, the proposed method has advantages such as high resolution (7.3) and wide linearity range (0.1-150 μg/mL, 0.2-300 μg/mL). Low LOD and LOQ values furnished in the method signify the high sensitivity over literature methods. Moreover, present method was applied to in vitro dissolution as well as stability studies of target analytes. The proposed AQbD method was found to be superior to literature methods due to its ability to simulate the interactive effects of variables on performance of the method. The present strategy of response surface methodology can overcome the limitations of reported methods such as high number of experiments, no variable-interaction study, less robust, and less feasibility for method transfer.
Conclusion
New RP-HPLC stability portentous method was developed for simultaneous analysis of dapagliflozin and saxagliptin in bulk and pharmaceutical dosage form using analytical quality by design approach. In this strategy, various constraints related to efficient separation of both analytes were considered and critical method parameters were optimized using response surface methodology. The developed method was validated for specificity, accuracy, linearity, precision, robustness, and it conformed all validation parameters of ICH Q2(R1) guidelines. The proposed HPLC method is highly robust for method transfer, regulatory flexibility within design space and allows continuous improvement.
Availability of data and materials
The data generated during this study is included in the supplementary file of this article.
Abbreviations
- ANOVA:
-
Analysis of variance
- API:
-
Active pharmaceutical ingredient
- AQbD:
-
Analytical quality by design
- CCD:
-
Central composite design
- CMP:
-
Critical method parameters
- C:
-
Coefficient of variation
- DoE:
-
Design of experiments
- FDA:
-
Food and drug administration
- HPTLC:
-
High performance thin-layer liquid chromatography
- ICH:
-
International conference on hormonization
- K1’:
-
Capacity factor of dapagliflozin
- K2’:
-
Capacity factor of saxagliptin
- LC-MS:
-
Liquid chromatography-tandem mass spectrometry
- LOD:
-
Limit of detection
- LOQ:
-
Limit of quantification
- MODR:
-
Method operable design region
- OFAT:
-
One factor at a time
- PDA:
-
Photo diode array
- RP-HPLC:
-
Reverse phase high performance liquid chromatography
- Rs:
-
Resolution
- RSD:
-
Relative standard deviation
- RSM:
-
Response surface methodology
- USP:
-
United States Pharmacopeia
- UV:
-
Ultra violet
References
Sisode PS, Jain VC, Prajapati N (2016) Pharmacology of combined treatment of saxagliptin hydrochloride and glibenclamide therapy to treat type-2 diabetes. Asian J Res Pharm Sci 6(1):59–61 https://doi.org/10.5958/2231-5659.2016.00009.6
Trupti T, Kadam N, Raotole N, Anita D, Samanta G (2019) A simultaneous determination of related substances by high performance liquid chromatography in a drug product using quality by design approach. J Chromatogr A 1432:26–38 https://doi.org/10.1016/j.chroma.2015.12.080
Gurrala S, Shivaraj, Subrahmanyam CVS, Anumolu PD, Saraf G (2019) Analytical quality by design assisted HPLC method for quantification of canagliflozin/metformin and stability studies. Indian J Pharm Educ Res 53(4s):699–709 https://doi.org/10.5530/ijper.53.4s.167
Parag D, Animesh M (2017) Analytical quality by design (AQbD): a new horizon for robust analytics in pharmaceutical process and automation. Int J Pharm Drug Anal 5(8):324–337
Maurice GE, Sylvester PA, Atim DA (2016) Plackett-burman design and response surface optimization of medium trace nutrients for glycolipopeptide biosurfactant production. Iran Biomed J 21(4):249–260 https://doi.org/10.18869/acadpub.ibj.21.4.249
Sandhu PS, Beg S, Katare OP, Singh B (2016) QbD-Driven development and validation of a HPLC method for estimation of tamoxifen citrate with improved performance. J Chromatogr Sci 54(8):1373–1384 https://doi.org/10.1093/chromsci/bmw090
Ramalingam P, Bhadraya K, Reddy YP (2015) Analytical quality by design: a tool for regulatory flexibility and robust analytics. Int J Anal Chem 2015:1–9. https://doi.org/10.1155/2015/868727
Raveendra BG, Kumar RA, Shaheen SD (2018) A novel stability-indicating method for the simultaneous estimation of saxagliptin and dapagliflozin in rat serum by using UV spectroscopy. Pharm Anal Acta 9(3):1–5 https://doi.org/10.4172/2153-2435.1000579
Shveta HP, Shailesh VL, Sachin BN (2018) Development and validation of UV-spectroscopic first derivative and high-performance thin layer chromatography analytical methods for simultaneous estimation of dapagliflozin propanediol monohydrate and saxagliptin hydrochloride in synthetic mixture. European J Biomed Pharm Sci 5(5):668–681
Suneetha A, Sharmila D (2019) Simultaneous estimation of saxagliptin and dapagliflozin in human plasma by validated high performance liquid chromatography - ultra violet method. Turk J Pharm Sci 16(2):227–233 https://doi.org/10.4274/tjps.galenos.2018.46547
Gundala A, Kvsrg P, Koganti B (2019) Application of quality by design approach in RP-HPLC method development for simultaneous estimation of saxagliptin and dapagliflozin in tablet dosage form. Braz J Pharm Sci 55:e18129 https://doi.org/10.1590/s2175-97902019000218129
Singh N, Bansal P, Maithani M, Chauhan Y (2018) Development and validation of a stability-indicating RP-HPLC method for simultaneous determination of dapagliflozin and saxagliptin in fixed-dose combination. New J Chem 42(4):2459–2466 https://doi.org/10.1039/C7NJ04260D
Deepan T, Dhanaraju MD (2018) Stability indicating HPLC method for the simultaneous determination of dapagliflozin and saxagliptin in bulk and tablet dosage form. Curr Issues Pharm Med Sci 31(1):39–43 https://doi.org/10.1515/cipms-2018-0009
Eswarudu MM, Aswini R, Srinivasa BP (2018) A novel RP-HPLC method for simultaneous estimation of dapagliflozin and saxagliptin in bulk and pharmaceutical dosage form. Int J Pharm Sci Res 9(12):5161–5167 https://doi.org/10.13040/IJPSR.0975-8232.9(12).5161-67
Nima S, Vanita M, Pragnesh P (2018) Stability indicating method development and validation for the simultaneous estimation of dapagliflozin and saxagliptin in synthetic mixture by HPLC. Pharma Sci Monit 9(2):112–128
Padmaja BR, Sivagami B, Chandrasekar R (2018) A highly validated RP-HPLC method development for the simultaneous estimation of dapagliflozin and saxagliptin in tablet dosage forms. Int J Pharm Sci Drug Res 10(5):372–378 https://doi.org/10.25004/IJPSDR.2018.100503
Phani RSCH, Prasad KRS, Useni RM (2017) A study of new method development, validation and forced degradation for simultaneous analysis of dapagliflozin and saxagliptin in pharmaceutical dosage form by HPLC method. Der Pharma Chem 9(20):96–103
Kumara Swamy G, Shruthi S, Rajkumar M, Sudheer KD (2017) A new stability indicating RP-HPLC method for simultaneous determination of saxagliptin and dapagliflozin in bulk and combined tablet dosage forms. Asian J Pharm Ana Med Chem 5(3):113–121
Surendran S, Paul D, Pokharkar S, Deshpande A, Giri S, Satheeshkumar N (2019) A LC-MS/MS method for simultaneous estimation of a novel anti-diabetic combination of saxagliptin and dapagliflozin using a polarity switch approach: application to in vivo rat pharmacokinetic study. Anal Methods 11(2):219–226 https://doi.org/10.1039/C8AY02087F
Goday S, Shaik AR, Avula P (2018) Development and validation of a LC-ESI-MS/MS based bioanalytical method for dapagliflozin and saxagliptin in human plasma. Indian J Pharm Educ Res 52(4):S277–S286 https://doi.org/10.5530/ijper.52.4s.108
El-Bagary RI, Elkady EF, Ayoub BM (2012) Spectrophotometric methods based on charge transfer complexation reactions for the determination of saxagliptin in bulk and pharmaceutical preparation. Int J Biomed Sci 8(3):204–208
Scheeren LE, Marcolino AIP, Adams AIH, Rolim CMB (2015) Stability indicating RP-LC-PDA method for the quantitative analysis of saxagliptin in pharmaceutical dosage form. Braz J Pharm Sci 51(2):461–466 https://doi.org/10.1590/S1984-82502015000200023
Abdel-Ghany MF, Abdel-Aziz O, Ayad M, Tadros MM (2015) Stability-indicating liquid chromatographic method for determination of saxagliptin and structure elucidation of the major degradation products using LC-MS. J Chromatogr Sci 53(4):554–564 https://doi.org/10.1093/chromsci/bmu084
Batta N, Pilli NR, Derangula VR, Vurimindi HB, Damaramadugu R, Yejella RP (2015) A rapid and sensitive LC-MS/MS assay for the determination of saxagliptin and its active metabolite 5-hydroxy saxagliptin in human plasma and its application to a pharmacokinetic study. Drug Res 65(3):133–140 https://doi.org/10.1093/chromsci/bmu084
Abdel-Aziz O, Ayad MF, Tadros MM (2015) Compatible validated spectrofluorimetric and spectrophotometric methods for determination of vildagliptin and saxagliptin by factorial design experiments. Spectrochim Acta A Mol Biomol Spectrosc 140:229–240 https://doi.org/10.1016/j.saa.2014.12.102
Gurav SB, Bhatia NM (2020) Development and validation of novel stability-indicating LC method for the determination of saxagliptin and metformin. Indian J Pharm Educ Res 54(2):S350–S357 https://doi.org/10.5530/ijper.54.2s.93
Rathod R, Ali F, Chandra A, Kumar R, Dahiya M, Singh GN (2020) Simultaneous determination of alogliptin, linagliptin, saxagliptin, and sitagliptin in bulk drug and formulation by uplc q-tof-ms. Curr Pharm Anal 17(1):95–105 https://doi.org/10.2174/1573412915666190708162012
Kant R, Bodla RB, Kapoor G, Bhutani R (2019) Optimization of a single HPLC-PDA method for quantifying metformin, gliclazide, pioglitazone, dapagliflozin, empagliflozin, saxagliptin, linagliptin and teneligliptin using central composite design. Bioorg Chem 91:103111 https://doi.org/10.1016/j.bioorg.2019.103111
Shah PA, Shah JV, Sanyal M, Shrivastav PS (2017) LC-MS/MS analysis of metformin, saxagliptin and 5-hydroxy saxagliptin in human plasma and its pharmacokinetic study with a fixed-dose formulation in healthy Indian subjects. Biomed Chromatogr 31(3):e3809 https://doi.org/10.1002/bmc.3809
Acknowledgements
The authors are thankful to the management of Gokaraju Rangaraju College of Pharmacy for providing necessary laboratory facilities.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
We assure that “all authors have read and approved the manuscript.” SG had analyzed the samples and completed this work under the supervision of SR and SCVS. PDA helped in experimental work and data analysis. All authors together contributed for this research work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
No competing interests to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: S1: Table.
Critical method parameters for optimization. S2: Table. Data of linearity studies. S3: Figure. Overlaid UV-absorption spectra of Dapagliflozin (20 μg/ml) Saxagliptin (10 μg/ml). S4: Figure. Chromatograms obtained under CCD runs. S5: Figure. Calibration plot of dapagliflozin (a) and saxagliptin (b). S6: Figure. Overlaid chromatogram - linearity studies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gurrala, S., Raj, S., Subrahmanyam, C.V.S. et al. Multivariate optimization of liquid chromatographic conditions for determination of dapagliflozin and saxagliptin, application to an in vitro dissolution and stability studies. Futur J Pharm Sci 7, 85 (2021). https://doi.org/10.1186/s43094-021-00229-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-021-00229-z