Abstract
Background
The plants Cynodon dactylon (C. dactylon), Emblica officinalis (E. officinalis), Kalanchoe pinnata (K. pinnata), and Bambusa nutans (B. nutans) have been reported to possess diuretic and antiurolithiatic potential against ethylene glycol and ammonium chloride along with in vitro calcium oxalate (CaOx) crystal growth inhibition property. Our previous research publications reported a rich presence of antioxidative phytocompounds like polyphenols and flavonoids in ethyl acetate fractions of these plants. This present study aims to explore antiurolithiatic potential of C. dactylon, E. officinalis, K. pinnata, and B. nutans ethyl acetate fraction following 7 days of sodium glyoxalate treatment on mice.
Results
Sodium glyoxylate treatment caused significant (P < 0.01–0.001) reduction in the urine magnesium and creatinine and elevation in oxalate, citrate, calcium, and phosphate levels. Ethyl acetate fraction of K. pinnata and B. nutans showed a highly significant antilithiatic effect by increasing urine volume, normalizing disrupted urine parameters, increasing LDH level, and decreasing kidney tissue oxalate content. E. officinalis and K. pinnata ethyl acetate fraction treatment showed a pronounced reversal of tubular dilation and damage of epithelial cell in kidney tissue with very less inflammatory cell infiltration.
Conclusion
The results signify the protective effect of K. pinnata and B. nutans ethyl acetate fraction rich with polyphenol and flavonoid on glyoxylate induced oxidative cell damage and morphological changes in mouse kidneys.
Similar content being viewed by others
Background
The plants Cynodon dactylon (Poaceae), Emblica officinalis (Euphorbiaceae), Kalanchoe pinnata (Crassulaceae), and Bambusa nutans (Graminae) have well-known traditional and Ayurvedic uses for diuretic potential, and diuretic effect is being screened and reported by us [1]. Diuretics effectively lowers urine calcium excretion and reduces kidney stone recurrence as forced diuresis showed acute symptomatic relief in patients of urolithiasis [2, 3]. Previously, we have reported the rich presence of antioxidative phytocompounds like polyphenols and flavonoids in ethyl acetate fractions of B. nutans shoot, E. officinalis fruit, and K. pinnata leaf that also showed excellent in vitro CaOx crystal growth inhibition [4]. Sohgaura et al. (2019) reported excellent antiurolithiasis potential of K. pinnata and E. officinalis along with the moderate protective effect of B. nutans and C. dactylon against urolithiasis induced by ethylene glycol and ammonium chloride combined treatment for 10 days [5]. These facts indicate the whole spectrum of antiurolithiatic potential of the ethyl acetate fraction of the selected plants is worth to be explored. The study aims at the screening of C. dactylon whole plant, E. officinalis fruit, K. pinnata leaf, and B. nutans shoot extract ethyl acetate fraction rich in flavonoid and polyphenol for antinephrolithiasis potential against glyoxylate administration.
Rodent models of CaOx urolithiasis are induced by either ethylene glycol alone or in combination with ammonium chloride. Ethylene glycol, glycolate, and glyoxylate are the main precursors of oxalate. These can induce experimental urolithiasis on rats and mice when administered by a suitable route [6]. In vivo, experimental induction of nephrolithiasis on rats is commonly done with calcium oxalate and other oxalate metabolic intermediates like glycolate and ethylene glycol [7,8,9]. The use of the CaOx in vivo model on rats remains controversial in concern to clinical manifestation [10]. Multifarious gene manifestation was found related to urolithiasis.
Putative CaOx inhibitors like Tamm-Horsfall protein, Heparan sulfate (HS)/heparan sulfate proteoglycan (HSPG), and bikunin are upregulated during the incidence of calcium oxalate nephrolithiasis in rat [11, 12]. Okada et al. reported successful formation and deposition of CaOx crystal in mouse kidneys by intraabdominal glyoxylate injection [6]. Subsequent morphological observation and functional analysis of renal macrophages and gene-related studies showed that the glyoxylate mice model is excellent for mimicking clinical signs of nephrolithiasis [13, 14]. Different established renal stone induction models in rodents like rats and mice have revealed that mice have a higher tolerance level than rats. Sodium glyoxylate mice model was selected for further evaluation of C. dactylon, E. officinalis, K. pinnata, and B. nutans ethyl acetate fractions protective and beneficial effects against nephrolithiasis.
Methods
Extraction of plant material
The plant material was made completely clean, dust free, and allowed to get dried under the shade for 15 to 30 days. Dried plant parts were pulverized with a mechanical pulverizer to form a coarse powder and stored in an airtight container for further studies. Drying, processing, and enrichment of ethyl acetate fraction from the hydro-methanolic extract was performed as described previously [4].
Experimental animal
An in vivo study was performed with due permission from the Institutional Animal Ethical Committee. The experimental study procedure and protocol were reviewed and approved by the Institutional Animal Ethics Committee (IAEC). Laboratory animals were taken care of as per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). Laboratory breed adult albino mice (25–30 g) of either sex were used. The mice were kept in polypropylene cages with paddy husk bedding maintained in hygienic condition at 22 ± 2°C temperature and 12-h light-dark cycle. The animals were fed with a standard pallet balanced diet and water ad libitum. Dose range 25, 50, and 100 mg/kg, p.o., was selected for E. officinalis, K. pinnata, and B. nutans, whereas 100, 300, and 500 mg/kg for C. dactylon based on the acute toxicity reported previously [1].
Study protocol
Glyoxylate-induced nephrolithiasis in mice was inducted, as reported by Tzou et al. [13]. The mice were randomly divided into fifteen groups comprising five animals in each group containing both male and female mice. All the animals of the respective groups had been treated as per the protocol daily for 7 days with ethyl acetate fraction (EA) of C. dactylon, E. officinalis, K. pinnata, and B. nutans. The animals are grouped as follows:
-
Group 1: water for injection 0.2 ml/25 g (vehicle control).
-
Group 2: Sodium glyoxylate 100 mg/kg, i.p. (negative control).
-
Group 3: Sodium glyoxylate 100 mg/kg, i.p.+ 750 mg/kg cystone (positive control).
-
Group 4: Sodium glyoxylate 100 mg/kg, i.p+ 25 mg/kg EA K. pinnata
-
Group 5: Sodium glyoxylate 100 mg/kg, i.p+ 50 mg/kg EA K. pinnata
-
Group 6: Sodium glyoxylate 100 mg/kg, i.p+ 100 mg/kg EA K. pinnata
-
Group 7: Sodium glyoxylate 100 mg/kg, i.p+ 25 mg/kg EA E. officinalis
-
Group 8: Sodium glyoxylate 100 mg/kg, i.p+ 50 mg/kg EA E. officinalis
-
Group 9: Sodium glyoxylate 100 mg/kg, i.p+ 100 mg/kg EA E. officinalis
-
Group 10: Sodium glyoxylate 100 mg/kg, i.p + 25 mg/kg EA B. nutans
-
Group 11: Sodium glyoxylate 100 mg/kg, i.p+ 50 mg/kg EA B. nutans
-
Group 12: Sodium glyoxylate 100 mg/kg, i.p+ 100 mg/kg EA B. nutans
-
Group 13: Sodium glyoxylate 100 mg/kg, i.p+ 100 mg/kg EA C. dactylon
-
Group 14: Sodium glyoxylate 100 mg/kg, i.p+ 300 mg/kg EA C. dactylon
-
Group 15: Sodium glyoxylate 100 mg/kg, i.p + 500 mg/kg EA C. dactylon
Sodium glyoxylate (100 mg/kg, i.p. in water for injection) was injected daily for 7 days to promote hyperoxaluria and CaOx deposition in the kidneys of groups 2 to 15 mice. All animals were allowed regular free access to food and water.
Collection and analysis of urine
Animals were individually kept in metabolic cages, and 24-h urine samples were collected on the 7th day after the last dosing with free access to water. The urinary volume was determined, and samples were analyzed for level of oxalate, citrate, calcium, phosphates, magnesium, and creatinine with the help of diagnostic kits (Span Diagnostics Ltd., India, and Lab-Care Diagnostics Ltd., India) using Autoanalyser (Star 21 Plus, Rapid Diagnostics, India).
Collection and analysis of kidney homogenate
The animals were sacrificed on the 8th day under ether anesthesia by cervical decapitation. Dissected kidneys were rinsed with phosphate-buffered saline (pH 7.4). Homogenized in 5 ml buffer containing 100 mM potassium phosphate (pH 7.0) and 2 mM ethylene diamine tetraacetic acid (EDTA) per gram of tissue, centrifuged at 10,000 rpm for 15 min at 4°C, and the supernatant removed for assay. Kidney tissue homogenate was processed for estimation of oxalate, protein, and LDH content. Proteins give an intensive violet-blue complex with copper salts in an alkaline medium when iodide is included as an antioxidant. Protein was estimated as the intensity of the color formed, which is proportional to the total protein concentration in the sample detected spectrophotometrically at 540 nm [15]. Oxalate was transformed into hydrogen peroxide by oxalate oxidase, which on reacting to the Trinder system, produces a blue-colored compound detected at 590 nm [16]. LDH activity was determined spectrophotometrically at 565 nm with lactate as substrate based on the method Wacker et al. with few modifications [17, 18].
Histopathology of the kidney
A part of the kidneys was fixed rapidly with 10% formalin. Following dehydration in an ascending series of ethanol (70, 80, 96, 100%), kidney samples were cleared in xylene and embedded in paraffin. Kidney tissue sections of 5 μm were stained with hematoxylin-eosin and observed for histopathological changes. A minimum of 10 fields was examined for each kidney slide for necrosis and the presence of CaOx crystals and photographed using an optical microscope at ×10 magnification [19].
Statistical analysis
The results were presented in terms of mean ± SEM. Experimental data were analyzed using one-way ANOVA followed by Turkey-Kramer multiple comparisons using the InStat-3 graph pad prism version. Differences between the values of the groups compared were considered significant at P < 0.05.
Result
Effect on urine parameters
Sodium glyoxylate treatment for 7 days promotes hyperoxaluria and CaOx deposition in the kidneys. Induction of nephrolithiasis following sodium glyoxylate treatment is confirmed by significant (p < 0.01–0.001) reduction in the urine biochemical parameters like magnesium and creatinine and urine volume elevation of oxalate, citrate, calcium, and phosphate. C. dactylon ethyl acetate fraction treated animals at 300 and 500 mg/kg doses showed significant (P < 0.05–0.01) increase in urine volume and decrease in oxalate, citrate, phosphates, and calcium. C. dactylon significantly (P < 0.05–0.01) increases urinary excretion of creatinine and magnesium at 500 mg/kg dose. The 50 and 100 mg/kg doses of E. officinalis ethyl acetate fraction showed a significant (P < 0.05–0.01) increase in urinary magnesium and creatinine and a decrease in oxalate, citrate, calcium, and phosphates level.
Ethyl acetate fraction of K. pinnata has significantly (P < 0.05–0.01) increased urine volume in treated animals at 50 and 100 mg/kg dose. Significant (P < 0.01–0.001) decrease in oxalate, citrate, phosphates, and calcium level and increase in magnesium, creatinine excretion was observed at both 50 and 100 mg/kg doses. B. nutans ethyl acetate fraction treated animals showed significant (P < 0.05) increase in urine volume only at 100 mg/kg dose. B. nutans at 50 and 100 mg/kg doses showed significant (P < 0.05–0.01) increase in urinary excretion of magnesium and creatinine and decrease in oxalate, citrate, calcium, and phosphates level (Table 1).
Effect on kidney tissue
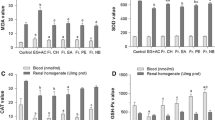
Glyoxylate-induced nephrolithiasis group showed a significant (P < 0.001) increase in LDH and oxalate level, whereas protein content was decreased in kidney homogenate. C. dactylon 500 mg/kg treated group showed a significant (P < 0.05–0.001) decrease in LDH and oxalate level along with an increase in protein level. E. Officinalis ethyl acetate fraction at 50 and 100 mg/kg treated doses showed significant (P < 0.05–0.001) decrease in LDH and oxalate level along with an increase in the protein level of kidney tissue homogenate. The effect of K. pinnata ethyl acetate fraction was highly significant (P < 0.01–0.001) in decreasing LDH and oxalate level along with an increase in protein level in kidney tissue homogenate at 50 and 100 mg/kg treated doses. Ethyl acetate fraction of B. nutans 50 and 100 mg/kg treated groups showed a significant (P < 0.05–0.001) decrease in LDH and oxalate along with an increase in protein level in kidney tissue homogenate (Fig. 1).
Kidney tissue parameters of glyoxalate induced nephrolithiatic mice treated with ethyl acetate fraction of C. dactylon, E. officinalis, K. pinnata, and B. nutans. EACD ethyl acetate fraction of C. dactylon, EAEO ethyl acetate fraction of E. Officinalis, EAKP ethyl acetate fraction of K. pinnata, EABN ethyl acetate fraction of B. nutans
Histopathology of the kidney
The photomicrograph of vehicle-treated animals showed normal kidney architecture. Kidney tissue section of animals treated with glyoxylate for 7 days showed deposition of microcrystals in tubules along with dilation, damage, and degeneration of epithelial cell lining by infiltration of inflammatory cells. Cystone 750 mg/kg treatment showed dilation of the epithelial cell with significant reversal of damage, degeneration, and crystal deposition. C. dactylon ethyl acetate fraction-treated groups at 300 and 500 mg/kg showed moderate reversal of these symptoms and less deposition of microcrystal in tubules. E. officinalis ethyl acetate fraction at 100 mg/kg dose showed a pronounced reversal of glyoxylate induced tubules epithelial cell lining dilation, damage, and degeneration with very less inflammatory cell infiltration. K. pinnata ethyl acetate fraction treated groups at 100 mg/kg showed a prompt decrease in tubular dilation and damage of epithelial cell in kidney tissue, but the effect on the infiltration of inflammatory cells and crystal deposition was moderate. B. nutans ethyl acetate fraction treated groups at 100 mg/kg dose showed moderate reversal of the pathological symptoms of glyoxylate induced cell damage and deposition of microcrystal in tubules with the presence of cellular degeneration (Fig. 2).
Kidney histopathology of glyoxylate-induced nephrolithiatic mice treated with ethyl acetate fraction of C. dactylon, E. officinalis, K. pinnata, and B. nutans. a Drinking water ad libitum (vehicle control). b Sodium glyoxylate 100 mg/kg, i.p. (negative control). c Cystone 750 mg/kg (positive control). d C. dactylon ethyl acetate fraction 100 mg/kg. e C. dactylon ethyl acetate fraction 300 mg/kg. f C. dactylon ethyl acetate fraction 500 mg/kg. g E. officinalis ethyl acetate fraction 25 mg/kg. h E. officinalis ethyl acetate fraction 50 mg/kg. i E. officinalis ethyl acetate fraction 100 mg/kg. j K. pinnata ethyl acetate fraction 25 mg/kg. k K. pinnata ethyl acetate fraction 50 mg/kg. l K. pinnata ethyl acetate fraction 100 mg/kg. m B. nutans ethyl acetate fraction 25 mg/kg. n B. nutans ethyl acetate fraction 50 mg/kg. o B. nutans ethyl acetate fraction 100 mg/kg
Effect on different urine parameter ratios
Ratios of different urine parameters were determined as an index of nephrolithiasis damage induced by glyoxylate treatment. These values comparatively showed the protective response of C. dactylon, E. officinalis, K. pinnata, and B. nutans ethyl acetate fractions. Vehicle control group showed calcium/oxalate, calcium/creatinine, phosphate/creatinine, and magnesium/creatinine ratio as 1.13, 0.14, 0.33, and 0.31, respectively, which are in the normal range. Glyoxylate treatment-induced nephrolithiasis disrupted these values with a decrease in calcium/oxalate and magnesium/creatinine ratio and an increase in calcium/creatinine and phosphate/creatinine ratio. C. dactylon has efficiently normalized calcium/oxalate, calcium/creatinine, and phosphate/creatinine ratio but not magnesium/creatinine ratio. E. officinalis has effectively normalized all four types of urine parameter ratios. K. pinnata and B. nutans have also normalized all four ratios but negligible effect on magnesium/creatinine ratio (Table 2).
Discussion
Several studies have highlighted the potential efficacy of complementary and alternative medicine for the prevention of chemical-related renal injury related to CaOx crystal deposition induced kidney damage. Mice are frequently used in all kinds of medical experiments. Mice were considered unsuitable as experimental urolithiasis model because hyperoxaluric stress does not seem to induce kidney stones in mice as it commonly does on rats. CaOx crystal deposition and renal oxidative stress induction were done in mice by intra-abdominal injection of glyoxylate (100 mg/kg/d) for 5 days [20]. This model has successfully replicated nephrolithiasis by the deposition of calcium oxalate crystal in mouse kidneys. Khan and Glenton have successfully induced hyperoxaluria by dietary administration of ethylene glycol combined with glyoxylate or hydroxyl proline in normocalciuric and hypercalciuric mice for 4 weeks. All mice developed calcium oxalate nephrolithiasis with kidney epithelial injury following treatment with glyoxylate. Results confirm that to create calcium oxalate nephrolithiasis in mice, hyperoxaluria along with hypercalciuria is required. Male mice, particularly on glyoxylate, had more renal injury and inflammatory cell migration into the interstitium around the crystal deposits [21]. Glyoxylate is metabolized to glycine by alanine glyoxylate aminotransferase (AGT) or to glycolate by glycolate reductase. Glycolate is further oxidized to oxalate by LDH [22]. In the case of deficiency of AGT and/or glycolate reductase, these two enzymes increased glyoxylate is finally oxidized to oxalate, which is responsible for massive hyperoxaluria leading to nephrocalcinosis [23]. These glyoxylate metabolites seem to modify normal tubular epithelium into a crystal-binding epithelium [24]. Conversion of glyoxylate to glycine is vitamin B6 dependent; thus, dietary vitamin B6 deficiency can also induce hyperoxaluria and hypocitraturia [25].
The kidney damage induced by glyoxylate was impressively alleviated after treatment with ethyl acetate fraction of C. dactylon, E. officinalis, K. pinnata, and B. nutans and cystone as positive control based on the results shown by mice urine biochemical parameter analysis, antioxidant enzyme LDH level in the kidney and histopathology. Ethyl acetate fraction of K. pinnata and B. nutans showed a highly significant antilithiatic effect by increasing urine volume and normalizing disrupted urine parameters, increased LDH, and kidney tissue oxalate content. Different urine parameter ratios were determined as an index of nephrolithiatic damage induced by glyoxylate treatment and to compare the protective response showed by the ethyl acetate fractions of C. dactylon, E. officinalis, K. pinnata, and B. nutans. Glyoxylate treatment-induced nephrolithiasis has disrupted these values with a decrease in calcium/oxalate and magnesium/creatinine ratio and an increase in calcium/creatinine and phosphate/creatinine ratios. E. officinalis ethyl acetate fraction has effectively normalized all four types of urine parameter ratios. Ethyl acetate fraction of K. pinnata and B. nutans has also normalized all four ratios but with nominal effect on magnesium/creatinine ratio, whereas ethyl acetate fractions of C. dactylon has efficiently normalized calcium/oxalate, calcium/creatinine, and phosphate/creatinine ratio but not magnesium/creatinine ratio. Consistent with the previous study reports, the glyoxylate-treated mice kidney showed widespread tubular injury. K. pinnata treated groups showed a prompt decrease in tubular dilation and damage of the epithelial cell, whereas B. nutans showed moderate reversal of the pathological symptoms of glyoxylate induced cell damage.
Recent studies have highlighted the therapeutic efficacy of E. officinalis, B. nutans, C. dactylon, and K. pinnata in CaOx crystal dissolution and growth inhibition. Phytochemical analysis revealed the highest flavonoid and polyphenol content in K. pinnata and lowest flavonoid content in B. nutans, though it has good polyphenol content. The study showed excellent CaOx crystal growth inhibition potential of B. nutans shoot followed by E. officinalis fruit and K. pinnata leaf. Kidney tubular cell injury related to CaOx crystal formation is linked to crystal aggregation and crystal-cell interactions. The reactive oxygen species lead to oxidative damage in tubular cells and further promote CaOx crystal formation [26]. Glyoxylate-treated mouse kidneys showed decreased superoxide dismutase and increased malondialdehyde. Many studies demonstrated that the accumulation of reactive oxygen species is intimately associated with renal tubular cell injury and the process of calcium crystallization [27]. Mitochondria are major sources of intracellular reactive oxygen species because they are sites of aerobic metabolism. Niimi et al. reported that reactive oxygen species generation is induced via the mitochondrial collapse in renal tubular cells exposed to calcium oxalate monohydrate crystals [28]. The internal structure of mitochondria in renal tubular cells underwent destruction and vacuolization, with decreased microvilli density in glyoxylate-treated mouse kidneys crystal-forming area. CaOx crystal formation overlays with glyoxylate administration induced cell injury and morphological changes in the renal tubular epithelial cells of mice [29]. It seems coadministration of antioxidant rich fractions of K. pinnata, B. nutans, and E. officinalis can regulate crystallization modulators’ expression, suppress inflammation, and upregulate the anti-inflammatory factor involved in CaOx crystal deposition.
The resistance mice show against kidney stone induction suggested that mice have a greater intrinsic ability to prevent stone formation following hyperoxaluric stress than rats. The differing response between mice and rats towards hyperoxaluric stress can be correlated to the fundamental molecular mechanism of kidney stone formation [6]. Crystal formation in mice could not be induced either by ethylene glycol or glycolate individual administration. The conversion of renal tubule retentive crystals into concrete stones is an important step in kidney stone formation containing osteopontin as a major component of the stone matrix. Mice showed a dramatic increase in osteopontin expression, a major stone-related protein, following glyoxylate administration. Osteopontin plays a crucial role in the morphological conversion of calcium oxalate crystals to stones in mouse kidneys [30]. In concert with all four plants previously reported beneficial role against ethylene glycol induced nephrolithiasis, these plants had also significantly protected mice from glyoxylate induced nephrolithiasis. Ethyl acetate fraction of K. pinnata and E. officinalis was most effective against ethylene glycol induced nephrolithiasis on rats, whereas K. pinnata and B. nutans were highly effective on mice glyoxylate nephrolithiasis. Among the four plants, K. pinnata ethyl acetate fraction had the highest content of total flavonoid and polyphenol, whereas B. nutans had the lowest flavonoid content through its polyphenol content was rich with excellent in vitro CaOx crystal growth inhibition potential [4]. B. nutans is reported to contain a rich presence of phenolic acids, such as ferulic acid and p-coumaric acid [31]. Bogucka-Kocka et al. reported a rich quantity of ferulic and caffeic acid in K. pinnata leaf [32]. The results signify that K. pinnata and B. nutans may have a modulating effect on the downregulation of osteopontin protein expression in mouse kidneys.
Peng et al. identified fifteen urinary metabolites as potential biomarkers related to amino acid metabolism, energy metabolism, and fatty acid metabolism after glyoxylate treatment on mice. Peng et al. reported a decrease in propionyl carnitine level accompanied by increased levels of sebacic acid and 3-hydroxysebacic acid, implying a decline in fatty acid transport and fatty acid β-oxidation in case of renal CaOx deposition [6]. Sebacic acid and 3-hydroxysebacic acid are medium-chain dicarboxylic acids derived from the ω-oxidation of fatty acids [33]. Propionylcarnitine is a short-chain acyl derivative of l-carnitine. Carnitine is an essential factor in regulating substrate flux and energy balance across cell membranes, possibly preventing cell injury [34]. Deoxyribonucleic acid (DNA) damage is the key feature of oxidative stress, and folic acid metabolism, purine metabolism, and pyrimidine metabolism were related to DNA injury and repair. Glyoxylate treatment decreases p-aminobenzoic acid, 1,3-dimethyluracil, 7-methylguanine, and deoxyuridine monophosphate [6]. Glyoxylate also disrupts tryptophan metabolism in kidney diseasing production of protective xanthurenic acid. Xanthurenic acid is a scavenger of peroxyl radicals in vitro and is a powerful antioxidant inhibiting lipid peroxidation [35]. This implies that the ethyl acetate fraction of K. pinnata and B. nutans can attenuate mitochondrial and DNA injury related to glyoxylate metabolism.
Conclusion
This study substantiates the antilithiatic effect of flavonoid and a polyphenol-rich fraction of K. pinnata and B. nutans against glyoxylate induced metabolic implications and CaOx crystal growth. Although the complex mechanism of glyoxylate-induced CaOx crystal deposition is not clearly elucidated, the present study provides two potential plants K. pinnata and B. nutans, with beneficial effects against nephrolithiasis on mice. The effect of K. pinnata and B. nutans can further be explored on downregulation of osteopontin, mitochondrial, and DNA damage related to glyoxalate induced nephrolithiasis in mice to elucidate the detailed mode of action.
Availability of data and materials
All data and materials are available on request.
Abbreviations
- C. dactylon :
-
Cynodon dactylon
- E. officinalis :
-
Emblica officinalis
- K. pinnata :
-
Kalanchoe pinnata
- B. nutans :
-
Bambusa nutans
- CaOx:
-
Calcium oxalate
- LDH:
-
Lactate dehydrogenase
- HS:
-
Heparan sulfate
- HSPG:
-
Heparan sulfate proteoglycan
- EA:
-
Ethyl acetate fraction
- EDTA:
-
Ethylene diamine tetra acetic acid
- EACD:
-
Ethyl acetate fraction of C. dactylon
- EAEO:
-
Ethyl acetate fraction of E. Officinalis
- EAKP:
-
Ethyl acetate fraction of K. pinnata
- EABN:
-
Ethyl acetate fraction of B. nutans
- AGT:
-
Alanine glyoxylate amino-transferase
- DNA:
-
Deoxyribonucleic acid
References
Sohgaura A, Bigoniya P, Shrivastava B (2018) Diuretic potential of Cynodon dactylon, Emblica officinalis, Kalanchoe pinnata and Bambusa nutans. J Pharmacogn Phytochem 7(3):2895–2900 Corpus ID: 221795201
Alexander RT, McArthur E, Jandoc R, Welk B, Fuster DG, Garg AX, Quinn RR (2018) Thiazide diuretic dose and risk of kidney stones in older adults: a retrospective cohort study. Can J Kidney Health Dis 5:2054358118787480
Tak B, Anuragi G, Sharma DC, Singh J, Durgawati D, Gupta R (2015) Role of forced diuresis in management of urinary calculi: an observational study. J Evol Med Dent Sci 4(100):16573–16579. https://doi.org/10.14260/jemds/2015/2468
Sohgaura AK, Bigoniya P, Shrivastava B (2018) In vitro antilithiatic potential of Kalanchoe pinnata, Emblica officinalis, Bambusa nutans and Cynodon dactylon. J Pharm Bioll Sci 10(2):83–89
Sohgaura AK, Bigoniya P, Shrivastava B (2019) Ameliorative effect of Kalanchoe pinnata, Emblica officinalis, Bambusa nutans and Cynodon dactylon on ethylene glycol and ammonium chloride induced nephrolithiasis. Pharmacologyonline 1:408–428
Okada A, Nomura S, Higashibata Y, Hirose M, Gao B, Yoshimura M, Yasunori I, Takahiro Y, Keiichi T, Kenjiro K (2007) Successful formation of calcium oxalate crystal deposition in mouse kidney by intra abdominal glyoxylate injection. Urol Res 35(2):89–99. https://doi.org/10.1007/s00240-007-0082-8
Joshi S, Wang W, Khan SR (2017) Transcriptional study of hyperoxaluria and calcium oxalate nephrolithiasis in male rats: inflammatory changes are mainly associated with crystal deposition. PLoS One 12(11):e0185009. https://doi.org/10.1371/journal.pone.0185009
Bilbault H, Jean-Philippe HaymannShah J, Patel B, Patel S, Patel R (2016) Effect of Hordeum vulgare Linn. Seeds on glycolic acid induced urolithiasis in rats. Pharmacog Comm 2(2):34–39
Sharma I, Khan W, Parveen R, Alam MJ, Ahmad I, Ansari MHR, Ahmad S (2017) Antiurolithiasis activity of bioactivity guided fraction of Bergenia ligulata against ethylene glycol induced renal calculi in rat. BioMed Res Int 2017:Article ID 1969525. https://doi.org/10.1155/2017/1969525
Yamaguchi S, Wiessner JH, Hasegawa AT, Hung LY, Mandel GS, Mandel NS (2005) Study of a rat model for calcium oxalate crystal formation without severe renal damage in selected conditions. Int J Urol 12(3):290–298. https://doi.org/10.1111/j.1442-2042.2005.01038.x
Marengo SR, Chen DH, Kaung HL, Resnick MI, Yang L (2002) Decreased renal expression of the putative calcium oxalate inhibitor Tamm-Horsfall protein in the ethylene glycol rat model of calcium oxalate urolithiasis. J Urol 167(5):2192–2197. https://doi.org/10.1016/S0022-5347(05)65127-0
Eguchi Y, Inoue M, Iida S, Matsuoka K, Noda S (2002) Heparan sulfate (HS)/heparan sulfate proteoglycan (HSPG) and bikunin are up-regulated during calcium oxalate nephrolithiasis. Kurume Med J 49(3):99–107. https://doi.org/10.2739/kurumemedj.49.99
Tzou DT, Taguchia K, Chi T, Stoller ML (2016) Animal models of urinary stone disease. Int J Surg 36(D):596–606. https://doi.org/10.1016/j.ijsu.2016.11.018
Taguchi K, Okada A, Hamamoto S, Unno R, Moritoki Y, Ando R, Mizuno K, Tozawa K, Kohri K, Yasui T (2016) M1/M2-macrophage phenotypes regulate renal calcium oxalate crystal development. Sci Rep 6:Article number 35167
Rifai N (2018) Tietz Fundamentals of clinical chemistry and molecular diagnostics, 8th edn. Saunders, Toronto
Laker MF, Hofmann AF, Meeuse BJ (1980) Spectrophotometric determination of urinary oxalate with oxalate oxidase prepared from moss. Clin Chem 26(7):827–830. https://doi.org/10.1093/clinchem/26.7.827
Wacker WE, Ulmer DD, Vallee BL (1956) Metalloenzymes and myocardial infarction. II. Malic and lactic dehydrogenase activities and zinc concentrations in serum. N Engl J Med 255(10):450–456
Prasirtsak B, Thitiprasert S, Tolieng V, Assabumrungrat S, Tanasupawat S, Thongchul N (2019) D-Lactic acid fermentation performance and the enzyme activity of a novel bacterium Terrilactibacillus laevilacticus SK5–6. Ann Microbiol 69(13):1537–1546. https://doi.org/10.1007/s13213-019-01538-8
Geraghty R, Wood K, Sayer JA (2020) Calcium oxalate crystal deposition in the kidney: identification, causes and consequences. Urolithiasis 48(5):377–384. https://doi.org/10.1007/s00240-020-01202-w
Peng Z, Chen W, Wang L, Ye Z, Gao S, Sun X, Guo Z (2015) Inhalation of hydrogen gas ameliorates glyoxylate-induced calcium oxalate deposition and renal oxidative stress in mice. Int J Clin Exp Pathol 8(3):2680–2689
Khan S, Glenton PA (2010) Experimental induction of calcium oxalate nephrolithiasis in mice. J Urol 184(3):1189–1196. https://doi.org/10.1016/j.juro.2010.04.065
Knight J, Jiang J, Assimos DG, Holmes RP (2006) Hydroxyproline ingestion and urinary oxalate and glycolate excretion. Kidney Int 70(11):1929–1934. https://doi.org/10.1038/sj.ki.5001906
Knight J, Holmes RP, Cramer SD, Takayama T, Salido E (2012) Hydroxyproline metabolism in mouse models of primary hyperoxaluria. Am J Physiol Renal Physiol 302(6):F688–F693. https://doi.org/10.1152/ajprenal.00473.2011
Vervaet BA, D’Haese PC, De Broe ME, Verhulst A (2009) Crystalluric and tubular epithelial parameters during the onset of intratubular nephrocalcinosis: illustration of the ‘fixed particle’ theory in vivo. Nephrol Dial Transplant 24(12):3659–3668. https://doi.org/10.1093/ndt/gfp418
Holmes RP, Assimos DG (1998) Glyoxylate synthesis, and its modulation and influence on oxalate synthesis. J Urol 160(5):1617–1624. https://doi.org/10.1016/S0022-5347(01)62363-2
Thamilselvan S, Khan SR, Menon M (2003) Oxalate and calcium oxalate mediated free radical toxicity in renal epithelial cells: effect of antioxidants. Urol Res 31(1):3–9. https://doi.org/10.1007/s00240-002-0286-x
Khan SR (2013) Reactive oxygen species as the molecular modulators of calcium oxalate kidney stone formation: evidence from clinical and experimental investigations. J Urol 189(3):803–811. https://doi.org/10.1016/j.juro.2012.05.078
Sun XY, Xu M, Ouyang JM (2017) Effect of crystal shape and aggregation of calcium oxalate monohydrate on cellular toxicity in renal epithelial cells. ACS Omega 2(9):6039–6052. https://doi.org/10.1021/acsomega.7b00510
Liu H, Ye T, Yang X, Liu J, Jiang K, Lu H, Xia D, Peng E, Chen Z, Sun F, Tang K, Ye Z (2019) H19 promote calcium oxalate nephrocalcinosis-induced renal tubular epithelial cell injury via a ceRNA pathway. EBioMedicine 50:366–378. https://doi.org/10.1016/j.ebiom.2019.10.059
Okada A, Hamamoto S, Taguchi K, Unno R, Sugino T, Ando R, Mizuno K, Tozawa K, Kohri K, Yasui T (2018) Kidney stone formers have more renal parenchymal crystals than non-stone formers, particularly in the papilla region. BMC Urol 18:19. https://doi.org/10.1186/s12894-018-0331-x
Tripathi YC, Jhumka Z, Anjum N (2015) Evaluation of total polyphenol and antioxidant activity of leaves of Bambusa nutans and Bambusa vulgaris. J Pharm Res 9(4):271–277
Bogucka-Kocka A, Zidorn C, Kasprzycka M, Szymczak G, Szewczyk K (2018) Phenolic acid content, antioxidant and cytotoxic activities of four Kalanchoë species. Saudi J Biol Sci 25(4):622–630. https://doi.org/10.1016/j.sjbs.2016.01.037
Yamaga M, Tani H, Yamaki A, Tatefuji T, Hashimoto K (2019) Metabolism and pharmacokinetics of medium chain fatty acids after oral administration of royal jelly to healthy subjects. RSC Adv 9(27):15392–15401. https://doi.org/10.1039/C9RA02991E
Longo N, Frigeni M, Pasquali M (2016) Carnitine transport and fatty acid oxidation. Biochim Biophys Acta 1863(10):2422–2435. https://doi.org/10.1016/j.bbamcr.2016.01.023
Peng Z, Chen W, Gao S, Su L, Li N, Wang L, Lou Z, Dong X, Guo Z (2014) Therapeutic effect of Xue Niao An on glyoxylate induced calcium oxalate crystal deposition based on urinary metabonomics approach. J Clin Biochem Nutr 55(3):184–190. https://doi.org/10.3164/jcbn.14-61
Acknowledgements
The authors are thankful to the Jaipur National University Jaipur for providing facilities such as a laboratory, library, journals, and Internet facility. The authors are grateful to Radhraraman College of Pharmacy, Bhopal, for providing the facility to perform experimental animal studies.
Plant authentication
The K. pinnata leaf, E. officinalis fruit, B. nutans shoot, and C. dactylon whole plant was collected between October 2014 and April 2015 from Rewa, Raisen, and Bhopal districts of M.P. Herbarium was prepared for all the four plants and authenticated by Dr. Zia UlHasan, Prof and Head, Herbarium Department, Department of Botany, Saifia College of Science Bhopal, and M.P. Voucher specimen no. 456/Bot/saifia/14 was allotted.
Funding
The authors declare that they have not received any funding for the study.
Author information
Authors and Affiliations
Contributions
PB: Conceptualized, designed the experiments, and edited the article text. AS: Performed the animal experiments, writing the original draft, review, and editing. BS: Checked the paper content and helped in data analysis. We declare that this work was done by the authors named in this article, and all liabilities pertaining to claims relating to the content of this article will be borne by the authors. The authors gave their individual critical revision and final approval of the version to be submitted.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experimental procedures and protocols used in the study were reviewed and approved by the Institutional Animal Ethical Committee (IAEC) in June 2014 (IAEC Approval No IAEC/RCP/JUN 2014/05), Radharaman College of Pharmacy, Bhopal (The registration number is 1169/PO/ReBi/S/08/CPCSEA). Laboratory animals were taken care of as per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). All experimental procedures were conducted in accordance with the ethical guidelines of CPCSEA, New Delhi.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bigoniya, P., Sohgaura, A.K. & Shrivastava, B. Antilithiatic effect of C. dactylon, E. officinalis, K. pinnata, and B. nutans ethyl acetate fraction on glyoxylate-induced nephrolithiasis. Futur J Pharm Sci 7, 79 (2021). https://doi.org/10.1186/s43094-021-00227-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-021-00227-1