Abstract
Background
Cardiovascular diseases have continued to be the leading cause of death globally. In addition, some of the drugs used in the treatment of the diseases present some adverse effects which limit the usefulness of such drugs. Thus, there is a need for novel drugs whose side effect is either minimal or non-existent. The presence of bioactive compounds in Cola hispida leaf is of great significance in the treatment and management of cardiovascular conditions. This study investigated the cardio-protective potential against doxorubicin (Dox)-induced cardiac infarction in rats.
Results
Dox induction resulted to muscle fiber degeneration in Dox-treated rats hence revealed significant (p < 0.05) elevation in the serum level of cardio biomarker enzymes and lipid peroxidation profile while significant (p < 0.05) fall in cardiac enzymatic antioxidant levels were observed relative to the normal control. Pre-treatment with ethyl acetate fraction of Cola hispida leaf expressed cardio-protective potentials against Dox-induced cardiotoxicity by significantly (p < 0.05) lowering the levels of cardiac biomarker enzymes towards normal, building up the activities of subdued antioxidant enzymes and depleting its malondialdehyde level. Histopathology photomicrograph of the heart tissues expressed myxomatous degeneration but was ameliorated through the administration of the fraction.
Conclusion
In accordance with the findings from this study, the administration of ethyl acetate fraction of Cola hispida leaf is effective against Dox-induced redox imbalance due to its enriched antioxidant phytoconstituents.
Similar content being viewed by others
Background
Cardiovascular diseases (CVD) are a class of disease conditions and structural problems that disrupt the cardiovascular system targeting the heart as well as the blood vessels of the heart and brain [1]. The sequential events leading to this CVD includes endothelial dysfunction, atherosclerosis plaque formation, and rupture of atherosclerotic lesion [2]. Cardiovascular diseases still maintain the principle reason of long-term morbidity and death among Western population [3]. Coronary artery disease (CAD) being the most prevalent cardiovascular disease condition involves the reduction of blood flow to the heart muscles as a result of plaque build-up in the heart [4]. Early detection of cardiotoxicity is measured by changes in the regional function of the heart using stress testing [5]. Several strategies adopted in the prevention of cardiotoxicity include dexrazoxane, beta blockers, angiotensin inhibitors (ACE), and probucol although their adverse effects limited their wider acceptance [6]. Doxorubicin (Dox), an anti-neoplastic drug, belonging to the anthracycline family expresses strong binding cooperativity to a phospholipid, cardiolipin resulting in an irreversible complex in the inner mitochondrial membrane. This disrupts the cardiolipin protein interface giving rise in the formation of superoxide anion radical, increasing the reactive oxygen species (ROS) level in the body system, hence induces different form of tissue toxicity and cardiomyocyte death [7,8,9,10]. The principle mechanism by which doxorubicin induces cardiotoxicity (DIC) varies depending on the diseases although mostly results from increased oxidative stress [7,8,9], iron metabolism [11], nitric oxide syntheses [8] and calcium homeostasis dysregulation [12].
However, there is rise in demand for alternative treatment of cardiotoxicity since nature’s chemical diversity and complexity has provided mankind with a wide range of molecules consisting diverse therapeutic benefits [13]. Cola hispida belongs to the family of Sterculiaceae, which is native to Sierra Leone, Ivory Coast, Ghana, and Nigeria. Although a lowland tree, it is also found in fertile areas, with altitudes of about 200 m [14]. Its make-up comprises of long, ovoid leaves of leathery texture bearing yellow flowers as well as a star-shaped fruit. The nuts have an after bitter taste and are enriched with 2–4% caffeine and some alkaloids. They serve as host gift to guest during bride price negotiation [15]. Traditionally, the nuts ease tiredness, hunger pangs, inhibits sleep, enhances mental activity, and as well used as dye while the pods are used in the production of fertilizers and soap and serve as a substitute in poultry feeds. In traditional medicine, the nuts when grounded and mixed with honey aids digestion and are used to relieve cough [16]. Since this species of cola seem quite rare and has not been clinically investigated, this paper seeks to investigate the cardio-protective effect of ethyl acetate fraction of Cola hispida leaf in doxorubicin-induced myocardial injury in rats.
Methods
Ethical approval
This research was approved by the ethical committee of our institution. The rats were handled according to the guidelines of the National Institute of Health (NIH) on the care and use of laboratory animals.
Drug and chemicals
All the chemicals, reagents, and drug used for the research were of analytical grade.
Collection of sample
The leaves of Cola hispida plant were freshly obtained from Ajuona Nsukka Local Government Area of Enugu State, without causing any damage to the plant. The leaves were identified by Ozioko Alfred, a taxonomist at Bioresources Development and Conservation Programme, Nsukka, Enugu State, Nigeria. The plant leaves were deposited in the herbarium of Bioresources Development and Conservation Programme, Nsukka, Enugu State, Nigeria, with a voucher number: InterCEDD/16304.
Plant material preparation
Cola hispida leaves were thoroughly washed using distilled water, kept at room temperature to dry, then grounded into a fine powder for extraction. A known quantity of the sample (750 g) was softened in 3.75 L methanol and kept for 48 h at room temperature. The mixture was filtered using Whatman No. 1 filter paper and then concentrated using the rotary evaporator to obtain chocolate-like semi-solid extract.
Solvent-solvent partitioning (fractionation) of Cola hispida methanol leaf extract
A quantity (20 g) of the crude extract was further re-extracted by the process of partitioning using 20% methanol, ethyl-acetate, and n-hexane in order of increasing polarity. This quantity (20 g) of the crude methanol extract was re-dissolved into 250 ml of 20% of the solvent followed by addition of n-hexane in the same proportion, stirred to mix and then allowed to settle on standing for 20 min for complete separation into two partitions; then, the n-hexane partition was collected in a flat bottom flask. The above process was repeated three times with the remaining 250 ml of 20% methanol until the n-hexane partition is colorless. Then, 250 ml of ethyl-acetate was added to the remaining partition of the 250 ml of 20% methanol; the ethyl-acetate partition was collected into flask. The process was repeated three times until the ethyl-acetate partition became colorless. The different solvent partitions were collected and concentrated, and the solvent (ethyl-acetate) which showed a better result in the pilot study was selected for further studies.
Screening of secondary metabolites
Phytochemical screening of the Cola hispida leaf constituents was in accordance with the method of Harborne [17] and Trease and Evans [18]. Quantitative analyses were done as described by Harborne [19] and Soni and Sosa [20].
Determination of cardioprotective and antioxidant properties of Cola hispida leaf fraction
A total of thirty (30) experimental albino rats (187–224 g) were purchased from Department of Zoology and Environmental Biology and kept in aluminum cages in an animal house with temperatures steadied at 25 °C and 55% relative humidity. All the experimental animals were allowed for 7 days of acclimatization with daily access to their feed and water all through the experiment. The advisory notes of the National Institute of Health on the management of research animals were maintained.
Treatment procedures
The thirty experimental albino rats were separated into six experimental groups (A, B, C, D, E, and F) in a random manner, each consisting of five experimental albino rats. A 100 mg kg−1 b.wt of vitamin E and varied oral administration of the fraction was given to groups C and D–F respectively while groups A and B received only distilled water and were also fed for 21 days after acclimatization.
Induction of myocardial injury
On the 21st day, after 2 h of fraction and vitamin E administration, group B–F were intraperitoneally treated with equal doses of 2.5 mg kg−1 b.wt of Dox while group A was administered only distilled water.
Group A: distilled water only
Group B: 2.5 mg kg−1 b.wt Dox only
Group C: 100 mg kg−1 b.wt vitamin E + 2.5 mg kg−1 b.wt Dox
Group D: 100 mg kg−1 b.wt, fraction + 2.5 mg kg−1 b.wt Dox
Group E: 200 mg kg−1 b.wt, fraction + 2.5 mg kg−1 b.wt Dox
Group F: 400 mg kg−1 b.wt, fraction + 2.5 mg kg−1 b.wt Dox
Collection of blood sample
This followed the procedure described by Agbafor et al. [21] which after an overnight fast, blood was collected through cardiac puncture after the rats were euthanized using chloroform. The blood samples were stored in a plain specimen container with no anticoagulant. The rats were humanely sacrificed, and the hearts were immediately surgically cut out, perfused with cold normal saline and homogenized using 0.25 M sucrose in 0.2 M phosphate buffer at pH 7.4.
Determination of cardioprotective property of Cola hispida
The Randox kits were used to determine the serum level of creatine kinase (CK), lactate dehydrogenase (LDH), and aspartate aminotransferase (AST) following manufacturer’s guidelines.
Heart homogenate preparation
The heart tissue was homogenized in 1.5% KCl with 10 mM phosphate buffer, pH 7.4 in ethylenediaminetetraacetic acid (EDTA), and centrifuged at 12,000 rpm for 20 min. An aliquot of the supernatants’ prepared tissue homogenate was drawn for each biochemical analysis.
Determination of antioxidant property
The concentration of malondialdehyde (MDA) of the tissue homogenate was measured following the method described by Wallin et al. [22]. Myocardial activities of the antioxidant enzymes: catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx) were assayed respectively by methods described by Fridovich [23], Aebi [24], and Paglia and Valentine [25].
Estimation of nutrient constituents of Cola hispida fraction
The method described by AOAC [26] and Pearson [27] was used to determine the vitamin and mineral presence respectively.
Preparation of tissue
The tissue samples were trimmed, washed, and dehydrated using ethanol in increasing percentage (60, 70, 80, and 90% proportions). The tissue samples were cleaned in xylene, immersed in paraffin, and broken up into sections at about 5-μ thickness. They were then treated with hematoxylin and eosin (H and E stain) and viewed at ×400 magnifications [28].
Data analysis
Analysis of data was done using SPSS version 21.0 on the test statistic, one-way analysis of variance (ANOVA), and Duncan Multiple Range Test to ascertain the statistical difference among the mean and the interaction between the variables. The statistical differences obtained were adjudged statistically significant at p<0.05.
Results
Preliminary phytochemical profiling of Cola hispida leaf extract
The phytochemical evaluation of methanol extract of Cola hispida leaves expressed the active ingredients, terpenoids, saponins, flavonoids, alkaloids, tannins, and glycosides in different proportions as shown in Table 1
Preliminary mineral and vitamin content profiling of Cola hispida leaf extract
The results showed the presence of the mineral elements potassium, iron, zinc, and copper, and the vitamins (A, C, and E). The concentration of potassium ions was higher than iron, zinc, and copper. At the same time, vitamin C showed highest concentration of non-enzymatic antioxidant level followed by vitamins E and A as presented in Table 2.
Effects of Cola hispida leaf ethyl acetate fraction on cardiac biomarker enzymes of albino rats
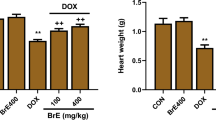
After the intraperitoneal administration of Dox to groups B–F, a significant (p<0.05) increase in the serum level of CK, LDH, and AST were observed in the Dox untreated group when compared with the control while pre-treatment with the graded doses of Cola hispida leaf fraction significantly reversed and ameliorated the cardiotoxicity of Dox-induced in albino rats as shown in Table 3. Moreover, pre-treatment with 100 mg kg−1 b.wt of vitamin E expressed a significant (p<0.05) decrease in the serum levels of the cardiac biomarker enzymes when compared with the control. However, a decrease in the serum level of CK, LDH, and AST was observed as the doses increased although at the highest dose of 400 mg kg−1 b.wt, the fraction significantly suppressed the toxic effect of Dox on LDH and AST more than the standard drug.
Effects of Cola hispida leaf ethyl acetate fraction on cardiac biomarker enzymes of albino rats
After the intraperitoneal administration of Dox to groups B–F, a significant (p<0.05) increase in the serum level of CK, LDH, and AST were observed in the Dox untreated group relative to the control while pre-treatment with the graded doses of Cola hispida leaf fraction significantly reversed and ameliorated the cardiotoxicity of Dox induced in albino rats as shown in Table 3. Moreover, pre-treatment with 100 mg kg−1 b.wt of vitamin E expressed a significant (p<0.05) decrease in the serum levels of the cardiac biomarker enzymes relative to the control (Table 3). However, a decline in the serum level of CK, LDH, and AST was noted as the doses increased. Although at the highest dose of 400 mg kg−1 b.wt, the fraction significantly suppressed the toxic effect of Dox on LDH and AST more than the standard drug.
Histopathological study of heart tissues
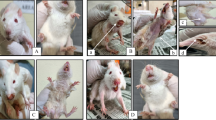
The photomicrograph of the heart tissue expresses variable degree of degeneration as represented in Fig. 1a–f. Group A having three tunics (intima, media, and adventitia) joined end to end revealed passive occlusion. Group B that received Dox only showed multifocal area with severe hyperemic condition, and degeneration of muscle fibre muscle fiber when compared to groups A, C, D, E and F. Plate C expresses mixed cellular infiltrates while plate D revealed minor loss of myofibrils with slender threads of muscle fiber. More so, plate E shows a myxomatous degeneration accompanied with thickened ventricular surface of mitral valves and F observed with homogenous mononuclear cells not associated with cardiomyopathy. Meanwhile, the group treated with 400 mg kg−1 b.wt fraction and 100 mg kg−1 b.wt vitamin E expressed some similarity with the normal control.
Discussion
This study focused on protective effects of ethyl acetate fraction of Cola hispida leaf in Dox-induced cardio-toxic rats. The phytochemistry of ethyl acetate fraction of Cola hispida leaf showed the presence of flavonoids, terpenoids, alkaloids, saponins, tannins, and glycosides. Dox being a cationic drug causes tissue (heart) toxicity and as such triggers lipid peroxidation through the generation of certain free radicals that harm the cells if not in control by the system [29]. This could damage the myocardial cells leading to decrease in CK, AST, and LDH serum level concentration or impairment in their activities and biological functions of the heart because these enzymes leak into the extracellular fluids. However, the leak can be avoided by preserving the myocardium membrane [30].
The present study showed significant (p < 0.05) increase of CK, AST, and LDH serum level relative to the normal control after Dox administration. This could be attributed to the enzymes leaking into the extracellular fluid as a result of myocardial cell membrane. Pretreatment of the albino rats with Cola hispida leaf fraction (100, 200, and 400 mg kg−1 b.wt) significantly (p<0.05) altered and reversed further increase in the serum level of CK, AST, and LDH brought about by Dox-induced toxicity. Moreover, Cola hispida leaf fraction showed protective capacity of the cell membranes from damage by preventing the leak of the cardiac biomarkers. This may give credit to the potency of Cola hispida leaves in cardio-protection of myocardial membrane. Vitamin E also helps in maintaining the integrity of the cardiac cells by its capacity to effectively break chain reaction of free radicals, hence exhibiting antioxidant properties to ameliorate lipid peroxidation while vitamin C which is crucial in regenerating vitamin E, beta-carotene, and other carotenoids also produce antioxidant capacity to lipid-rich tissues, hence provide a robust cell membrane [31, 32]. The significant (p<0.05) reduction in the cardiac MDA concentrations after the administration of different doses of the fraction indicate cardio-protective potency of Cola hispida leaf fraction against the Dox-induced lipid peroxidation [33]. This protective effect of Cola hispida leaf could be attributed to its antioxidant property, thus ethyl acetate fraction of Cola hispida leaf could be used to restore affected biochemical profiles induced by Dox exposure or any other cardiotoxic substance to enhance membrane stabilization [34]. Also, the presence of phytochemicals and secondary metabolites could be responsible for the antioxidant activity of the fraction [26, 35] (Table 4).
The decrease in SOD, CAT, and GPx serum levels in the heart tissues of rats treated with Dox only may be due to Dox metabolite overload hence impairing the antioxidant activity of these enzymes since heart tissue is especially prone to free radical damages due to its low amounts of detoxifying enzymes [36]. Pre-treatment with varying doses of the fraction prior to Dox significantly (p<0.05) reversed the Dox-induced reduction in activity of the antioxidant enzymes. Administration of vitamin E and the graded fraction showed significantly (p˂0.05) expressed increase in these antioxidant enzyme activities suggesting protective attributes of Cola hispida leaves phytoconstituents [37]. Meanwhile, the presence of vitamins C and E in Cola hispida leaf extract could have enhanced the enzymatic antioxidant activities. The iron, copper, zinc, and magnesium ions present in the Cola hispida extract are co-factors to some of these enzymes hence may have enhanced the antioxidant activity. The dose-dependent reversal activity of the fraction on myocardial necrosis to normal histoarchitectural structure of the heart may suggest membrane stabilization and cytoprotective attributes of Cola hispida leaf fraction.
Conclusion
The decrease in glutathione peroxidase, superoxide dismutase, and catalase as well as increased AST, LDH, and CK of experimental albino rats treated with Dox only showed cardiovascular impairment resulting from damage to the cardiac cell membrane. However, the increase in activities of these enzymes when vitamin E and Cola hispida leaf fraction were administered suggests the restoration of the cell membrane integrity and antioxidant property by Cola hispida leaves and could therefore serve as a robust drug to enhance cardioprotective potency of the heart.
Availability of data and materials
All data and material are available upon request.
Abbreviations
- CVD:
-
Cardiovascular diseases
- CAD:
-
Coronary artery disease
- CK:
-
Creatine kinase
- LDH:
-
Lactate dehydrogenase
- AST:
-
Aspartate aminotransferase
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- GPx:
-
Glutathione peroxidase
- MDA:
-
Malondialdehyde
References
Tian D, Meng J (2019) Exercise for prevention and relief of cardiovascular disease: prognoses, mechanisms, and approaches. Oxid Med Cell Longev:1–11. https://doi.org/10.1155/2019/3756750
Kyoung HP, Woo JP (2015) Endothelial dysfunction: clinical implications in cardiovascular disease and therapeutic approaches. J Korean Med Sci 30(9):1213–1225. https://doi.org/10.3346/jkms.2015.30.9.1213
Patricia M, Sol GO, José MV, Martín A, Victor MV, Maria DM (2019) Targeting early atherosclerosis: a focus on oxidative stress and inflammation. Oxid Med Cell Longev 8:1–32. https://doi.org/10.1155/2019/8563845
Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H (2016) Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet 388(10046):761–775. https://doi.org/10.1016/S0140-6736(16)30506-2
National Heart, Lung and Blood Institute (2017). https://www.nhlbi.nih.gov/health/health-topics/topics/hdw/signs. Accessed 4th August 2020.
Lipshultz SE, Scully RE, Lipsitz SR (2010) Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia. Long-term follow-up of a prospective, randomised, multicentre trial. Lancet Oncol 11(10):950–961. https://doi.org/10.1016/s1470-2045(10)70204-7
Raj S, Franco SI, Lipshultz SE (2014) Anthracycline-induced cardiotoxicity: a review of pathophysiology, diagnosis, and treatment. Curr Treat Options Cardiovasc Med 16(6):315–322. https://doi.org/10.1007/s11936-014-0315-4
Ghigo A, Li M, Hirsch E (2016) New signal transduction paradigms in anthracycline-induced cardiotoxicity. Biochimica et BiophysicaActa. 1863(7):1916–1925. https://doi.org/10.1016/j.bbamcr.2016.01.021
Varricchi G, Ameri P, Cadeddu C, Ghigo A, Madonna R, Marone G, Mercurio V, Monte I, Novo G, Parrella P, Pirozzi F, Pecoraro A, Spallarossa P, Zito C, Mercuro G, Pagliaro P, Tocchetti CG (2018) Antineoplastic drug-induced cardiotoxicity: a redox perspective. Front Physiol 9:167. https://doi.org/10.3389/fphys.2018.00167
Qinggong WT, Wei D, Xuejuan Z, Xiaoyu J, Tao Y, Wanpeng Y, Zhijuan L, Jianxun W (2019) miR-499-5p attenuates mitochondrial fission and cell apoptosis via p21 in doxorubicin cardiotoxicity. Front Genet 9:734. https://doi.org/10.3389/fgene.2018.00734
Octavia Y, Kararigas G, Boer M (2017) Folic acid reduces doxorubicin-induced cardiomyopathy by modulating endothelial nitric oxide synthase. J Cell Mol Med 21(12):3277–3287. https://doi.org/10.1111/jcmm.13231
Mitry MA, Edwards JG (2016) Doxorubicin induced heart failure: Phenotype and molecular mechanisms. Int J Cardiol Heart Vasc 10:17–24. https://doi.org/10.1016/j.ijcha.2015.11.004
Gordon MC, David JN (2013) Natural products: a continuing source of novel drug leads. Biochim. Biophys. Acta 1830(6):3670–3695. https://doi.org/10.1016/j.bbagen.2013.02.008
Burdock GA, Carabin IG, Crincoli CM (2009) Safety assessment of Kola nut extract as a food ingredient. Food Chem Toxicol 47(8):1725–1732. https://doi.org/10.1016/j.fct.2009.04.019 PMID: 19394393
BIN O, Pritchett W (2003) The Igbos and their traditions (PDF), p 1 Accessed on 30 July 2019
Odebunmi EO, Oluwaniyi OO, Awolola GV, Adediji OD (2009) Proximate and nutritional composition of Kola nut (Cola nitida), bitter cola (Garcinia cola) and alligator pepper (Afromomum melegueta). Afr J Biotechnol 8(2):308–310. https://doi.org/10.4314/ajb.v8i2.59797
Harborne JB (1973) Phytochemical methods: a guide to modern techniques of plant analysis. Chapman and Hall Ltd, London, p 279
Trease GE, Evans WC (2002) Pharmacognosy. 15th Edn. Saunder Publishers, London, pp 42–44 221 -229, 246 – 249, 404 -306, 331-332, 391-393
Harborne JB (1998) Phytochemical methods: a guide to modern technique of plant analysis. Champman and Hall, London
Soni A, Sosa S (2013) Phytochemical analysis and free radical scavenging potential of herbal and medicinal plant extracts. J Pharmacogn Phytochem 2(4):22–24 Corpus ID: 41059142
Agbafor KN, Ezeani C, Akubugwo EI, Obiudu IK, Uraku AJ, Ogbanshi ME, Edwin N, Ugwu OPC (2015) Cardioprotective effect of leaf and root extracts of Newbouldia leavis against carbon tetrachloride induced cardiotoxicity in albino rats. Eur J Med Plant 9(3):1–7. https://doi.org/10.3923/jas.2017.407.414
Wallin B, Rosengren B, Shertzer HG, Camejo G (1993) Lipoprotein oxidation and measurement of TBARS formation in a single microliter plate: its use for evaluation of antioxidants. Anal Biochem 208(1):10–15. https://doi.org/10.1006/abio.1993.1002
Fridovich I (1989) Superoxide dismutase: an adaptation to a pragmatic gas. J Biol Chem 264(14):7761–7764 PMID: 2542241
Aebi HE (1983) Catalase. In: Bergmeyer HU Ed., Methods of enzymatic analysis. Verlag Chemie, Weinhem, pp 273–286.
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70(1):158–169 PMID: 6066618
AOAC (2003) Official Methods of Analysis. (17th edition). Association of Official Analytical Chemists, Virginia U.S.A, pp 408–426
Pearson D (1976) The chemical analysis of food, 17th edn. Churchill Livingstone, London, pp 3–4 ISBN: 0443014116
Drury RA, Wallington A, Cameroun SR (1967) Carleton’s histological techniques. Oxford University Press, New York, pp 1–420 Bookmark: https://trove.nla.gov.au/version/12440997
Canan GN, Laurent D (2018) Updates in anthracycline-mediated cardiotoxicity. Front Pharmacol. 9:1262. https://doi.org/10.3389/fphar.2018.01262
Balakrishna S, Shiva SR, Shivalinge GK (2015) Cardioprotective effects of ethanolic leaf extract of Ipomoea batatas on doxorubicin induced cardiotoxicity in rats. Asian J Pharm Clin Res 8(2):444–450 Corpus ID: 53139183
Kurutas EB (2015) The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J 15(1):71–81. https://doi.org/10.1186/s12937-016-0186-5
Njoku UO, Ogugofor MO, Nwodo OFC (2017) Treatment with methanol extract of Ficus capensis stem bark protects against changes in biomarker levels of carbontetrachloride-induced cardiotoxicity of rats. J Appl Sci 17:407–414. https://doi.org/10.3923/jas.2017.407.414
Renu K, Abilash VG, Tirupathi PB, Arunachalam PS (2017) Molecular mechanism of doxorubicin-induced cardiomyopathy–an update. Eur J Pharmacol 818:241–253. https://doi.org/10.1016/j.ejphar.2017.10.043
Patrice T, Rozec B, Sidoroff A, Blanloeil Y, Despins P, Perrigaud C (2016) Influence of vitamins on secondary reactive oxygen species production in sera of patients with resectable NSCLC. Diseases 4(3):25. https://doi.org/10.3390/diseases4030025
Akintehinse OV, Alli-Smith YR, Olasehinde RO, Omiyale BO, Enye LA, Owoseni JS, Fakunle JB (2016) Phytochemical screening and proximate analysis of young Cola acuminata leaves. Unique Res J Med Med Sci 4(5):029–034
Ying W, Robyn B, Alycia N, Siegfried H (2018) Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. J Cell Biol 217(6):1915–1928. https://doi.org/10.1083/jcb.201708007
Cinzia F, Francesco F, Manuela B, Stefano P, Antonio F, Daniela A, Sandro N, Giorgia V, Roberto N, Simone B, Claudio T, Ravirajsinh NJ (2019) Beneficial role of phytochemicals on oxidative stress and age-related diseases. BioMed Res Int 87:482–493
Acknowledgements
The authors wish to acknowledge Mr. Alfred Ozioko who authenticated the plant material, and Mr. Mbaoji of Department of Pure and Industrial Chemistry, University of Nigeria Nsukka, for his assistance.
Plant authentication
The plant leaves were identified by Mr. Ozioko Alfred, a taxonomist at Bioresources Development and Conservation Programme, Nsukka, Enugu State, Nigeria. The plant leaves were deposited in the herbarium of Bioresources Development and Conservation Programme, Nsukka, Enugu State, Nigeria, with a voucher number: InterCEDD/16304.
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
NOU and OMO designed the study. UCL fed the experimental animals and administered the plant fraction and standard drug. OMO and UCL collected the blood sample from the experimental animals while UCL and OMO evaluated the different biomarkers under study. OMO carried out the statistical analysis of the data. NOU interpreted the results. All the authors contributed in preparing the manuscript. All the authors have read and approved the manuscript for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This research was approved by the ethical committee of Faculty of Biological Sciences, University of Nigeria Nsukka, Enugu State, Nigeria, with an approval number: UNN/FBS/EC/1035. The rats were handled according to the guidelines of the National Institute of Health (NIH) on the case and use of laboratory animals.
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Umenwanne, C.L., Ogugofor, M.O. & Njoku, O.U. Ethyl acetate fraction of Cola hispida leaf protects against doxorubicin-induced myocardial injury in male albino rats. Futur J Pharm Sci 7, 61 (2021). https://doi.org/10.1186/s43094-021-00207-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-021-00207-5