Abstract
Background
The fruits of Xylopia aethiopica and seeds and leaves of Piper guineense are commonly used as spices. Due to their wide use in nutrition and traditional medicine, there is need to examine the biochemical and histological effects of ethanolic extracts of fruits of Xylopia aethiopica and seeds and leaves of Piper guineense on liver and kidney function in male albino rats. The test animals (groups 2, 3 and 4) received 100 mg/kg each of the corresponding extract for 21 days. Group 1 served as normal control and received a placebo of normal saline. The animals were later fasted overnight, sacrificed and their blood collected through cardiac puncture for biochemical indices.
Results
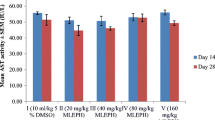
AST activity decreased significantly (p < 0.05) in rats of group 2 compared to the normal control; ALP decreased significantly (p < 0.05) in animals of group 3; while ALT increased significantly (p < 0.05) in group 4. The concentrations of urea and potassium showed significant decrease (p < 0.05) in animals of group 3. Creatinine increased significantly (p < 0.05) in group 2. Concentrations of total protein, albumin, globulin, sodium and chlorides in the test animals did not show any significant alteration. WBC count increased significantly (p < 0.05) in rats of group 3 and non-significantly (p > 0.05) in groups 2 and 4. PCV increased significantly in rats of group 4 and non-significantly in groups 2 and 3. RBC count and Hb levels increased non-significantly (p > 0.05) in all the test groups compared to group 1. The histoarchitectural states of the liver and kidneys showed no apparent alteration by the different extracts.
Conclusion
The extracts have no apparent toxic effect on the liver and kidneys of the experimental rats, although fruits’ extract of Xylopia aethiopica showed possibility of exhibiting mild liver toxicity.
Similar content being viewed by others
Background
Medicinal plants are widely known to exhibit different positive effects in animals, especially humans. Most spices are used for the management or treatment of some diseased conditions in herbal medicine. Some of which include fruits of Xylopia aethiopica (X. aethiopica) and seeds and leaves of Piper guineense (P. guineense).
P. guineense (Family: Piperaceae) a tropical plant of West African origin [1] is popularly known as “African black pepper.” In Nigeria, it is commonly called “Uziza” in Igbo and “Iyere” in Yoruba. Its seeds and leaves are used as spices in preparation of certain types of foods such as the popular “hot soup” or “pepper soup” usually consumed by nursing mothers after childbirth to aid uterine contraction and consequently, placenta expulsion or the expulsion of some remains in the woman’s womb [2]. In traditional medicine, the leaves have been associated with management of problems of infertility in women and for treating respiratory ailments [3]. Its parts are used in herbal medicine for treatment of rheumatic pains. Researchers have reported that the seeds and leaves possess antiparasitic and antimicrobial activities [4, 5]. Some people use the seeds as an aphrodisiac. Uhegbu et al. [1] reported the effect of aqueous extract of the seeds on antioxidant enzymes, liver marker enzymes and indices of haematology in albino rats.
X. aethiopica (Family: Annonaceae) popularly called “African pepper” is reported to grow in forest zones and often along rivers and in arid areas [6]. The colour of the matured fruit usually changes from green to brownish-black after drying. In Nigeria, it is commonly called “Uda” in Igbo, “Erunje” in Yoruba and “Kimba” in Hausa [7]. The fruits are also used in preparation of hot soup usually given to nursing mothers after childbirth. Fleischer [8] reported that the fruit has antipyretic and anti-inflammatory properties, while Lajide [9] and Burkil [10] reported that fruit extracts are useful in bronchitis, oedema and dysentery. The analgesic and anti-helminthic activities of the fruit have been reported [11], while another study reported its use as purgative, carminative and in treating cough [12]. The use of the dried root for treating toothache and pyorrhea and the use of the back extract in the management of rheumatism, stomach aches and asthmatic attack has been reported [13, 14]. In Nigeria, a formulation of the leaves and roots are used for treating fever [10]. The fruit of X. aethiopica contains some constituents that could synergistically improve other food materials that are used in nutrition and appreciable level of certain mineral that supports the catalysis of some enzymes and maintenance of homeostasis and immune function [7].
X. aethiopica- and P. guineense-treated rats showed that these spices have hypo-kalaemic, hypolipidaemic and no or low hepatotoxic effects [15]. The seeds’ extract possesses hepatoprotective and antioxidant properties comparable to that of Livolin forte [16]. Cold-water fruit extract of X. aethiopica has been reported to cause a significant increase in creatinine level of rabbits, indirectly affecting the renal function, while ethanolic and the hot-water extracts had no significant effect on the kidney function of the animals [17]. It is believed that the use of ethanol in extraction may give good yield of their chemical constituents. It is important that the biochemical and histological effects of these plant materials be investigated to ascertain their likely effects on its consumers. There is therefore the need to investigate the comparative biochemical and histological effects of ethanolic extracts of fruits of X. aethiopica and seeds and leaves of P. guineense on liver and kidney of male albino rat. Fruits of X. aethiopica and seeds and leaves of P. guineense are widely used as spices in nutrition. Due to their wide use nutrition and traditional medicine, there is need to examine their effects as designed in this current study. This will enable the consumers note the possible effects on liver and kidney.
Methods
Plant materials
Seeds and leaves of P. guineense and fruits of X. aethiopica (Figs. 1, 2 and 3) used in this study were purchased from New Market in Wukari, Taraba State, Nigeria.
Preparation of extracts
The plant materials were cleaned, air-dried and pulverized to fine powder using laboratory manual blender. The powder of each sample was macerated in 70% ethanol for 2 days with intermittent shaking and filtered. The filtrate was then concentrated using rotary evaporator. The extracts were re-dissolved in normal saline and stored in a refrigerator prior to administration.
Experimental animals
Twenty healthy male albino rats, aged 7 weeks, weighing 87–103 g, were used in this study. They were allowed to acclimatize for 14 days prior to the start of experiment. All animals were allowed access to feed and water ad libitum throughout the experiment. Standard laboratory protocols for animal studies were followed. All methods were performed in accordance with the relevant guidelines and regulations.
Experimental design
A modified method of Uhegbu et al. [1] was used. The animals were randomly distributed into four groups of five animals each. Rats of group 1 served as normal control and were administered a placebo of normal saline, while rats of groups 2, 3 and 4 served as test animals. Animals of group 2 were administered ethanolic seeds’ extract of P. guineense (100 mg/kg body weight); group 3 animals received ethanolic leaves’ extract of P. guineense (100 mg/kg body weight); and group 4 animals received ethanolic fruits’ extract of X. aethiopica (100 mg/kg body weight). The different plant extracts were administered to the corresponding test animals orally for a period of 21 days. This is because many people consume the plant materials for a moderate or a long period, hence the 21-day period in this study.
Blood collection
After administration of the extracts, the animals were starved overnight, anaesthetized with chloroform and sacrificed by cervical dislocation. Blood samples were collected by cardiac puncture using a 10-mL hypodermic syringe. The blood samples were dispensed into two different types of tubes. The first part was dispensed into sample tubes containing an anti-coagulant for haematological analysis. The second part was dispensed into a clean plain sample tubes and allowed to clot for about 15 min and centrifuged at 4000 rpm for 10 min. The serum was separated from the clot by simple aspiration with Pasteur pipette and dispensed into clean tubes for serum biochemical analysis.
Biochemical and histological analysis
Serum biochemistry was carried out on each sample. The concentrations of aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), total protein, albumin, electrolytes (sodium, potassium and chlorides), urea and creatinine were determined using auto-analyser (Cobas C111 Chemistry analyzer). The level of the selected haematological indices including haemoglobin (Hb), red blood cells (RBC) count, packed cell volume (PCV) and white blood cells (WBC) count were determined using haematological auto-analyser (Abacus 380). After the animals were sacrificed; the liver and kidneys of all animals in the four groups were harvested and examined histologically (Stain: haematoxylin and eosin; magnification × 200). Photomicrographs of different liver and kidney sections were taken.
Statistical analysis
The biochemical results were analysed statistically with the use of one-way analysis of variance (ANOVA) and further with Duncan’s multiple comparison using Statistical Package for Social Sciences (SPSS) version 21. The means were compared for significance at p ≤ 0.05 and the group results were presented as mean ± SD (n = 5).
Results
The results of the study are presented in Tables 1, 2, 3, 4 and 5 and Figs. 4, 5, 6, 7, 8, 9, 10 and 11.
Discussion
Selected liver maker enzymes AST, ALT and ALP levels were reduced in the rats administered ethanolic extracts of seeds and leaves of P. guineense but increased in the rats administered ethanolic extract of fruits of X. aethiopica (Table 1). The results suggest that seeds and leaves extract of P. guineense have the ability to protect organs such as the liver and the kidneys. This result is in agreement with the report of Uhegbu et al. [1]. The seeds’ and the leaves’ extracts may contain chemical constituents that may possess hepato-protective or renal-protective effects. Administration of fruits’ extract of X. aethiopica elevated the level of the enzymes above normal values, thereby suggesting a toxic state and possible infiltration of the liver or kidney tissues. The histoarchitectural states of the liver (Figs. 4, 5, 6 and 7) and kidneys (Figs. 8, 9, 10 and 11) agree with this result. This may arise as a result of a malfunctioning cell membrane of the organs, thereby suggesting cellular leakage of the enzymes into the blood [18, 19]. It is possible that despite the acclaimed medicinal effects of fruits’ extract of X. aethiopica, it may contain certain toxic chemicals that may lead to toxic effects when administered for a long period as served in this study. Fruits’ extract of Xylopia aethiopica causes infiltration on the liver cells’ membrane.
The concentration of albumin in the experimental animals (when compared to the normal control) increased non-significantly in the group administered seeds’ extract of P. guineense and the group administered fruits’ extract of X. aethiopica but reduced non-significantly in the group administered leaves’ extract of P. guineense. Globulin reduced non-significantly in all the groups administered the different extracts. This result (Table 2) showed seeds’ extract of P. guineense and fruits’ extract of X. aethiopica may encourage protein synthesis more than leaves’ extract of P. guineense. This result also showed that consumption of the three plant materials in nutrition may aid or stabilize processes associated with protein biosynthesis, thereby supporting effective liver function. Liver damage and its synthetic functions may be assessed using serum protein level [20], since the hepatocytes are mostly responsible for synthesizing most proteins distributed in the plasma [21]. The result therefore suggests that the liver may be functioning properly.
The liver and kidneys work in synergy to maintain homeostasis in the body. This ensures the proper excretion of waste materials and reabsorption of some useful materials by the kidneys. Creatine is produced in the liver before being distributed into circulation. Creatine phosphate metabolism produces the waste product creatinine which should be excreted by the kidneys. When creatinine and urea are retained in the blood, it shows a possible impairment of the kidneys [22]. The results of this study (Table 3) showed that creatinine was retained in the animals administered seeds’ extract of P. guineense and fruits’ extract of X. aethiopica, but was not retained in the animals administered leaves’ extract of P. guineense when compared with the normal control animals. This showed the constituents of seeds’ extract of P. guineense and the fruits’ extract of X. aethiopica may possess renal toxicity. Seeds’ extract of Piper guineense caused impaired kidney function. Renal diseases that reduce glomerular filtration may result in retention of urea which is the major end product of protein catabolism [23]. The result of this study showed urea was properly excreted in all groups administered the different extracts when compared to the control. This result also showed that leaves’ extract of P. guineense encouraged elimination of urea by the kidney better than the other extracts. Increase in the level of blood urea has been reported to result from inability of impaired kidney to filter urea up to normal levels [24].
Healthy functioning of the kidneys, heart and liver can be assessed using the electrolytes balance in the blood. When the level of serum or plasma electrolytes are abnormal, it is believed that the kidney function has been impaired [25]. Also, electrolyte balance could show the possibility of proper maintenance of homeostasis. The results of this study (Table 4) showed that the concentrations of the serum electrolytes (chlorides and sodium) were not significantly altered in all groups administered the different plant extracts, except potassium which reduced significantly in the group administered leaves’ extract of P. guineense when compared with the control. This shows that the different plant extracts do not adversely interfere with electrolytes balance, thereby suggesting a possible good interaction between the liver and the kidneys.
WBC count has been reported to be a common immune function index which aids in defence against diseases and its causative pathogens and in promoting the immune system [18]. Metcalf [26] reported that white blood cells originate from pluripotent haemopoietic stem cells. Stress is known to influence the rate of white blood cells production, while green leafy vegetables are believed to improve the compositions of blood. In this study, administration of the three plant extracts caused an increase in the level of WBC count when compared to the control (Table 5). This shows the three plant extracts are able to stimulate WBC production. Leaves’ extract of P. guineense increased the production of the WBC more than seed extract of P. guineense and fruit extract of X. aethiopica. Leaves’ extract of Piper guineense caused leukocytosis.
There was a non-significant increase in the level of RBC counts in all the groups administered the extracts compared with the control. This shows the plant extracts could mildly promote erythropoiesis. The result also showed that leaves’ extract of P. guineense may cause an increase in the production of RBC more than seed extract of P. guineense and fruit extract of X. aethiopica. There was an increase in the levels of Hb and PCV in all the test animals compared to the values in control animals (Table 5). Consumption of plant foods usually increase Hb synthesis due to their high vitamins and minerals content [27] that may stimulate the production of globin which is a component of Hb. This increase is believed to be as a result of the chemical constituents of the plant materials. The increase in values of Hb and PCV (Table 5) supports the use of these plant materials in preparation of the popular hot soup or pepper soup usually eaten by women immediately after childbirth in Africa, especially Nigeria. The plant materials may cause an increase in RBC, Hb and PCV, thereby stimulating blood production in order to replenish the lost blood during childbirth. It is possible that this positive effect may be due to action of the chemical constituents of the extracts. Imo et al. [7] reported the presence of different phytochemicals which could possess different biological and physiological actions in fruits of X. aethiopica. These results also showed that consumption of the three plant materials in nutrition may prevent anaemia and certain diseases from affecting the animals. When the number of healthy RBC drops, the level of haemoglobin will also drop [28], which may result in reduction in oxygen delivery to cells and tissues which maybe characterized by weakness, tiredness, shortness of breath and inability to adequately perform physical exercise [29]. The result of this study showed that fruits’ extract of Xylopia aethiopica caused polycythemia.
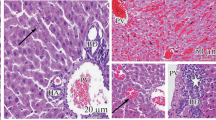
Photomicrographs of normal liver showed normal hepatic architecture. The hepatocyte, portal tract and sinusoids are normal (Fig. 4). Also, livers of rats administered ethanolic seeds’ extract of P. guineense (100 mg/kg bw) showed normal sinusoids, portal tract and hepatocytes (Fig. 5). Photomicrographs from liver section of rat administered ethanolic leaves’ extract of P. guineense (100 mg/kg bw) showed normal sinusoids and hepatocytes. Portal tract is normal, though appeared small (Fig. 6). Photomicrographs from liver section of rat administered ethanolic fruits’ extract of X. aethiopica (100 mg/kg bw) showed normal sinusoids, hepatocytes and central vein. Some areas around the periportal region appear to be mildly dilated (Fig. 7).
Photomicrograph of normal kidney showed normal histoarchitecture of the renal tissue. The normal glomerulus (G), collecting ducts (Cd), Bowman’s space (Bs), Bowman’s capsule (Bc) and distal convoluted tubule (DCT) are shown (Fig. 8), while kidneys of rats administered ethanolic seeds’ extract of P. guineense (100 mg/kg bw) showed normal glomerulus, collecting ducts and Bowman’s capsule. Some of the Bowman’s spaces are mildly enlarged (Fig. 9). Photomicrograph of kidney section from rat administered ethanolic leaves’ extract of P. guineense (100 mg/kg bw) showed normal glomerulus, collecting ducts and Bowman’s capsule. The Bowman’s spaces are mildly enlarged (Fig. 10), while kidney section from rat administered ethanolic fruits’ extract of X. aethiopica (100 mg/kg bw) showed normal glomerulus, Bowman’s spaces, collecting ducts and Bowman’s capsule (Fig. 11).
Conclusion
The results of this study suggest that the three plant extracts have no apparent toxic effect on the liver and kidneys of the experimental rats, though fruits’ extract of X. aethiopica showed possibility of exhibiting mild liver toxicity. Constituents of seeds’ extract of P. guineense and the fruits’ extract of X. aethiopica may also possess renal toxicity. The histoarchitectural state of the liver and kidney sections showed no apparent alteration by the different extracts, thereby showing a correlation with the results of the biochemical analysis. The inclusion of these three plant materials in nutrition may help in processes associated with protein biosynthesis and blood formation, thereby supporting effective function of the liver cells. Fruits’ extract of Xylopia aethiopica may cause infiltration on the liver cells’ membrane and polycythemia, and seeds’ extract of Piper guineense may cause impaired kidney function, while leaves’ extract of Piper guineense may cause leukocytosis. It is possible that the extracts may contain chemical constituents that may possess hepatoprotective or renal-protective effects.
Availability of data and materials
Available upon request.
Abbreviations
- AST:
-
Aspartate transaminase
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine transaminase
- WBC:
-
White blood cell
- RBC:
-
Red blood cell
- PCV:
-
Packed cell volume
- Hb:
-
Haemoglobin
- S:
-
Sinusoids
- PT:
-
Portal tract
- CV:
-
Central vein
- H:
-
Hepatocytes
- G:
-
Glomerulus
- Cd:
-
Collecting ducts
- Bs:
-
Bowman’s space
- Bc:
-
Bowman’s capsule
- DCT:
-
Distal convoluted tubule
References
Uhegbu FO, Imo C, Ugbogu AE (2015) Effect of aqueous extract of Piper guineense seeds on some liver enzymes, antioxidant enzymes and some hematological parameters in albino rats. Int J Plant Sci Ecol 1(4):167–171
Udoh FV, Theodore YL, Braide VB (1999) Effects of extract of seed and leaf of Piper guineense on skeletal muscle activity in rat and frog. Phytother Res 13:106–110
Mbongue FGY, Kamtchouing P, Essame OJL, Yewah PM, Dimo T, Lontsi D (2005) Effect of the aqueous extract of dry fruits of Piper guineense on the reproductive function of adult male rats. Indian J Pharmacol 7:30–32
Ngane AN, Biyiti L, Bouchet PH, Nkegfact A, Zolo PHA (2003) Antifungal activity of Piper guineense of Cameroun. Fitoter 4(5):464–468
Ekanem AP, Wang M, Simon JE, Obiekezie AI, Morah F (2004) In vivo and in vitro activities of seed extract of Piper guineense Schum. and Thonn. Against skin and gill Monogenean parasites of Gold fish (Carassius auratus auratus). Phytother Res 3:97
Aguoru CU, Pilla C, Olasan JO (2016) Phytochemical screening of Xylopia aethiopica with emphasis on its medicinally active principles. J Med Plants Res 10(22):306–309
Imo C, Yakubu OE, Imo NG, Udegbunam IS, Onukwugha OJ (2018) Chemical composition of Xylopia aethiopica fruits. Am J Physiol Biochem Pharmacol 7(2):48–53
Fleischer T (2003) Xylopia aethiopica A Rich: a chemical and biological perspective. J Univ Sci Technol 23:24–31
Lajide L (1995) Termite anti-feedant activity of Xylopia aethiopica. J Phytochem 40(4):1105–1112
Burkil HM (1999) The useful plants of wet tropical Africa, 4th edn. Macmillan Press, New York, pp 34–36
Esuoso KO, Odetoun SM (2005) Proximate chemical composition and possible industrial utilization of Blighia sapida seed and oils. J Phytotherapy Res 72(7):311–313
Anika OC, Unimke AA, Tiku DR, Isu NR (2017) Antibacterial screening of two indigenous Nigerian spices; Piper guineense and Xylopia aethiopica. Microbiol Res J Int 22(1):1–11
John-Dewole OO, Agunbiade SO, Alao OO, Arojojoye OA (2012) Phytochemical and antimicrobial studies of extract of the fruit of Xylopia aethiopica for medicinal importance. J Biotechnol Pharmaceutical Res 3(6):118–122
Tona L, Kanbu K, Nigimbi N, Cimanga K, Vietinck AJ (1999) Anti-amoebic and phytochemical screening of some Congolese medicinal plants. J Ethnopharmacol 61(1):57–65
Nwaichi EO, Igbinobaro O (2012) Effects of some selected spices on some biochemical profile of Wister Albino rats. Am J Environ Eng 2(1):8–11
Oyinloye BE, Osunsanmi FO, Ajiboye BO, Ojo OA, Kappo AP (2017) Modulatory effect of methanol extract of Piper guineense in CCl4-induced hepatotoxicity in male rats. Int. J. Environ. Res. Public Health 14:955
Brown H, Nnatuanya IN, Obisike UA (2016) Evaluation of the effect of Xylopia aethiopica on renal function indices of rats. Eur J Pharmaceutical Med Res 3(2):30–35
Imo C, Uhegbu FO, Imo CK, Ifeanacho NG (2013) Ameliorating effect and haematological activities of methanolic leaf extract of Gongronema latifolium in acetaminophen induced hepatic toxicity in wistar albino rats. Int J Biosci 3(11):183–188
Imo C, Uhegbu FO, Ifeanacho NG, Egbeigwe O, Ezekwe AS (2014) Biochemical and histological changes associated with methanolic leaf extract of Gongronema latifolium in acetaminophen-induced hepatic toxicity in Wistar Albino rats. Int J Biomol Biomed 4(2):1–7
Nair SP (2006) Protective effect of Tefroli- a polyherbal mixture (tonic) on cadmium chloride induced hepatotoxic rats. Pharmacognosy Magazine 2(6):112–128
Imo C, Uhegbu FO, Imo NG, Aroworo KA, Kukoyi AJ, Zachariah SS (2017) Effects of ethanolic extracts of Datura metel parts on liver function of male Albino Rats. FUW Trends Sci Technol J 2(2):848–852
Okpala JC, Sani I, Abdullahi R, Ifedilichukwu HN, Igwe JC (2014) Effects of nbutanol fraction of Gongronema latifolium leave extract on some biochemical parameters in CCl4- induced oxidative damage in Wistar albino rats. Afr J Biochem Res 8(2):52–64
Imo C, Uhegbu FO (2015) Renalprotective effect of ethanolic leaf extract of Gongronema latifolium Benth in acetaminophen-induced renal toxicity in albino rats. Am Chem Sci J 8(3):1–10
Shaikh IA, Gautam RK (2014) Effect of organophosphate pesticide, nuvan on serum biochemical parameters of fresh water catfish Heteropneustes fossilis (Bloch.). Int Res J Environ Sci 3(10):1–6
Crook MA (2007) The kidneys. In: Clinical chemistry and metabolic medicine, 7th edn. Bookpower, Britain, pp 36–57
Metcalf D (1993) The hematopoietic regulators: redundancy or subtlety? Blood 82(1):3515–3523
Morebise O, Fafunso MA, Mankinde JM, Olayide OA, Awe E (2002) Anti-inflammatory property of Gongronema latifolium. Phytother Res 16:575–577
Imo C, Arowora AK, Awache I, Abdullahi RZ (2016) Haematological effects of ethanolic leaf, seed and fruit extracts of Datura metel on male albino rats. FUW Trends Sci Technol J 1(2):509–512
Janz TG, Johnson RL, Rubenstein SD (2013) Anaemia in the emergency department: Evaluation and treatment. Emerge Med Pract 15(11):1–15
Acknowledgements
Not applicable.
Plant authentication
Fruit of X. aethiopica was identified and authenticated at the herbarium unit of the Biological Science Department, Ahmadu Bello University, Zaria, Nigeria, by Mr. Umar Gallah and a voucher specimen number 1026 deposited accordingly. Seeds and leaves of P. guineense used were identified and authenticated at the herbarium unit of Forestry Research Institute of Nigeria, Ibadan, by Adeniji, K. A and voucher specimen number 112178 deposited accordingly.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
CI, NGI and GCD designed the experiment. CI, KAA, CSE, NGI and GCD carried out the laboratory work. CI, KAA, CSE, JI, OEY, NGI and GCD interpreted the results, sourced for literatures and developed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Standard laboratory protocols for animal studies were followed as approved by the Research Ethics Committee, Department of Biochemistry, Federal University Wukari, Nigeria, with reference number FUW/BCH/005.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Imo, C., Arowora, K.A., Ezeonu, C.S. et al. Biochemical and histological effects of ethanolic extracts of fruits of Xylopia aethiopica and seeds and leaves of Piper guineense on liver and kidney function in male albino rats. Futur J Pharm Sci 7, 35 (2021). https://doi.org/10.1186/s43094-021-00187-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-021-00187-6