Abstract
Background
A simple, economical, sensitive, authentic, and faithful technique was used for the study of simultaneous evaluation of Ertugliflozin and Metformin in tablet dosage forms. The Chromatographic analysis was performed isocratically by using a Phenomenex C18 column (150 mm × 4.6 mm, 5 μm), and a mobile phase containing acetonitrile and 0.1% OPA buffer, with a proportion of 40: 60% v/v was passed throughout the column maintained at a temperature of 30 °C with a flow rate of 1 ml/min. Approximately, 10 μl of drug solution was injected and the analytes were eluted at 220.0 nm.
Results
The elution times of Ertugliflozin and Metformin were 2.226 min and 2.955 min, respectively. The percentage RSD of Ertugliflozin and Metformin are 0.60 and 0.59, respectively. The percentage recoveries of Ertugliflozin and Metformin were determined to be 100.55% and 99.40%, respectively. LOD, LOQ values of Metformin and Ertugliflozin obtained were found to be 0.10, 0.2 μg/ml and 0.03, 0.09 μg/ml, respectively. Regression equation of Ertugliflozin was y = 11287x + 1191 and y = 46270x + 161.0 for Metformin. All verification parameters are within the range according to the ICH guidelines, and the degradation products are also within the limits, which show that the method is stable.
Conclusion
In the currently developed RP-HPLC analytical method, the elution time and run time is reduced, which proves that the method is economical and widely acceptable, also simple and practical, which can be used in routine quality control tests in the industry.
Similar content being viewed by others
Background
Ertugliflozin has a place with the class of strong and particular inhibitors of the sodium-subordinate glucose cotransporters (SGLT) [1]; all the more explicitly the sort 2 which is liable for about 90% of the glucose reabsorption from glomerulus [2, 3]. It became FDA endorsed as monotherapy and in combination with sitagliptin or metformin hydrochloride on 22 December 2017.
In grown up patients enduring with type 2 diabetes, Ertugliflozin as a monotherapy has been seemed to reinforce the glycemic regulation. Ertugliflozin, in combination with metformin hydrochloride, is demonstrated to enhance glycemic control in sufferers with diabetes type 2 who are not controlled on a recurring of Ertugliflozin or metformin or in patients who are now dealt with both
Metformin
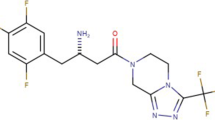
Metformin has a place with the classification biguanides oral anti-diabetes drug [4]. This is the primary essential drug for the determination of type 2 diabetes, particularly in overweight and hefty people and those with typical kidney function. Its utilization in gestational diabetes has been constrained by security concerns despite the fact that in any event one examination has been directed which indicated no worries for youngsters prenatally presented to Metformin as long as 2 years old. It is additionally utilized in the treatment of polycystic ovary disorder and has been explored for different ailments where insulin obstruction might be a significant factor. Metformin works by suppressing glucose creation from three-carbon particles (like propionic acid), a result of dietary fiber aging in the large intestine and pyruvate, a by-product of glucose breakdown in the muscles by the liver [5,6,7]. FDA approved a set combination of Ertugliflozin and Metformin for the regulation of blood glucose levels in patients with type II diabetes [8,9,10]. Structures of Ertugliflozin and Metformin were given as Figs. 1 and 2.
Methods
Ertugliflozin and Metformin were procured as gift samples from Startech laboratories.
Segluromet formulation combination of Metformin and Ertugliflozin was obtained from local pharmacy store.
Acetonitrile, Methanol of HPLC grade, and O-phosphoric acid of analytical grade were obtained from Rankem laboratories.
Chromatographic conditions
Equipment
Waters HPLC 2695 chromatographic System set with quaternary pumps, Photo Diode Array detector and Auto sampler. Chromatograms were recorded using Empower 2 Software.
Chromatographic conditions
Mobile phase: 0.1%OPA buffer: Acetonitrile (60:40% v/v)
Column: Phenomenex (4.6 mm × 150 mm, 5 μm)
Flow rate: 1.0 ml min−1
Column temperature: 30 °C
Programming: Isocratic
Wavelength: 220 nm
Run time: 10 min
Sample volume: 10 μl
Solvent
Acetonitrile (ACN) and water in the ratio 50:50% v/v is used as solvent.
Both standard and sample solutions were filtered through 0.45 μm before injecting into the HPLC.
Preparation of standard solutions
Standard stock solution of 15 μg ml−1 of Ertugliflozin and 1000 μg ml−1 of metformin were prepared by weighing accurately 0.75 mg of Ertugliflozin and 50 mg of Metformin and transferring to 50 ml volumetric flask. Three-fourth volume of diluent was added and kept for sonication for 25 min and finally they were made up to the mark using the diluent. Working standard solution of conc 100 μg/ml of Metformin and 1.5 μg/ml of Ertugliflozin was obtained by pipetting out 1 ml of stock solution and transferring it to 10 ml volumetric flask and making up to the mark with the diluent.
Preparation of sample solutions
Weigh 20 tablets, calculate the average weight. Weight equivalent to one tablet was transferred to volumetric flask of 500 ml, next 50 ml of diluent was applied and sonicated for 25 min, further volume was made up to the mark using diluent to obtain concentration of 1000 μg/ml of metformin and 15 μg/ml of Ertugliflozin. Then, 1 ml of filtered sample stock solution was transferred to 10ml volumetric flask and made up with diluent to obtain concentration of 100 μg/ml of Metformin and 1.5 μg/ml of Ertugliflozin.
Buffer
0.1%OPA buffer: 0.1 ml orthophosphoric acid diluted to 100 ml using water of HPLC grade.
Validation
System suitability
System suitability parameters were evaluated and analyzed to check system performance using standard solutions of Ertugliflozin (1.5 ppm) and Metformin (100 ppm).
Linearity
Linearity is to produce test results that are exactly proportional to the analyte concentration in the sample. It is obtained with in the concentration range of 0.375–2.25 μg/ml for Ertugliflozin and 25–150 μg/ml for metformin. The calibration graph is plotted with X-axis concentration and Y-axis peak areas and seen in Figs. 3 and 4.
Specificity
The interference in the optimized method is verified. There is no evidence of interference of peaks in blank and placebo at retention times of these drugs in this method. So, the developed method is specific.
Accuracy
Accuracy is a measure of how close is the experimental value to the true value. Accuracy is determined by calculating the recovery values accuracy is done at intervals of 50%, 100%, and 150% levels. % recovery and % RSD were taken into consideration.
Precision
Precision is defined as the closeness of agreement between a series of measurements obtained from multiple sampling of the homogeneous sample. It is estimated by injecting six replicates of Ertugliflozin of conc 1.5 ppm and metformin of conc 100 ppm.
LOD and LOQ
Limit of detection (LOD) and limit of quantification (LOQ) of Ertugliflozin and Metformin were determined from the calibration curve method using the following equations:
Where α is standard deviation, s is the slope.
Robustness
Even though there are small deliberate changes in method like flow rate, mobile phase ratio, and temperature there is no change in the result and the results are within range as per ICH Guide lines.
Assay
Segluromet bearing the label claim Metformin 500 mg and Ertugliflozin 7.5 mg. Assay was performed with the above formulation. Average % Assay for Metformin and Ertugliflozin obtained was found to be 101.32 and 99.46%, respectively.
Forced degradation studies
This was achieved by following recommendations of an international conference on harmonization [11,12,13].
Standard samples of Ertugliflozin and Metformin were degraded under different stress conditions like acidic, alkali, oxidative, thermal, photostability, and neutral conditions.
Samples for acidic and alkali degradation were refluxed at 60 0C for 30 min with 2N HCl and 2N NaOH. Oxidative degradation is carried out by using 20% H2O2 and kept aside for 30 min at 60 0C. Thermal degradation is done by placing the sample in an oven at 105 °C for 6 h. For photostability, studies the sample solutions were subjected to UV light by placing the sample in photo stability chamber for 7 days or 200 W h/m2 .The sample solutions were refluxed in water for 6 h at 60 0C .All the samples were finally diluted to obtain concentration of 1.5 μg/ml of Ertugliflozin and 100 μg/ml of Metformin. Ten microliters was injected into the system and the chromatogram is recorded to assess the stability of sample. Chromatograms of acid, alkali,peroxide,thermal,U.V and water stress conditions were depicted as Figs. 5, 6, 7, 8, 9 and 10
Results
Optimized method
Strategy streamlining has done by actualizing experimentation technique in such a manner to get a chromatogram with good resolution(R), proficiency, acknowledged number of theoretical plates, and tailing factor. In this strategy, a few preliminaries have been finished by changing organic phase proportion and flow rate. At last, the strategy with Phenomenex 150 (4.6 × 150 mm, 5 μm), mobile phase composition of 0.1%OPA buffer: Acetonitrile (60:40 v/v) and a flow rate of 1 mL/min was chosen as advanced technique. Various trails were carried out using different compositions of mobile phase and different columns based on the literature review [14,15,16,17,18] and optimized chromatogram was shown in Fig. 11.
Validation
Validation is done in accordance with the guidelines framed by the ICH [19].
System suitability
All the system suitability parameters like tailing factor should be less than 2, theoretical plates greater than 2000, resolution more than 2, % RSD of peak areas less than 2 were evaluated to show that the values were reproducible. In the current method, all the parameters were established within limits which prove that the values are reproducible and the results were summarized in (Table 1).
Linearity
Linearity was determined over the range of (25–150 μg/ml) for Metformin and (0.375–2.25 μg/ml) for Ertugliflozin. Regression equation obtained for Metformin was y = 11287x + 1191 and for Ertugliflozin it was y = 46270x + 16.
Precision
System precision
Percentage RSD values for both intra and inter day precision were within the range that is less than 2. The results were summarized in the (Table 2).
Accuracy
Accuracy results at various levels of concentration were summarized in the (Table 3).
Sensitivity
The present method can detect and quantify the analyte at lower concentration (Table 4).
LOD and LOQ values were calculated using the formulae
Where α is the standard deviation of the response of regression lines and s is the slope obtained from the calibration curve.
Robustness
In view of the outcomes, the developed analytical method was proved to be robust (Table 5).
Assay
Average % Assay for Metformin and Ertugliflozin obtained was found to be 101.32 and 99.46% correspondingly and the results were summarized in (Table 6).
Degradation
Degradation studies show that Metformin and Ertugliflozin were well separated from the degradation products that were formed in different stress conditions. As per the ICH guidelines, % degradation should be less than 20% to consider the particular method as stable. In this method, % degradation was found to be less than 10% which proves that the method was stable and specific. Thus, the developed analytical method is stable and able to separate both Metformin and Ertugliflozin even in the presence of degraded products. % amount degraded is shown in (Table 7).
Discussion
The stability indicating [11,12,13] RP-HPLC technique assumes a vital role in assurance of stability, both subjective and quantitative estimation of API and formulation. Till date, numerous explanatory techniques [14,15,16,17,18] have been created for Ertugliflozin and Metformin individual and in blend with other anti-diabetic drugs. Subsequently, endeavors were made to build up a successful soundness demonstrating RP-HPLC strategy. Various parameters and variable effects on peak shape, RT, and resolution were studied well. Column was also opted in such a way that which gave the best result. Analytes were eluted before 5 min, RT in the existing technique was 2.226 min for Ertugliflozin and 2.955 min for Metformin, speaks to the strategy with great and successful maintenance time and can be treated as practical as it lessens solvent utilization and analyte run time which proves it is economical and simple. Correlation coefficient of the developed method was very close to 1.0, which supports the sensitivity of the method. Intra and inter day analysis was also performed which proves that the developed method was precise. Validity of the developed method was proved by investigating and evaluating the parameters like linearity, accuracy, precision, sensitivity, and repeatability. Forced degradation studies were also carried out by exposing the drugs to acidic, alkaline, thermal, oxidative, photolytic, and neutral conditions to indicate the stability of the current method. The currently developed method is able to separate the active pharmaceutical ingredient from any degradants which proves that the method is stable. Consequently, fast examination of increasingly number examples is possible. The determined and measurable results of the approval parameters were not out of as far as possible expressed by ICH.
Conclusion
Development of an accurate and efficient analytical method to determine and evaluate the quality of the drug substances are some of the critical activities carried out during process research and development to meet the requirements of different regulatory authorities. A simple, accurate, precise method was developed for the simultaneous estimation of the Ertugliflozin and Metformin in tablet dosage form. Forced degradation studies were finished by applying a few pressure conditions to evaluate the strength of the method. The proposed technique was effectively isolating both the drug and its degradation products with good resolution and measures the dynamic substance at minute fixation levels. The developed method was explicit, delicate. Consequently, the proposed technique can be adjusted to ordinary examination in pharmaceutical industry.
Availability of data and materials
All data and material are available upon request.
Abbreviations
- ERT:
-
Ertugliflozin
- MET:
-
Metformin
- RT:
-
Retention time
- LOD:
-
Limit of detection
- LOQ:
-
Limit of quantification
- PPM:
-
Parts per million
- RSD:
-
Relative standard deviation
- PDA:
-
Photo diode array
- OPA:
-
Orthophosphoric acid
References
FDA label: Merck & Co.Inc. SEGLUROMET™ (Ertugliflozin and Metformin hydrochloride) tablets, for oral use. Initial US Approval,(2017): https://www.Accessdata.fda.gov/drugsatfda_docs/label/2017209806s1b1.pdf.
Drug Profile for Ertugliflozin. Available from: https://www.drugbank.ca/drugs/DB11827.
Cinti F, Moffa S, Impronta F, Cefalo CM, Sun VA, Sorice GP (2017) Spotlight on ertugliflozin and its potential in the treatment of type 2 diabetes. Drug Design, Development and Therapy 11:2905–2919. https://doi.org/10.2147/DDDT.S114932
Drug Profile for Metformin. Available from: https://www.drugbank.ca/drugs/DB00331.
Wang YW, He SJ, Feng X, Cheng J, Luo YT, Tian L (2017) Metformin: a review of its potential indications. Drug Design Development Therap 11:2421–2429. https://doi.org/10.2147/DDDT.S141675
Rena G, Hardie DG, Pearson ER (2017) The mechanisms of action of metformin. Diabetologia 60(9):1577–1585. https://doi.org/10.1007/s00125-017-4342-z
Madiraju AK, Qiu Y, Perry RJ, Rahimi Y, Zhang XM, Zhang D (2018) Metformin inhibits gluconeogenesis via a redox dependent mechanism in vivo. Nature Medicine 24(9):1384–1394. https://doi.org/10.1038/s41591-018-0125-4
Madiraju AK, Erion DM, Rahimi Y, Zhang XM, Braddock DT, Albright RA (2014) Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nat Med 510(7506):542–546. https://doi.org/10.1038/nature13270
FDA approves Segluromet.(2017) https://www.drugs.com/newdrugs/fda-approves-segluromet-ertugliflozin-metformin hydrochloride-type-2-diabetes. Accessed 22 Dec 2017.
Ertugliflozin/Metformin (By Mouth). Pubmed Health, 2018. https://www.ncbi,nml.nih.gov/pubmedhealth/PMHT0031142/.Accessesed Feb 2018
ICH harmonised tripartite guideline (2003) Stability testing of new drug substances and products, Q1A (R2). International Conference on Harmonization, pp 1–18
International Conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use (2003) ICH Harmonised tripartite guideline. In: Stability testing of new drug substances and products Q1A (R2)
International Conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use (1996) ICH Harmonised tripartite guideline. In: Stability testing: photostability testing of new drug substances and products Q1B
Harsha Hruditha P, Jayachandra Reddy P (2020) Development and validation of stability indicating HPLC method for simultaneous estimation of Metformin and Ertugliflozin. Int J Chem Pharm Sci 8(2):45–48
Jagadeesh K, Annapurna N (2019) Stability indicating method development and validation of metformin and ertugliflozin by high-performance liquid chromatography with PDA detection and its application to tablet dosage form. Asian J Pharm Clin Res 12(3):353–358
Wajahat S, Ahmed A, Khan G, Anas S, Absar A, Qureshi (2020) Analytical method development and validation for simultaneous estimation of ertugliflozin and metformin HCl in bulk and pharmaceutical dosage form by HPLC. Int J Pharm Sci Res 11(1):226–232
China Babu D, Madhusudhana C, Mastanamma SK (2019) A new stress indicating RP-HPLC method development and validation for the simultaneous estimation of ertugliflozin and metformin in bulk and its tablet dosage form. Indian drugs 56(2):39–46
Nizami T, Shrivastava B, Sharma P (2018) Analytical method development and validation for simultaneous estimation of Ertugliflozin and Metformin in tablet dosage form by RP-HPLC method. Int J Pharm Life Sci 9(7):5854–5859
ICH harmonized Triplicate guideline (2005): Validation of analytical procedures : text and methodology Q2 (R1).
Acknowledgements
This research would not have been possible without the support of Dr. Tulja Rani and my co authors Mounika and Ratna. I am so grateful to the assistance provided by Dr. Tulja Rani. Finally, it is with true pleasure that I acknowledge the contributions provided by my co-authors in the preparation of this manuscript.
Funding
No funding and self-financed.
Author information
Authors and Affiliations
Contributions
S.B and G.T conducted the literature survey, designed the work, developed, and validated a new RP-HPLC method. M.M and M.R compiled, analyzed, and interpreted the data. S.B and G.T wrote the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
No competing interests to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sunkara, B., Gampa, T., Markanti, M. et al. Stability indicating method development and validation for simultaneous estimation and quantification of Ertugliflozin and Metformin in bulk and tablet dosage form. Futur J Pharm Sci 7, 32 (2021). https://doi.org/10.1186/s43094-021-00179-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-021-00179-6