Abstract
Background
High fat-fructose diet is a proinflammatory diet that increases risk of hepatocytes and myocytes steatosis and fibrosis. Finding anti-inflammatory strategies to fight these harmful effects is paid attention to nowadays. This study compared the effects of two widely anti-inflammatory interventions—metformin and intermittent fasting on myocytes and hepatocyte injury induced by proinflammatory diet and tracking possible underlying mechanisms. In this work, rats fed high fat-fructose diet were subdivided into untreated group, treated by metformin, and/or intermittent fasting.
Results
Metformin (300 mg/kg/day) and intermittent fasting (3 days/week) specially their combination for 4 weeks showed significant improvement in insulin resistance, lipid profile, antioxidants (p < 0.05), as well as enhanced hepatocytes and myocytes repair and reduced collagen deposition through upregulation of mitophagy-related genes: PINK1, PARKIN, LAMP2, and PPAR-α (p < 0.05).
Conclusions
Intermittent fasting has beneficial metabolic and molecular therapeutic effects against proinflammatory diet-induced injury. Their results are like those of metformin sparing its adverse effects. Their combination showed additional effects against diet-induced myocytes and hepatocyte injury by upregulation of mitophagy-related genes without the need of increasing the dose of metformin.
Graphic abstract

Similar content being viewed by others
1 Background
According to the dietary inflammation index (DII), Western-style diet (high fat-high fructose) is a proinflammatory diet that is characterized by an overall excessive energy intake, increased risk of insulin resistance, dyslipidemia, and obesity [1], which in turn leads to excessive oxidative stress, and pathological changes in many organs, including the liver and skeletal muscle [2].
Metformin, a class of insulin sensitizers, is generally considered the first-line oral treatment in type 2 diabetes mellitus primarily due to its antihyperglycemic efficacy and its favorable effect on the body weight and insulin resistance, as well as its anti-inflammatory, anti-oxidative, and hypolipidemic effects [3].
Additionally, medical nutrition therapy including different dietary patterns has been recommended to improve glycemic control and reduce related complications.
Intermittent fasting (IF) has become an extremely common dietary protocol for weight loss, comprising alternating fasting and non-fasting periods [4]. It has a potential impact on cellular processes that could be through DNA repair, reducing inflammation, and oxidative stress defense [5]. Despite the growing support to use of IF as a nonpharmacological approach to improve health, its underlying molecular mechanisms are not completely investigated.
Mitophagy, a mitochondrial-lysosomal degradative pathway, is an important adaptive cellular consequence that occurs under normal physiological conditions and in response to different stressors to maintain cellular homeostasis [6]. The mitophagy process has a crucial role in the degradation of damaged or dysfunctional mitochondria, in addition to removing mutated mitochondrial DNA through different signaling pathways [7].
Both metformin and IF are considered anti-inflammatory interventions against proinflammatory diet but there is no sufficient data about the efficacy of IF as a potentially beneficial adjuvant intervention with metformin. Hence, this study aimed to compare their possible protective effects against hepatocytes and myocytes injury induced by a proinflammatory high fat-fructose diet and track mitophagy as a possible underlying mechanism and its interplay with other cellular processes, such as oxidative stress, and cellular repair.
2 Methods
2.1 Animals
In this study, 30 male Wistar rats (5-week-old, 100–120 gm) were purchased and bred in the animal house of the National Research Center, Cairo, Egypt. The experiment design and procedures were conducted according to ARRIVE GUIDELINES and approved by the Institutional Animal Care and Use Committee, Cairo University (CU-III-F-36-21). Rats freely accessed food and water except in intermitting fasting groups and were kept at controlled temperature and 12-h light/dark cycle.
2.2 Experimental design
After 3 days of acclimatization, the animals were randomly allocated into the following groups (6 rats each): Control group fed commercial chow (carbohydrates 64%, protein 20%, fat 5% w/w) for 8 weeks while the other groups were fed proinflammatory diet (high fat-fructose = HFF) containing lard and fructose (code: L11770, alpha chemika, India): 30% and 20% (w/w), respectively. These were added to commercial chow for 8 weeks and were further subdivided as follows: without intervention (HFF group), received metformin (MET group), underwent intermittent fasting (IF group) or combined metformin and intermitting fasting (MET-IF group). Metformin and intermittent fasting started in the last 4 weeks of the experiment.
2.3 Protocol of intervention
Metformin (Amoun Pharmaceutical Company, El-Obour City, Egypt) was freshly prepared by being dissolved in distilled water and was given by gavage in daily doses of 300 mg/kg for 4 weeks [8]. The other rats received a matched volume of distilled water by oral gavage.
Intermittent fasting was performed by a 24-h deprivation of food on three alternate days/week in the span of 4 weeks (Rats in IF and MET-IF groups fasted on days 2, 4, and 6 of the weeks but allowed for free access to water). Rats had unlimited access to food on non-fasting days.
2.4 Animal euthanasia and specimen collection
At the end of 8 weeks, the overnight fasted animals were anesthetized with a mixture of ketamine and xylazine (IP 40 and 10 mg/kg, respectively). Retroorbital blood was withdrawn and left for half an hour at room temperature and then centrifuged at 3000 rpm for fifteen minutes. The supernatant serum was stored at − 20 °C for further biochemical analysis. Neck dislocation was performed under anesthesia, a midline abdominal incision was done to extract the liver, while the soleus muscles were dissected from the hindlimbs, both were preserved for molecular (stored at − 80 °C) and histopathological studies (fixed in 10% formol saline).
2.5 Biochemical measurements
2.5.1 Real-time quantitative PCR (qPCR)
Total RNA was obtained from homogenates of liver and muscle tissue using the SV Total RNA Isolation System from Promega, a company based in Madison, WI, USA. The RNA was then subjected to reverse transcription using the miScript II RT kit from Qiagen, a company located in Valencia, CA, USA. Subsequently, reverse transcription-polymerase chain reaction (RT-PCR) was carried out using the Bioline kit (SensiFASTTM SYBRR Hi-ROX One-Step Kit, catalog no. PI-50217 V), followed by amplification of PCR. The amplification program was as follows: 95 °C for 2 min, and 40 cycles of 95 °C for 15 s, 60 °C for 30 s and 72 °C for 1 min [9]. The primer sequence utilized for PINK1, PARKIN, LAMP2, and PPAR-alpha in this study is provided in Table 1. Data were turned into to cycle threshold (Ct). The values of the Ct were recorded for both the evaluated gene (lncRNA-AF085935) and the housekeeping gene (18rs) on the PCR datasheet. The relative quantitation (RQ) of each target gene was determined using the delta-delta Ct (ΔΔCt) calculation. The 2-∆∆Ct method was employed to calculate the RQ of each gene.
2.5.2 Estimation of serum oxidative stress markers
The evaluation of serum malondialdehyde (MDA) and reduced glutathione (GSH) levels was conducted according to the instructions provided by the manufacturer, as documented in a previous study [10]. The Elabscience® Colorimetric Assay Kit (Catalog no. EBCK025S, TBA method) was used to determine MDA concentrations, while the BioVision Colorimetric Assay Kit (Catalog no. K261100) was employed for the determination of GSH concentrations.
2.5.3 Estimation of serum total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL), and low-density lipoprotein (LDL)
The levels of serum TC, TG, and HDL were assessed using a commercially accessible kit under the instructions provided by the manufacturer, as previously stated [11, 12]. Serum LDL level (measured in mg/dl) was determined using Friedewald’s formula: LDL = [TC–HDL–TG/5] [13].
2.5.4 Estimation of serum glucose and insulin and Homeostasis Model of Insulin Resistance (HOMA-IR)
Fasting serum glucose levels were evaluated using the oxidase–peroxidase method, utilizing test materials provided by “Diamond Diagnostics.” Fasting serum insulin levels were estimated through the usage of an ELISA kit provided by Monobind Inc. from Lake Forest, California, USA. Insulin resistance was assessed by calculating HOMA-IR: HOMA-IR = Fasting insulin (µU/ml) × Fasting glucose (mmol/L)/22.5 [14].
2.6 Histological studies
Dissected specimens from the lower limb and liver were fixed and then placed in xylene for 10 min before being embedded in paraffin wax. Sections of 5 μm thickness were processed for hematoxylin and eosin staining for microanatomy and general architecture of the tissues, and Masson’s trichrome stain was used to visualize the collagen fibers that stained blue. Proliferating cell nuclear antigen (PCNA) was detected by immunohistochemical staining using the avidin–biotin-peroxidase complex technique. PCNA antibody (PC10) is a mouse monoclonal antibody (Abcam, Catalog no.ab29, RRID: AB_303394). PCNA-positive cells show brown nuclear deposits [15].
2.6.1 Morphometric study to estimate the percentage area of collagen fibers and PCNA-positive immunoreactivity
A Leica Qwin 500 LTD (Cambridge UK) computer-assisted image analyzer was used. Light microscopy was used to examine the slides, and parameters were measured in 10 nonoverlapping high-power fields (× 400) that were randomly selected using the binary mood.
2.7 Statistical analysis
SPSS version 26 was used in the current work for data analysis. For comparison between groups, one-way ANOVA was used for normally distributed data and Kruskal–Wallis for non-normally distributed data, followed by Bonferroni’s post hoc test with a p-value < 0.05 was considered significant. The mean and the standard deviation were used for data representation.
3 Results
3.1 Intermittent fasting (IF) prevents proinflammatory diet-induced weight gain
A high fat-fructose diet resulted in significant weight gain (Table 2) compared to the body weight of rats fed the normal commercial chow (p-value < 0.05). However, intervention by IF (but not metformin) resulted significantly in less weight gain compared to the untreated HFF group (p-value < 0.05).
3.2 IF maximizes metformin metabolic effects
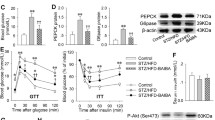
The metabolic hazards of a proinflammatory diet in the HFF group were detected by significant (p-value < 0.05) insulin resistance, dyslipidemia, and disturbance of the equilibrium between oxidants/antioxidants compounds (Fig. 1) compared to the normal diet in the control group. However, these effects were remarkably mitigated in all treated groups (p-value < 0.05).
Effect of metformin and intermittent fasting on a glycemic panel, b oxidative stress, c lipid profile. Rats fed on high fat-fructose diet exhibited significant insulin resistance, dyslipidemia and increased redox status as compared to control rats. Rats treated with intermittent fasting and metformin showed significant improvement in the forementioned parameters as compared to HFF group. The mean ± SD was used to represent data. p < 0.05 is considered significant to * control, # HFF, $ MET, @ IF groups
3.3 Upregulation of mitophagy-related genes and attenuation of hepatic triglyceride content
As shown in Fig. 2, the high fat-fructose diet significantly downregulated the mitophagy-related gene (PINK1, PARKIN, LAMP 2, PPAR-α) compared to the regular diet (p-value < 0.05). Both metformin and IF significantly (p < 0.05) upregulate the expression of these genes compared to the untreated group.
Effect of metformin and intermittent fasting on expression of mitophagy-related genes in rat liver and skeletal muscle: a PINK1, b PARKIN, c LAMP2, d PPAR-α. A significant improvement in expression of mitophagy-related genes in the experimental treated groups as compared to HFF group. The mean ± SD was used to represent data. p < 0.05 is considered significant to * control, # HFF, $ MET, @ IF groups
Notably, a significant decline (p-value < 0.05) in liver triglyceride levels was detected in the IF, MET, and MET-IF groups compared to the HFF group (Table 2).
3.4 The morphological studies and immunohistochemical staining
In the HFF group, obvious pathological changes in both liver (Fig. 3) and skeletal muscle (Fig. 4) were characterized by distorted architecture, inflammatory cell infiltration, as well as significant (p-value < 0.05) collagen deposition (Fig. 5, 6) and negative PCNA immunostaining (Fig. 7, 8) compared to the control group. Both metformin and IF ameliorated these pathological changes and the combination of both interventions in the MET-IF group successfully restored the normal histological appearance and resulted in a significant decrease in collagen deposition and positive PCNA immunostaining (p-value < 0.05). These findings suggest that intermittent fasting enhances the antifibrotic effect and cellular repair of metformin.
Photomicrographs of sections in rat liver (H&E X400). a Control group showed normal liver architecture. Liver cords radiating from the central vein (CV) with blood sinusoids (BS) in between. The hepatocytes show acidophilic cytoplasm and pale vesicular nuclei with prominent nucleoli (arrows), b HFF group showed marked disrupted architecture and vacuolated hepatocytes due to fat accumulation (arrows). Inflammatory cells (Asterix) and pyknotic nuclei (curved arrows) are seen, c MET group showed some vacuolated hepatocytes (arrows) and localized inflammatory cells (Asterix), d IF group showed less vacuolation of hepatocytes (arrows) with localized eosinophilic exudate (curved arrow) and few inflammatory cells (Asterix), e MET-IF group showed preservation of the hepatocytes with vesicular nuclei (arrows). Abbreviations: CV central vein, BS blood sinusoids
Photomicrographs of sections in rat skeletal muscle (H&E X400). a Control group showed muscle fibers separated by narrow endomysium (star), b HFF group showed splitting and fragmentation of some muscle fibers (arrow heads). Note the presence of some central nuclei (circles) and hyper-eosinophilic muscle fibers (black arrow), c MET group muscle bundles are separated by wide perimysium (Asterix). Multiple peripheral nuclei (arrows) and some inflammatory cells can be noted (blue arrows), d IF group showed splitting of some muscle fibers (arrowhead) and a swollen myofiber (bifid arrow), e MET-IF group. Note the presence of multiple sarcolemma nuclei (bent arrow)
Photomicrographs with Masson’s trichrome in sections in rat liver (A) and skeletal muscle (B) (X400). Arrows in photos indicate collagen fibers deposition, a Control group showed minimal collagen fibers, b HFF group showed marked collagen fibers deposition with increased density, c MET group showed some collagen fibers deposition in the liver and minimal collagen fiber deposition in the muscle, d IF group showed moderate collagen fibers deposition, e MET-IF group showed apparently minimal collagen fibers. Abbreviations: P portal area
Percentage area of collagen deposition in hepatocytes and skeletal myocytes in rats. A significant increase (p-value < 0.01) in collagen deposition in the untreated HFF group compared to all experimental groups. The mean ± SD were used to represent data. p < 0.05 is considered significant to * control, # HFF, $ MET, @ IF groups
Immunohistochemical staining of rat liver (A) and skeletal muscle (B) (PCNA immunostaining × 400). Arrows in photos (a, c, d, e) indicate positive PCNA immunostaining (Brown nuclear deposits), a control group displayed positive PCNA immunostaining of some nuclei, b HFF group showed negative PCNA immunostaining, c MET group displayed positive PCNA immunostaining of many nuclei. Note: Aggregation of positive hepatocytes nuclei are seen (Asterix) in A, d IF group showed positive PCNA immunostaining of some nuclei, e MET-IF group showed positive PCNA immunostaining of most of the nuclei
Percentage area of PCNA immunostaining in liver and skeletal muscle in rats. A significant decrease (p-value < 0.01) in cellular proliferation in the untreated HFF group indicated by negative PCNA immunostaining compared to the other experimental groups. While treated groups showed significant increase in cellular proliferation that exceeds normal level (p-value < 0.05). The mean ± SD were used to represent data. p < 0.05 is considered significant to * control, # HFF, $ MET, @ IF groups
4 Discussion
In the current study, a proinflammatory diet for 8 weeks led to significant metabolic disorders and several pathological changes in the liver and skeletal muscle. Many theories were suggested to identify the causes of such abnormalities as malfunctioning mitophagy with decreased mitochondrial biogenesis and its impact on cellular homeostasis [16].
Mitophagy is mediated through specific proteins that enhance the supererogatory mitochondrial removal, aiming to preserve healthy mitochondria. This is related to different pathways involving mainly PINK1 and PARKIN proteins. Wang et al. [17] stated that mitophagy surrounds the damaged mitochondrial fragments by tagging them with PINK1, which is a continuously degraded protein in the outer mitochondrial membrane that becomes stable if phosphorylated, then phosphorylated PARKIN mediates the ubiquitylation of the outer membrane for autophagosome formation and is fused with the lysosome for degradation [18]. The damaged mitochondria are usually eliminated by mitophagy before the production of excessive ROS [19].
The present study revealed the relation of a proinflammatory diet to mitophagy through the reduction in hepatic and muscle PINK1 and Parkin proteins. Marked mitochondrial oxidative damage occurred due to decreased activity of antioxidant defense led to excess ROS, excessive mitochondrial DNA damage, and mitophagy impairment. This interferes with insulin signaling directly by inducing insulin resistance and indirectly by enhancing mitochondrial damage. This results in a reduction of mitochondrial function and density with the accumulation of lipotoxic intermediates [20].
Mitophagy being lysosomal dependent can also be assessed by measuring lysosomal-associated membrane proteins (LAMPs 1/2) that play a key role in keeping lysosomal membrane integrity. A previous study by Zummo et al. [21] revealed that deficient LAMP1 could impair autophagic flux, resulting in damaged mitochondria aggregations. Increased oxidative stress causes lysosomal dysfunction through the degradation of LAMP, which impairs autophagy and mitochondrial homeostasis [22]. This is consistent with this work that showed down-regulation of hepatic LAMP 2 expression in rats in the HFF group that exhibit high oxidative stress.
The nuclear peroxisome proliferator-activated receptors (PPAR) are well known to be key regulators for many genes involved in cellular metabolism and the autophagy process [23]. This could analyze its expression in tissues with high metabolic rates, such as the liver and skeletal muscle [24], in addition to its presence in cells of the immune system (e.g., macrophages, monocytes, and lymphocytes) [25, 26]. Moreover, previous studies explored its crucial role against fat accumulation and related inflammations [27, 28]. It also potentiates hepatic mitochondrial integrity by controlling the redox status [29]. In the current study, a fat-fructose-rich diet vigorously down-regulates hepatic PPAR-α expression. Despite numerous works of literature demonstrating similar findings, the mechanisms by which a proinflammatory diet affects PPAR-α levels and activity are still under-representative [30].
The histopathological findings in the current study endorse the cellular injury and impaired autophagy caused by increased oxidative stress in rats fed a high fat-fructose diet. The morphological examination revealed markedly disturbed architecture of both hepatic and skeletal muscle tissues and vacuolated hepatocytes, indicating fat accumulation in the HFF group. Additionally, significant collagen deposition in the hepatic and skeletal muscle tissues of rats in the HFF group suggests that a proinflammatory diet can initiate fibrotic changes in hepatic and skeletal muscles that may rely on impaired autophagy and inflammation induced by oxidative stress.
Moreover, the hepatic and muscle tissues in the HFF group showed negative immunostaining for PCNA, a marker of cellular proliferation with a crucial role in DNA replication and repair. This was in accordance with previous research that reported mRNA and DNA damage and a decrease in testicular PCNA levels in rats fed an enriched fat diet [31]. Contrary to our finding, Rivière and his colleagues reported that a high fructose diet initially decreased PCNA levels in mice, followed by a subsequent increase in their levels in the olfactory epithelium [32]. In addition, another study reported that a high-fat diet increased positive PCNA immunostaining in rat prostate [33].
The therapeutic effect of metformin and IF was obvious by the significant reduction in insulin resistance and lipid panels with the elevation of serum antioxidants. However, to the best of our knowledge, the precise pathways and mechanisms are not so obvious. Different hypotheses have been reported to explain such mechanisms as the activation of PPAR-γ coactivator (PGC)-1α [34] and AMPK pathways that are involved in the regulation of mitochondria biogenesis and the autophagy process [35, 36].
On the contrary, Chausse et al. [37] observed different effects of IF on different tissues, with an increase in oxidative damage markers in the liver and brain without any change in the mitochondrial bioenergetics of the skeletal muscle. So, the impact of IF on mitochondrial dynamics and ultrastructure still needs to be elucidated.
Furthermore, metformin and IF exert a significant upregulation in the expression of genes regulating mitophagy, including PINK1, PARKIN, LAMP2, and PPAR-α, which, in turn, improve mitochondrial bioenergetics and decrease ROS formation.
Prior studies reported the protective effect of metformin against fibrosis in experimental and clinical studies [38, 39] through stimulation of AMPK and inhibition of TGF-β1 signaling pathways, as well as attenuation of ROS production and enhancement of mitochondrial function and autophagy [40].
In the current work, both metformin and intermittent fasting significantly minimized the hepatic collagen deposition and enhanced PCNA immunostaining that significantly exceeded the physiological level in the control rats, so this can be considered physiological compensation after the restoration of redox balance, which is important for the DNA repair process and cellular proliferation. So, the beneficial effects of metformin and IF on cellular fibrosis and proliferation are mostly due to their antioxidant properties and upregulation of mitophagy-related genes.
The impact of metformin and intermittent fasting on weight gain was assessed in the current work. Interestingly, metformin for 4 weeks does not seem to prevent weight gain, despite its robust metabolic changes. The favorable effect of metformin on body weight may require a longer duration. On the other hand, only intermittent fasting successfully precluded the weight gain in both the IF and MET-IF groups without significant differences in rat weight between these groups, providing strong evidence that the reduction of weight was driven by intermittent fasting and not by metformin. These findings emphasize the magnitude of intermittent fasting in maintaining a normal body weight and protecting against weight gain despite the continuation of the obesogenic proinflammatory diet.
5 Conclusion
Intermittent fasting has beneficial metabolic and molecular effects that meet those of metformin against proinflammatory diet-induced hepatocyte injury. Furthermore, the combination of intermittent fasting with metformin is safe and does not cause hypoglycemia. Applying intermittent fasting with metformin robustly upregulated the mitophagy genes, improved cellular repair, and prevented fibrosis in the liver and skeletal muscle, which in turn hindered the adverse metabolic effects of the proinflammatory diet. As intermittent fasting exhibited a therapeutic effect in equality to that of metformin, as shown in this study, this opens up the possibility of safely reducing the metformin dose to a lower effective dose and getting the same result as the usual dose when combined with intermittent fasting.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AMPK:
-
Adenosine mono-phosphate-dependent kinase
- GSH:
-
Glutathione GSH
- HDL:
-
High-density lipoprotein
- HFF:
-
High fat-fructose
- HOMA-IR:
-
Homeostasis model of insulin resistance
- IF:
-
Intermittent fasting
- LAMP:
-
Lysosomal-associated membrane protein
- LDL:
-
Low-density lipoprotein
- MDA:
-
Malondialdehyde
- mtDNA:
-
Mitochondrial DNA
- PARKIN:
-
Parkin RBR E3 ubiquitin-protein ligase
- PCNA:
-
Proliferating cell nuclear antigen
- PGC:
-
Peroxisome proliferator-activated receptor-γ coactivator
- PINK1:
-
PTEN-induced kinase 1
- PPAR:
-
Peroxisome proliferator-activated receptor
- qPCR:
-
Quantitative real-time quantitative PCR
- ROS:
-
Reactive oxygen species
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
- TGF:
-
Transforming growth factor
References
Gill PA, Inniss S, Kumagai T et al (2022) The role of diet and gut microbiota in regulating gastrointestinal and inflammatory disease. Front Immunol 13:866059. https://doi.org/10.3389/fimmu.2022.866059
Yustisia I, Tandiari D, Cangara MH et al (2022) A high-fat, high-fructose diet induced hepatic steatosis, renal lesions, dyslipidemia, and hyperuricemia in non-obese rats. Heliyon 8(10):e10896. https://doi.org/10.1016/j.heliyon.2022.e10896
Meng XM, Ma XX, Tian YL et al (2017) Metformin improves the glucose and lipid metabolism via influencing the level of serum total bile acids in rats with streptozotocin-induced type 2 diabetes mellitus. Eur Rev Med Pharmacol Sci 21(9):2232–2237
Anton SD, Moehl K, Donahoo WT et al (2018) Flipping the metabolic switch: understanding and applying the health benefits of fasting. Obesity 26(2):254–268. https://doi.org/10.1002/oby.22065
Washburn RL, Cox JE, Muhlestein JB et al (2019) Pilot study of novel intermittent fasting effects on metabolomic and trimethylamine N-oxide changes during 24-hour water-only fasting in the FEELGOOD Trial. Nutrients 11(2):246. https://doi.org/10.3390/nu11020246
Springer MZ, Macleod KF (2016) In brief: mitophagy—mechanisms and role in human disease. J Pathol 240(3):253–255. https://doi.org/10.1002/path.4774
Thomas RL, Gustafsson AB (2013) Mitochondrial autophagy—an essential quality control mechanism for myocardial homeostasis. Circ J 77(10):2449–2454. https://doi.org/10.1253/circj.cj-13-0835
Cheng J, Xu L, Yu Q et al (2022) Metformin alleviates long-term high-fructose diet-induced skeletal muscle insulin resistance in rats by regulating purine nucleotide cycle. Eur J Pharmacol 933:175234. https://doi.org/10.1016/j.ejphar.2022.175234
Hazman M (2022) Gel express: a novel frugal method quantifies gene relative expression in conventional RT-PCR. Beni-Suef Univ J Basic Appl Sci 11:11. https://doi.org/10.1186/s43088-022-00194-3
Wills ED (1987) Evaluation of lipid peroxidation in lipids and biological membranes. In: Snell K, Mullock B (eds) Biochemical toxicology: a practical approach. IRL Press
Covaci A, Voorspoels S, Thomsen C, van Bavel B, Neels H (2006) Evaluation of total lipids using enzymatic methods for the normalization of persistent organic pollutant levels in serum. Sci Total Environ 366(1):361–366. https://doi.org/10.1016/j.scitotenv.2006.03.006
Allain CC, Poon LS, Chan CS et al (1974) Enzymatic determination of total serum cholesterol. Clin Chem 20(4):470–475
Krishnaveni P, Gowda VM (2015) Assessing the validity of Friedewald’s formula and Anandraja’s formula for serum LDL-cholesterol calculation. J Clin Diagn Res JCDR 9(12):BC01-BC4. https://doi.org/10.7860/JCDR/2015/16850.6870
Matthews DR, Hosker JP, Rudenski AS et al (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7):412–419. https://doi.org/10.1007/BF00280883
Bancroft JD, Gamble M (2008) Theory and practice of histological techniques, 6th edn. Churchill Livingstone, Elsevier
Tappy L, Lê KA (2010) Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev 90(1):23–46. https://doi.org/10.1152/physrev.00019.2010
Wang Y, An H, Liu T et al (2019) Metformin improves mitochondrial respiratory activity through activation of AMPK. Cell Rep 29(6):1511-1523.e5. https://doi.org/10.1016/j.celrep.2019.09.070
Eiyama A, Okamoto K (2015) PINK1/Parkin-mediated mitophagy in mammalian cells. Curr Opin Cell Biol 33:95–101. https://doi.org/10.1016/j.ceb.2015.01.002
Koyano F, Okatsu K, Kosako H et al (2014) Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature 510(7503):162–166. https://doi.org/10.1038/nature13392
Anderson EJ, Lustig ME, Boyle KE et al (2009) Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Investig 119(3):573–581. https://doi.org/10.1172/JCI37048
Zummo FP, Krishnanda SI, Georgiou M et al (2022) Exendin-4 stimulates autophagy in pancreatic β-cells via the RAPGEF/EPAC-Ca2+-PPP3/calcineurin-TFEB.axis. Autophagy 18(4):799–815. https://doi.org/10.1080/15548627.2021.1956123
Yamashima T, Seike T, Oikawa S et al (2023) Hsp70.1 carbonylation induces lysosomal cell death for lifestyle-related diseases. Front Mol Biosci 9:1063632. https://doi.org/10.3389/fmolb.2022.1063632
Soto-Avellaneda A, Morrison BE (2020) Signaling and other functions of lipids in autophagy: a review. Lipids Health Dis 19(1):214. https://doi.org/10.1186/s12944-020-01389-2
Mandard S, Müller M, Kersten S (2004) Peroxisome proliferator-activated receptor alpha target genes. Cell Mol Life Sci CMLS 61(4):393–416. https://doi.org/10.1007/s00018-003-3216-3
Marx N, Mackman N, Schönbeck U et al (2001) PPAR alpha activators inhibit tissue factor expression and activity in human monocytes. Circulation 103(2):213–219. https://doi.org/10.1161/01.cir.103.2.213
Neve BP, Corseaux D, Chinetti G et al (2001) PPAR alpha agonists inhibit tissue factor expression in human monocytes and macrophages. Circulation 103(2):207–212. https://doi.org/10.1161/01.cir.103.2.207
Montagner A, Polizzi A, Fouché E et al (2016) Liver PPARα is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut 65(7):1202–1214. https://doi.org/10.1136/gutjnl-2015-310798
Todisco S, Santarsiero A, Convertini P et al (2022) PPAR alpha as a metabolic modulator of the liver: role in the pathogenesis of nonalcoholic steatohepatitis (NASH). Biology 11(5):792. https://doi.org/10.3390/biology11050792
Houghton MJ, Kerimi A, Tumova S et al (2018) Quercetin preserves redox status and stimulates mitochondrial function in metabolically-stressed HepG2 cells. Free Radic Biol Med 129:296–309. https://doi.org/10.1016/j.freeradbiomed.2018.09.037
Inci MK, Park SH, Helsley RN et al (2023) Fructose impairs fat oxidation: Implications for the mechanism of western diet-induced NAFLD. J Nutr Biochem 114:109224. https://doi.org/10.1016/j.jnutbio.2022.109224
Moradi-Ozarlou M, Moshari S, Rezaei Agdam H et al (2021) High-fat diet-induced obesity amplifies HSP70-2a and HSP90 expression in testicular tissue; correlation with proliferating cell nuclear antigen (PCNA). Life Sci 279:119633. https://doi.org/10.1016/j.lfs.2021.119633
Rivière S, Soubeyre V, Jarriault D et al (2016) High fructose diet inducing diabetes rapidly impacts olfactory epithelium and behavior in mice. Sci Rep 6:34011. https://doi.org/10.1038/srep34011
Tikoo K, Vikram A, Shrivastava S et al (2017) Parental high-fat diet promotes inflammatory and senescence-related changes in prostate. Oxid Med Cell Longev. https://doi.org/10.1155/2017/4962950
Antarianto RD, Kadharusman MM, Wijaya S et al (2023) The impact of prolonged and intermittent fasting on PGC-1α, Oct-4, and CK-19 liver gene expression. Curr Aging Sci 16(1):49–55. https://doi.org/10.2174/1874609815666220627155337
Rena G, Hardie DG, Pearson ER (2017) The mechanisms of action of metformin. Diabetologia 60(9):1577–1585. https://doi.org/10.1007/s00125-017-4342-z
Qi Z, Ding S (2012) Transcriptional regulation by nuclear corepressors and PGC-1α: implications for mitochondrial quality control and insulin sensitivity. PPAR Res. https://doi.org/10.1155/2012/348245
Chausse B, Vieira-Lara MA, Sanchez AB et al (2015) Intermittent fasting results in tissue-specific changes in bioenergetics and redox state. PLoS ONE 10(3):e0120413. https://doi.org/10.1371/journal.pone.0120413
Juban G, Saclier M, Yacoub-Youssef H et al (2018) AMPK activation regulates LTBP4-dependent TGF-β1 secretion by pro-inflammatory macrophages and controls fibrosis in Duchenne muscular dystrophy. Cell Rep 25(8):2163-2176.e6. https://doi.org/10.1016/j.celrep.2018.10.077
Pinyopornpanish K, Leerapun A, Pinyopornpanish K et al (2021) Effects of metformin on hepatic steatosis in adults with nonalcoholic fatty liver disease and diabetes: insights from the cellular to patient levels. Gut Liver 15(6):827–840. https://doi.org/10.5009/gnl20367
Wu M, Xu H, Liu J, Tan X, Wan S, Guo M et al (2021) Metformin and fibrosis: a review of existing evidence and mechanisms. J Diabetes Res. https://doi.org/10.1155/2021/6673525
Acknowledgments
Not applicable.
Funding
No funding source.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Nermeen Bastawy, Omnia Mohamed, Nermeen Bakr, Doaa Ghareeb, Laila Rashed, Mai Abdelaziz, Hanan Abdalla, Dina Hisham. The first draft of the manuscript was written by Ghada Farouk, Nermeen Bakr, and Nermeen Bastawy. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All the experiment procedures were conducted according to the regulations of the Institutional Animal Care and Use Committee (CU-IACUC), Cairo University (CU-III-F-36-21).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bastawy, N., Soliman, G.F., Sadek, N.B. et al. Metformin and intermittent fasting mitigate high fat-fructose diet-induced liver and skeletal muscle injury through upregulation of mitophagy genes in rats. Beni-Suef Univ J Basic Appl Sci 13, 88 (2024). https://doi.org/10.1186/s43088-024-00548-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43088-024-00548-z