Abstract
Background
Throughout the storage of blood, the red cells undergo alterations known as “storage lesions,” which involve shape changes and the formation of microparticles (MPs). Studies of the formation of red cell microparticles (RMPs) emphasize the prospective application of RMPs as a quality control measure in the preparation and storage of blood components in the future. In the present study, twenty packed RBC units in citrate phosphate dextrose adenine-1 (CPDA1) were collected from volunteers and stored for 35 days. Over 35 days of storage, samples were collected at six distinct time points weekly and evaluated for the presence of RMPs. MPs were separated by the ultracentrifugation method. Electron microscopy was used to characterize the morphology and size of the isolated microparticles, and flow cytometry was performed to determine the percentage of RMPs that expressed glycophorin A (CD235a) and Annexin V antigens. RMPs' procoagulant activity (PCA) was assessed using a plasma recalcification test. RMP concentration in accordance with ABO blood grouping was assessed by using various types of donated blood groups.
Results
RMPs progressively increased over storage. The procoagulant activity (PCA) exhibited a significant increase during storage, as evidenced by a shorter plasma recalcification time (P value = 0.001). A significant negative correlation (P value = 0.001) between plasma recalcification time and Annexin V-positive microparticles, as well as a dual-positive Annexin V/CD235a population, was identified, indicating a strong correlation between the direct quantitative assay by flowcytometry and the functional assay through the PCA.
Conclusion
RMPs increase on storage with increased PCA. Finding ways to reduce these microparticles in packed RBC units is crucial for reducing the risk of transfusion-related coagulopathy.
Similar content being viewed by others
1 Background
Cell-derived microparticles (MPs) are small membrane microvesicles with a diameter of less than 1 μm, released by cells following apoptosis, activation, and injury. The aging of red blood cells (RBCs) involves the release of MPs, which are a significant component of storage lesions [1]. Throughout the storage of blood, RBCs exhibit storage lesions involving alteration in shape, release of microparticles, and oxidative damage to membrane components. The quantity of RMPs in RBC concentrates depends on the manufacturing techniques, the different additive solutions, and the variability of donors [2]. The existence of RMPs in blood units can cause severe transfusion reactions such as thrombosis, inflammation, and immunomodulation on behalf of their procoagulant and pro-inflammatory abilities [3, 4]. The procoagulant activity of RMPs arose from the presence of a negatively charged phospholipid on their outer surface (phosphatidyl serine), which is important for the production of thrombin [5]. New methodologies are needed to monitor and quantify the release of MPs in RBC units and assess their possible influence on blood transfusions. This leads us to study the changes in RMPs in packed red blood cell transfusion units as a potential safety biomarker by determining their ultrastructure, quantity, and procoagulant activity in relation to storage duration. Monitoring MP production in packed RBC units and quantification were done by using flow cytometry; the procoagulant activity of RMPs was done by coagulation tests; and the correlation of procoagulant activity and RMPs was assessed. A scanning electron microscope was used to identify the morphology and size of the RMPs.
2 Methods
2.1 Participants
After obtaining informed consent from 20 healthy blood donor volunteers, packed RBCs were processed by the blood bank in accordance with current standards and stored at 4 °C for 35 days. Selected donors had normal coagulation profiles tested by the prothrombin time (PT), activated partial thromboplastin time (APTT), and platelet count.
2.2 Packed RBC preparation
An amount of 450 ± 50 ml of whole blood was collected by phlebotomy in JMS CPDA1 blood collection bags with 63 ml of citrate phosphate dextrose adenine‑1 (CPDA1) as an anticoagulant. After the collection of a unit of whole blood in the primary bag, packed RBCs were separated from platelet-rich plasma by centrifugation at 1500 g for 10 min at 4 °C. Platelet-rich plasma was then transferred to a satellite bag. Leucocytes and platelets were removed by laboratory filter log 4. (Macropharma, France). The whole blood unit was processed for component separation within 6 h of collection.
2.3 Sample collection and RBC microparticles isolation
Samples were collected from CPDA1 blood units under aseptic conditions at different intervals throughout the storage, every week, i.e., days 1, 7, 14, 21, 28, and 35 days. This was done to investigate the production of MPs from red blood cells during storage and to assess their procoagulant activity (PCA) following the guidelines of the International Society of Thrombosis and Hemostasis [6]. Collected samples were doubled centrifuged at 3000 g for 15 min at 4 °C to obtain supernatant-containing microparticles for further flow cytometric analysis, and then an extra centrifugation step at 18,000 g for 45 min at 4 °C was performed to obtain a concentrated pellet of microparticles for coagulation and electron microscopic studies.
2.4 Flow cytometric identification of red blood cell microparticles
Flow cytometric analysis was performed using BD Accuri C6 flow cytometer on freshly prepared MPs. Dual staining for the MPs was done by adding 2 µl of antihuman CD235a-PE monoclonal antibody (BioLegend catalog number 349106) and 5 µl of annexin V FITC monoclonal antibody (BioLegend catalog number 640906) to 50 µl of supernatant-containing MPs and incubated for 20 min in the dark. Control tubes were used by incubating samples with phosphate-buffered saline (PBS). Data were collected using logarithmic amplification, after acquiring at least 10.000 events; the area of interest was determined by back gating.
2.5 Coagulation assay
After obtaining a concentrated pellet of MPs, 100 μl of PBS solution was added, and the prepared suspension was used to determine its procoagulant activity through a plasma recalcification time test by using the coagulation analyzer (BFT II Analyzer). 50 μl of MPs suspension was added to 50 μl of fresh normal plasma and incubated at 37 °C for 180 s, then 50 μl of prewarmed CaCl2 was added to start the reaction, and the coagulation time was recorded.
2.6 Electron microscopic examination of red blood cell microparticles.
Samples are then mounted on carbon-coated adhesive material and examined by FEI-Philips electron microscopy.
2.7 Statistical analysis
The data were statistically described in terms of mean and standard deviation (± SD). The comparison between different groups in the present study was done using the Tamhane test as a post hoc test followed the one-way analysis of variance test (ANOVA). The correlation between quantitative variables was done using the Spearman correlation coefficient. P < 0.05 was considered statistically significant. All statistical calculations were done using IBM SPSS statistics (V. 28.0.1.1., IBM Corp., Armonk, NY, USA).
3 Results
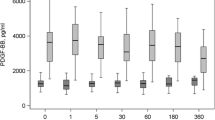
Our results show that the percent of expression of CD235a on MPs shows no significant difference from day 1 to day 35 (P value = 0.5). On the other hand, Annexin V expression on MPs shows a highly significant increase on day 35 compared to day 1 (P value = 0.001). On studying the coexpression of CD235a and Annexin V on MPs, we detected a highly significant increase in their coexpression on day 35 compared to day 1 (P value = 0.001). In addition, we found that CT showed a highly significant shortage on day 35 compared to day 1 (p value = 0.001). (Table 1, Figs. 1, 2, 3 and 4).
On comparing day 1 with other days, we did not find any significant difference regarding CD235a expression, while Annex V expression shows a significant difference on days 21, 28 and 35(P value = 0.02, 0.01 and 0.001, respectively). On the other hand, there was a highly significant difference in CD235a and Annex V coexpression on most days except day 7. In addition, CT shows a significant difference only on day 35 (P value = 0.001). Regarding the comparison of day 7 with other days, we found significant differences regarding CT on all days except day 14. In contrast, CD235a expression and Annex V expression show no significant difference except for day 21 in Annex V expression. CD235a and Annex V coexpression show a significant difference on most days except day 35. Days 14, 28 and 35 show a significant difference in CT compared to other days. On the other hand, CD235a and Annex V show no significant difference when comparing days 14, 28, and 35 to other days. CD235a and Annexin V coexpression show a highly significant difference only when comparing day 14 with days 21 and 28 as shown in Table 2.
A significant negative correlation between the expression of Annexin V and the coexpression of Annexin V/CD 235a with the coagulation time (P = 0.001) was found, and this confirms the correlation between the MPs count and its procoagulant activity as shown in Table 3 and Figs. 5 and 6.
On comparing RMP concentration in accordance with ABO blood group type, where all results obtained at day 21, we found that lower RMP concentration was in units donated by type AB donors (mean of 2.5 ± 1.3 RMPs/µL) compared with type B (mean of 8 ± 8.3 RMPs/µl, P = 0.45), type A (mean of 10.6 ± 2.3 RMPs/µL, P = 0.04), while O donors (mean of 14 ± 4.9 RMPs/µL, P = 0.05) show the highest level of RMPs concentration (Fig. 7).
3.1 Scanning electron microscopic (SEM) study of isolated microparticles
Examination of the RBCs microparticles isolated from stored RBCs bag by scanning electron microscope shows globular structures with a high degree of symmetry ranging in size from 550: 916 nm MPs (Fig. 8).
4 Discussion
Providing useful blood components is the goal of blood preservation. Understanding the functional alterations that take place during storage may help develop better preservation techniques, enhancing the safety of blood products [7]. Storage lesions refer to the changes that occur in blood components during the storage process, which can affect their quality and functionality. One of the parameters that can be used to assess storage lesions is the development of microparticles (MPs), which are tiny vesicles released by cells during storage [6, 8].
In the present study, electron microscopy was used to characterize the morphology and size of isolated microparticles, and flow cytometry was used to characterize the percentage of RMPs based on the expression of glycophorin A (CD235a) and Annexin V antigens. The procoagulant activity (PCA) of RMPs was tested by plasma recalcification test. RMPs concentration in accordance with ABO blood grouping was assessed by using various types of donated blood groups. Our findings align with prior research indicating that storage lesions cause red cell disintegration and microparticle production [9]. The percentage of RMPs rapidly grew during storage, reaching statistical significance around day 14 [10].
Controversy has arisen regarding the clinical effect of blood storage, where some studies suggested that storing lesions for 14 days or more has a negative influence on mortality and morbidity [11]. Whereas others did not indicate such an effect [12]. The duration of storage required for red cell microparticle formation was studied, and a clear difference between results of different studies was observed. A previous study found that RMPs gradually increased after day 10 and continued to do so over time [13]. On the other hand, other studies proved that RMP formations increased after that time, which could be due to the difference in processing techniques and the variation of types of the storage blood bags and the additives that were used [14].
It was found that the MP concentration exhibited variability among different donors. The cause of this variation remains unknown; however, factors such as donor age and gender, and ABO blood group could potentially play a role and should be further examined. Additionally, the selection of the isolation method and preanalytical factors represents a significant source of variability and artifacts in MP analysis [15,16,17].
Our results showed that RMPs in stored packed RBC units, especially those expressing Annexin V, exhibit procoagulant properties as evidenced by a reduction in clotting time starting from day 21 of storage. The observed reduction in clotting time around day 21 of storage suggests that the procoagulant activity of RMPs becomes more pronounced with prolonged storage duration. Clinically, this finding highlights the importance of considering the age of stored PRBC units when transfusing patients, especially those at higher risk of thrombosis. Understanding the kinetics of RMP-mediated coagulation may have implications for the optimal timing of transfusion and the management of patients requiring blood products.
A previous study has reported a similar temporal pattern of procoagulant activity in stored PRBC units [18]. Other studies reported a different timing where a reduction in CT started earlier, on day 7 of storage [17]. This controversy depends on many factors, such as storage conditions, sample size, and assay methods used for clotting time measurement.
Our results also showed a strong correlation between PCA in stored packed RBCs and the quantity of RMPs assayed by flow cytometry. Consistent with these results, the RMPs’ procoagulant effect is well-documented and represents one of the most investigated aspects of their impact on illness outcomes. RBC lysates have been known to reduce plasma clotting time since 1961 [19]. In agreement with our results, Hashemi Tayer et al stated that RBC-derived microvesicles especially those expressing Annexin V exhibit procoagulant properties [18]. Reducing MVs in RBC concentrates may minimize the risk of transfusion-induced thrombotic events. According to Wesseling et al. (2016), filtering RBC lysate with a 0.22-μm filter eliminated its thrombogenic potential. The result suggests that RBC membranes and possibly RMPs play a significant role in thrombogenesis, as opposed to soluble proteins. Phosphatidyl serine on the outer membrane mediates the procoagulant activity of RMPs [20]. In the presence of calcium, the negatively charged PS binds to gammacarboxyglutamic acid-rich domains of coagulation factors, forming tenase (Xase) and prothrombinase (PTase) complexes [21, 22]. Moreover, it is found that microparticles contain tissue factor, which plays an important function in coagulation [23]. Tissue factor, also known as coagulation factor III or tissue thromboplastin, plays a crucial role in the extrinsic pathway of the coagulation cascade resulting in thrombin generation and clot formation [24]. Additionally, it is observed that RMPs can stimulate the formation of thrombin independent of tissue factor through the intrinsic pathway of coagulation [25]. RMPs were also shown to promote phenotypic changes in platelets, which enhance surface expression of P-selectin and activated GPIIb/IIIa when platelets were exposed to RMPs [1].
On comparing the concentration of RMPs in different blood groups on day 21, we found that AB donors had the lowest level of RMPs followed by blood group B and then blood group A while O donors showed the highest level of RMPs concentration. In contrast to our results, Gamonet et al. found that blood groups B and AB donor packed RBC units appear to be most affected by storage [26]. This discrepancy in results may be due to that ABO and RhD blood phenotypes differ between ethnic groupings. When creating evidence-based blood collection and management procedures, it is important to account for the diverse distribution of ABO and RhD blood groups across ethnic groups [27]. It was found that the MP concentration exhibited variability among different donors. Factors such as donor age and gender, and ABO blood group could potentially play a role and should be further examined. The rationale for relating the concentration of RMPs to different blood groups in our research was to study the prevalence of the blood groups that have a higher rate of microparticle formation. Hence our findings might provide information to guide clinical practice and/or blood transfusion services in the region.
In conclusion, MP studies are quickly developing and can be used to analyze blood products by monitoring and quantifying their release during RBC storage as a future quality control measure during blood component preparation and storage to maintain their functional integrity during storage and to be safe for transfusion to patients in need. We need to better understand the functional and clinical significance of diverse types of MPs, including their cellular origin, structural characteristics, protein composition, and triggering factors. Future research should focus on reducing microparticles in aged RBC units to improve patient outcomes and minimize transfusion-related problems.
Availability of data and material
No supplementary data.
Abbreviations
- MPs:
-
Microparticles
- RMPs:
-
Red cell microparticles
- CPDA1:
-
Citrate phosphate dextrose adenine-1
- PCA:
-
Procoagulant activity
- PT:
-
Prothrombin time
- APTT:
-
Activated partial thromboplastin time
- PBS:
-
Phosphate-buffered saline
References
Kim Y, Goodman MD, Jung AD et al (2020) Microparticles from aged packed red blood cell units stimulate pulmonary microthrombus formation via P-selectin. Thromb Res 185:160–166
Noulsri E, Palasuwan A (2018) Effects of donor age, donor sex, blood-component processing, and storage on cell-derived microparticle concentrations in routine blood component preparation. Transfus Apher Sci 57:587–592
Gradziuk M, Radziwon P (2017) Methods for detection of microparticles derived from blood and endothelial cells. Acta Haematol Pol 48:316–329
Lan X, Chen Y, Bi Q et al (2023) Effects of storage duration of suspended red blood cells before intraoperative infusion on coagulation indexes, routine blood examination, and immune function in patients with gastrointestinal tumors. Pak J Med Sci 39(1):182–187
Lalic-Cosic S, Dopsaj V, Kovac M et al (2021) Phosphatidylserine exposing extracellular vesicles in pre-eclamptic patients. Front Med (Lausanne). 8:761453
Gamonet C, Mourey G, Aupet S et al (2017) How to quantify microparticles in RBCs? A validated flow cytometry method allows the detection of an increase in microparticles during storage. Transfusion 57(3):504–516. https://doi.org/10.1111/trf.13989
Levin G, Sukhareva E, Lavrentieva A (2016) Impact of microparticles derived from erythrocytes on fibrinolysis. J Thromb Thrombolysis 41:452–458
Kostova EB, Beuger BM, Klei TR et al (2015) Identification of signaling cascades involved in red blood cell shrinkage and vesiculation. Biosci Rep 35:1–16
Kheansaard W, Phongpao K, Paiboonsukwong K et al (2018) Microparticles from β thalassemia/HbE patients induce endothelial cell dysfunction. Sci Rep 8:13033
Rana M, Arafat Y, Naseem O et al (2020) Levels of red blood cells derived microparticles in stored erythrocyte concentrate. Afr J Pharm Pharmacol 14:185–191
Wang D, Sun J, Solomon SB (2012) Transfusion of older stored blood and risk of death: a meta-analysis. Transfus 52(6):1184–1195
Mc Quilten ZK, French CJ, Nichol A, Higgins A et al (2018) Effect of age of red cells for transfusion on patient outcomes: a systematic review and meta-analysis. Transfus Med Rev 32(2):77–88
Jy W, Ricci M, Shariatmadar S et al (2011) Microparticles in stored red blood cells as potential mediators of transfusion complications. Transfusion 51(4):886–893
Ransford K, Samuel AB, Max AA et al (2020) Comediation of erythrocyte haemolysis by erythrocyte-derived microparticles and complement during malaria infection. Adv Hematol 2020:1–5. https://doi.org/10.1155/2020/1640480
Almizraq R, Tchir JD, Holovati JL et al (2013) Storage of red blood cells affects membrane composition, microvesiculation, and in vitro quality. Transfusion 53(10):2258–2267
Rubin O, Crettaz D, Canellini G et al (2008) Microparticles in stored red blood cells: an approach using flow cytometry and proteomic tools. Vox Sang 95:288–297
Fouda R, Aboul Enein A, El-Desoukey N et al (2020) Impact of storage, leukofiltration, and ascorbic acid fortification on red cell-derived microparticles in stored packed red blood cells: a flow cytometric and procoagulant study. J Appl Hematol 11(2):51–58
Hashemi Tayer A, Amirizadeh N, Ahmadinejad M (2019) Procoagulant Activity of red blood cell-derived microvesicles during red cell storage. Transfus Med Hemother 46(4):224–230
Bradlow BA (1961) Liberation of material with platelet-like coagulant properties from intact red cells and particularly from reticulocytes. Br J Haematol 7:476–495
Wesseling MC, Wagner-Britz L, Nguyen DB et al (2016) Novel insights in the regulation of phosphatidylserine exposure in human red blood cells. Cell Physiol Biochem 39:1941–1954
Vallier L, Cointe S, Lacroix R et al (2016) Microparticles and fibrinolysis. Semin Thromb Hemost 43:129–134
Thangaraju K, Neerukonda SN, Katneni U, Buehler PW (2021) Extracellular vesicles from red blood cells and their evolving roles in health, coagulopathy and therapy. Int J Mol Sci 22(1):153. https://doi.org/10.3390/ijms22010153
Gong Y, Kim SO, Felez J et al (2001) Conversion of Glu-plasminogen to Lys-plasminogen is necessary for optimal stimulation of plasminogen activation on the endothelial cell surface. J Biol Chem 276:19078–19083
Said AS, Rogers SC, Doctor A (2018) Physiologic impact of circulating RBC microparticles upon blood-vascular interactions. Front Physiol 8:1120
Koshiar RL, Somajo S, Norstrom E (2014) Erythrocyte-derived microparticles supporting activated protein C-mediated regulation of blood coagulation. PLoS ONE 9:e104200
Gamonet C, Desmarets M, Mourey G et al (2020) Processing methods and storage duration impact extracellular vesicle counts in red blood cell units. Blood Adv 4:5527–5539
Liu J, Zhang S, Wang Q et al (2017) Frequencies and ethnic distribution of ABO and RhD blood groups in China: a population-based cross sectional study. BMJ 7:e018476
Acknowledgements
This work is based upon Research project (Grant No 109 SH) supported by Theodor Bilharz Research Institute (TBRI). The role of central lab, blood bank and electron microscopy lab in Theodor Bilharz Research institute is much appreciated. We are grateful for Dr. Rady Elaraby, TBRI. For his efforts in statistical analysis of the results.
Funding
This work is based upon Research project (Grant No 109 SH) supported by Theodor Bilharz Research Institute (TBRI).
Author information
Authors and Affiliations
Contributions
Nagwa AbdelKhalek ElKhafif. Put the conception and design of the study; Ayat SalahEldin Hassan, Rabab Fouad Yassin and Noha Abdelaal Amin performed the practical work of the study; Ayat SalahEldin Hassan and Rabab Fouad Yassin revised and analysed the results; Nagwa AbdelKhalek ElKhafif and Ayat SalahEldin Hassan analysed and interpreted the electron microscopy data; Ayat SalahEldin Hassan and Rabab Fouad Yassin were primarily responsible for writing the manuscript, drafting of the paper, revising it critically for intellectual content; and the final approval of the version to be published; and all authors agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study received approval from the ethics committee of Theodor Bilharz Research Institute, Giza-Egypt under federal wide assurance number FWA00010609. Written consent was granted in all donors.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing financial or non financial interests, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hassan, A.S.M., ElKhafif, N.A., Amin, N.A. et al. Procoagulant activity of red blood cell microparticles in stored packed red blood cell units and its relation to ABO blood grouping. Beni-Suef Univ J Basic Appl Sci 13, 51 (2024). https://doi.org/10.1186/s43088-024-00509-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43088-024-00509-6