Abstract
Background
Despite the fact that natives of Southeast Asia have been consuming Prismatomeris glabra for decades for a variety of health benefits, research on this species is not as extensive as that on other species due to its limited distribution. The purpose of this study was to determine the cytotoxicity and identify the bioactive compounds of P. glabra crude leaf extracts against the MCF-7 cell line.
Results
We first examined the potential cytotoxic activity of P. glabra using the MTT assay against the MCF-7 cell line to determine the IC50 of the plant extracts at various concentrations at three time points (24 h, 48 h, and 72 h). Across all time points, the MTT assay revealed that the aqueous extract exhibited the lowest IC50 values (p < 0.05) compared to the ethanol and methanol extracts. All plant extracts exerted the ability to induce cell death in the MCF-7 cell line at all time points, and the optimal time for P. glabra to manifest its antiproliferative activities and promote cell death was 48 h. LC–MS analysis was conducted to reveal the components in plant extracts. Forty compounds were discovered in P. glabra's extracts, with the majority being flavonoids and triterpenoids. Five similar compounds were present in all three extracts. Further research should be conducted on these compounds to unveil a compound that fulfils the criteria as a promising anticancer agent. This research is of the utmost importance, as it provides a fundamental framework for the identification of alternative therapies for breast cancer and contributes implicitly to the development of new drugs.
Conclusions
This study discovered that P. glabra crude leaf extracts have the potential to inhibit the MCF-7 cell line by inducing cell death.
Similar content being viewed by others
1 Background
Cancer is the second-leading cause of death worldwide, accounting for an estimated 9.6 million deaths in 2018. The most common types of cancer diagnosed worldwide are breast, colorectal, lung, cervix, and thyroid [1]. It has been reported that breast cancer is the most prevalent cancer worldwide, and this includes the Malaysian population [2]. Furthermore, it is the second leading cause of death among women [3]. According to the American Cancer Society breast cancer treatment will vary depending on the patient's type, stage, and overall health, and will typically include surgery, radiation, chemotherapy, hormone therapy, targeted therapy, and immunotherapy [4]. The standard modalities possess numbers of constraints including toxicity, side effects, inconsistent patient treatment results [5], multiple resistance mechanisms [6,7,8], limited therapeutic efficacy [9, 10], costly [9].
For decades, it was discovered that plant parts are abundant in medicinal compounds that can be used to treat a wide range of human and animal ailments [11]. The vast majority of pharmaceuticals available today are derived from plants [12]. Herbal demand is increasing for a variety of reasons, including efficacy claims for relieving disease symptoms and providing a cure, being more cost-effective, and offering a safer treatment option than other therapies [13]. Moreover, unlike synthetic chemical-based drugs, direct plant-based medicines have no adverse effects [14]. But the growth of the herbal market has to deal with problems like conservation, proper scientific research based on traditional knowledge, quality control, and proper documentation [15]. Not only that, but the safety level of any natural product must be addressed and documented [16]. Anticancer drugs are derived from a variety of medicinal plants, and these drugs offer numerous benefits. Bioactivities found in medicinal plants can inhibit the growth of cancer cells in a variety of ways [17]. In recent years, it has been shown that phytochemicals or synthetic drugs derived from natural resources like Taxus baccata (paclitaxel), topoisomerase I inhibitors (irinotecan, etoposide, and doxorubicin), and Madagascar periwinkle (including vincristine, vinblastine, vinorelbine, and vindesine) have beneficial effects in various of cancer types [18]. More than 50% of cancer drugs available in clinical trials today are derived from natural sources [19].
There are 15 Prismatomeris species that grow in tropical Asia [20, 21]. Several studies on Prismatomeris species such as Prismatomeris connata (P. connata), Prismatomeris tetrandra (P. tetrandra), and Prismatomeris malayan (P. malayan) have found bioactive compounds including anthraquinones, glycosides, triterpenoids, and iridoids [22,23,24,25]. These compounds have demonstrated as antitumor, cytotoxic, anticancer, antimalarial, antifungal, antituberculosis, and antiplasmodial properties [24,25,26,27]. Each species of Prismatomeris, such as P. connata, P. tetrandra, and Prismatomeris fragrans (P. fragrans), has unique uses. As an example, traditional Chinese medicine uses the root of P. connata to treat pneumoconiosis, leukocytic, hepatitis, leukaemia, and anaemia [28]. Meanwhile, in Chinese traditional medicine as well, P. tetrandra roots were used to treat anaemia, gingival bleeding, hepatitis, and leucocythemia [29]. The roots of the P. fragrans plant were used in traditional medicine as a tonic derived from a water decoction [30]. Prismatomeris glabra (P. glabra) (Rubiaceae) [31, 32], or locally known as Ajisamat, Haji samat, or Tongkat Haji Samat, is typically distributed in tropical and subtropical areas up to 700 m altitude in Southeast Asia, including Peninsular Malaysia, Sumatra, and Borneo [20, 22, 23, 33]. The research on P. glabra is not as widely studied as that on other species due to its limited distribution. Two studies have discovered the presence of anthraquinones in P. glabra [32, 34]. In 2021, the presence of sterols and flavonoids was identified in P. glabra [35]. According to Mohamad et al. the cytotoxicity effect of P. glabra is contributed by the presence of anthraquinones. Anthraquinones have also been proven to have anticancer properties in a few studies [34, 36]. The phenolic hydroxyl (OH) group of anthraquinones is the main factor in their antitumor properties [22]. Feng et al. have showed that hydroxylation at the C-1 position of anthraquinones leads to a substantial elevation of cytotoxicity activity, which implies the importance of the phenolic OH group for the antitumor activity of anthraquinones. Furthermore, the position of hydroxyl groups (C-5 and C-8) in anthraquinone monomers is vital for antitumor efficacy [37]. In anthraquinone molecules, C-1 and C-3 are the critical functional sites for antitumor activities [38]. Anthraquinones have also demonstrated antioxidant properties [39] and are capable of inducing apoptosis [27]. The natives make extensive use of P. glabra for a variety of health benefits, including increased stamina, protection against tropical diseases, increased vitality [20], enhanced ergogenic effects [35], and increased sexual desire [40,41,42]. In light of the aforementioned shortcomings of standard modalities, this study intends to provide a fundamental framework to identify alternative therapies derived from natural sources. This study represents original research that utilizes three solvents for in vitro analysis and identification of bioactive compounds in the crude extract of P. glabra. To the best of our knowledge, this is the first study to report on the biological activity of anthraquinones against MCF-7 cells. We hypothesized that the anthraquinones in this study would demonstrate similar effects as in previous studies, but the level of efficacy of their antitumor properties would be subjected to the position of the phenolic OH group in their molecular structures. Here, we present our findings of the cytotoxicity effect of P. glabra’s extracts against the MCF-7 cell line at three time points (24 h, 48 h, and 72 h) as well as the identification of the potential bioactive compounds present in P. glabra’s extracts using liquid chromatography-mass spectrometry (LC–MS).
2 Methods
2.1 Extraction of Prismatomeris plants
Prismatomeris plants were collected from Tasik Kenyir, Hulu Terengganu, Terengganu Darul Iman, Malaysia, and identified with the accession number PG0001. The procedure for plant extraction was executed in accordance with Salleh et al. (2015), with minor adjustments [20]. P. glabra leaf was air-dried at room temperature and ground into a fine powder. Ten (10) grams of powdered P. glabra leaf was soaked in three types of solvents in 100 mL of 95% ethanol, and 100% methanol and distilled water (aqueous extract). Ethanol and methanol extracts were soaked for 24 h. Meanwhile, for the aqueous extract, 10 g of powdered P. glabra leaf were boiled in 100 mL of distilled water for 10 min. The suspension from the macerated and boiling process was filtered using Whatman filter paper No. 1, and rotary evaporation was used to remove the solvent. The filtrate was allowed to dry out at room temperature, and the resulting crude extracts (ethanol, methanol, and aqueous extracts) was stored at − 20 °C prior to analysis [43].
2.2 Cell culture
The MCF-7 cell line (ATCC® HTB-22™) (human breast adenocarcinoma) was obtained from the American Type Culture Collection (ATCC, Manassas, VA) and the culture procedure adhered to the guidelines provided by the manufacturer. The cell line was cultured in a 25 cm2 cell culture flask at 37 °C in a humidified atmosphere of 95% air and 5% CO2 in Roswell Park Memorial Institute (RPMI) 1640 medium (Nacalai Tesque Inc., Kyoto, Japan) supplemented with 10% (v/v) fetal bovine serum (FBS; TICO Europe, Netherlands), 1% (v/v) penicillin–streptomycin (Gibco, Thermo Fisher Scientific, USA), and 300 mg/litre of L-glutamine (Nacalai Tesque, Inc., Kyoto, Japan). The growth of the cell culture was monitored daily under an inverted light microscope (Nikon, Japan) for a number of reasons, including a change in the medium’s colour, contamination, and the morphology of the cell line. The old medium was replaced every 2 days with fresh, complete medium after a washing step using phosphate-buffered saline (PBS). When cells reached 90% confluence, they were washed with 3 mL of PBS three times following discarding the old media from the flask. This is to ensure all traces have been removed from the culture medium before detaching the cells with 2 mL of trypsin, and incubating at 37 °C for 5–7 min. The cells were examined microscopically under an inverted light microscope to ensure all cells were detached from the surface of the flask before adding 3 mL of fresh media. The fresh media (3 mL) was added into the flask, and the cell suspension was transferred into a 15 mL Falcon tube (Greiner Bio-One, Austria) using a 5 mL serological pipette (Greiner Bio-One, Austria). The suspension was centrifuged for 5 min at 12,000 rpm and 24 °C. The supernatant was gently discarded using a sterile Pasteur pipette (Nest Scientific, USA) without disturbing the cell pellet at the bottom of the Falcon tube. The fresh media (1 mL) was added into the cell pellet, and the cell suspension was mixed uniformly and gently. The cells are ready for subculturing, treatment, or storage.
2.3 Cell viability assay
The MTT (3-[4, 5-dimethylthiazaol-2-yl]—2, 5-diphenyltetrazolium bromide, Sigma-Aldrich Canada) assay was carried out to evaluate the percentage of cell viability of the MCF-7 cell line following treatment with different concentrations of crude extracts of P. glabra. The cell viability assay was conducted according to Beheshti et al. (2021) with certain adjustments [44]. At 90% cell confluence, the MCF-7 cell line was seeded in a flat-bottomed 96-well plate (Nest Scientific, USA) at a density of 2.0 × 104 cells/well in a volume of 100 µL for 24 h before being treated with complete medium and dimethylsulfoxide (DMSO) (controlled cells also called as untreated group) and various concentrations (500 µg/mL, 250 µg/mL, 125 µg/mL, 62.5 µg/mL, 31.3 µg/mL, and 15.6 µg/mL) of ethanol, methanol, and aqueous extracts. The cells were treated with complete medium only (untreated group) and various concentrations of extracts for 24 h, 48 h, and 72 h in triplicates. A volume of 10 µL of MTT solution was added to each well and incubated for 4 h at 37 °C. Post the incubation period, the mixture were removed and 100 µL of DMSO was added and was left incubated for 5 min. Finally, the absorbance was measured at 570 nm with reference to 630 nm using a microplate reader (TECAN, Switzerland). The experiment was repeated in triplicate. The percentage of cell viability was calculated according to the following formula [45]:
The half-maximal inhibitory concentration (IC50) of each P. glabra crude extract was determined from the dose–response graph. The concentration of P. glabra extracts that reduced cell viability by 50% was measured by graphing triplicate data points over a range of concentrations.
2.4 Microscopic examination of cellular morphology
The microscopic examination of cellular morphology was performed according to Khazaei et al. (2017), with certain adjustments [46]. In order to examine the induction of cell death through cellular morphological changes in the MCF-7 cell line following the cytotoxicity assay, cells were seeded at a density of 1 × 104 cells per well into a flat-bottomed 96-well containing 100 μL of fresh complete medium per well and incubated overnight at 37 °C in a CO2 incubator. Then, the old media was discarded. 100 µL of complete media (untreated group) and 100 uL of each P. glabra crude extract at 500 μg/mL (treated group) were added and incubated for 24 h, 48 h, and 72 h, respectively. The image of cell morphology for each group was observed and photographed. The morphological changes of treated cells were compared with those of untreated cells that served as the negative control.
2.5 Chemical profiling analysis using liquid chromatography–mass spectrometry (LC–MS)
A LC–MS QTOF system was selected to analyse samples following Lawal et al. (2016), with some modifications [47]. The LC–MS analysis of the extracts was conducted on an Agilent Technologies 6520 system (Agilent Technologies, Santa Clara, California, United States), equipped with a column ZORAX Eclipse Plus C18 Rapid Resolution HT (2.1 × 100 mm). The mobile phases were (a) distilled water and 1% formic acid, and (b) acetonitrile and 1% formic acid. The column temperature was set at 40 °C. The run time was 48 min, with an injection volume of 2 µL and a flow rate of 0.25 mL/min. The compounds were identified using the Metlin database.
2.6 Statistical analysis
The statistical analysis was performed using the GraphPad PRISM software version 8.0.2 (GraphPad Software, Inc., San Diego, CA, USA). The differences between the means were assessed with the one-way and two-way analysis of variance (ANOVA) and Tukey’s post hoc test. The dose-dependent curve analysis for the determination of IC50 was analysed and plotted based on the mean ± standard error of the mean (SEM) of the percentage cell viability. The difference with p values less than 0.05 were considered statistically significant (*p < 0.05, **p < 0.01 and ***p < 0.001).
3 Results
3.1 The crude leaf extracts of P. glabra
In this study, P. glabra leaf was extracted by the maceration technique using three solvents: ethanol, methanol, and distilled water (aqueous). The results showed that the aqueous extract had the highest extraction yield at 17.6%, followed by the methanol extract at 15.7%, and finally the ethanol extract at 6.5% (Fig. 1).
3.2 Cytotoxicity effect
The cytotoxic effects of ethanol, methanol, and aqueous extracts of P. glabra were studied against the MCF-7 cell line using the MTT assay. The cytotoxicity assay was evaluated based on time-dependent inhibition activity for three time points (24 h, 48 h, and 72 h) in triplicates. The IC50 values for all extracts for three time points are shown in Table 1. The cytotoxic effects of all extracts after 24 h of treatments were expressed as a percentage of cell viability in the MCF-7 cell line (Fig. 2). The MCF-7 cell line was treated with different concentrations of ethanol, methanol, and aqueous extracts, i.e., 100, 200, 300, 400, and 500 µg/mL. The cytotoxicity of the ethanol extract was dose-dependent, showing the highest cell viability (92.78 ± 9.33%) at 100 µg/ml and the lowest cell viability (44.46 ± 6.16%) at 500 µg/mL (Fig. 1). The IC50 value of the ethanol extract against the MCF-7 cell line after 24 h of treatment was 467.80 ± 10.88 µg/mL (Table 1 and Fig. 3A). The cytotoxicity of the methanol extract was dose-dependent, showing the highest cell viability (117.37 ± 12.10%) at 100 µg/mL and the lowest cell viability (19.03 ± 1.56%) at 500 µg/mL (Fig. 2). The IC50 value of the methanol extract against the MCF-7 cell line after 24 h of treatment was 318.34 ± 7.22 µg/mL (Table 1 and Fig. 3A). Similarly, the aqueous extract also showed itself to be dose-dependent, with an IC50 of 159.20 ± 31.72 µg/mL following 24 h of treatment (Table 1 and Fig. 3A).
The cytotoxicity effect of ethanol, methanol, and aqueous P. glabra crude leaf extracts against the MCF-7 cell lines after three different time points of treatment where A 24 h, B 48 h, and C 72 h. The error bars represent the standard error of the mean (SEM). Data are expressed as a mean percentage of viable cells ± SEM of at least three replicates in three independent tests; The asterisk symbols (*), (**), and hash symbol (#) indicate p < 0.05, **p < 0.01 and #p < 0.001, respectively. The differences were determined by two-way ANOVA and Tukey’s post hoc test
Bar chart illustrates the IC50 value of MCF-7 cell lines post-treatment with LE, LM, and LW after A 24 h, B 48 h, and C 72 h. Ethanol extract (LE), methanol extract (LM), and an aqueous extract (LW). Data are expressed as means ± SEM, n = 3; *p < 0.05, **p < 0.01 and ***p < 0.001. The differences were determined by one-way ANOVA and Tukey’s post hoc test
The cytotoxicity of the ethanol, methanol, and aqueous extracts of P. glabra leaf against the MCF-7 cell line after 48 h showed a dose-dependent manner. The cytotoxicity of the ethanol extract at 100 µg/mL showed the highest percentage of cell viability (92.11 ± 10.04%), while at 500 µg/mL, it showed only 21.45 ± 2.60% of cell viability (Fig. 2). The IC50 value of the ethanol extract against the MCF-7 cell line following 48 h of treatment was 251.52 ± 12.5 µg/mL (Table 1, Fig. 3B). At 100 µg/mL of the methanol extract, the cell viability of the MCF-7 cell line was 91.64 ± 7.97 µg/mL. While, 6.97 ± 1.28% of the cell viability of the MCF-7 cell line following 48 h of treatment with 500 µg/mL of the methanol extract. The IC50 value of the methanol extract against the MCF-7 cell line following 48 h of treatment was 206.02 ± 11.1 µg/mL (Table 1, Fig. 3B). Likewise, the cytotoxicity of the aqueous extract against the MCF-7 cell line also showed a dose-dependent manner with an IC50 value of 110.93 ± 5.33 µg/mL following 48 h of treatment (Table 1, Fig. 3B).
After 72 h, the cytotoxicity of the ethanol, methanol, and aqueous extracts of P. glabra leaf against the MCF-7 cell line also depicted a dose-dependent manner. At a concentration of 100 µg/ml of the ethanol extract, the cell viability was 140.78 ± 5.29%. An increase in the ethanol extract concentration at 200, 300, 400, and 500 µg/mL reduced the cell viability of the MCF-7 cell line after 72 h of treatment to 41 ± 9.42%, 24.63 ± 4.28%, 20.78 ± 3.13%, and 20.81 ± 2.86%, respectively (Fig. 2). The IC50 value of the ethanol extract against the MCF-7 cell line after 72 h of treatment was 214.92 ± 10.62 µg/mL (Table 1, Fig. 3C). For the cytotoxicity of the methanol extract against the MCF-7 cell line, it also showed a reduction in the percentage of cell viability with increasing the concentration of the methanol extract (Fig. 3C), with an IC50 value of 184.70 ± 11.77 µg/mL (Table 1, Fig. 3C). Similar pattern of reduction in the percentage of cell viability as increasing the concentration of the aqueous extract. The IC50 value of the aqueous extract was 158.01 ± 8.49 µg/mL which is lower compared to the IC50 values of the ethanol and methanol extracts following 72 h of treatment (Table 1, Fig. 3C).
The data showed that, compared to the methanol and ethanol extracts, the aqueous extract had significantly lower IC50 values across the time points of treatment (p < 0.05). After 24 h, 48 h, and 72 h of treatment, the IC50 values were 159.20 ± 31.72 µg/mL, 110.93 ± 5.33 µg/mL, and 158.01 ± 8.49 µg/mL, respectively. However, both methanol and ethanol extracts were less potent against the MCF-7 cell line, with IC50 values of 184.70 ± 11.77 µg/mL and 214.92 ± 16.62 µg/mL, respectively, after 72 h of treatment.
3.3 Microscopic observation of morphological changes in MCF-7 cell line
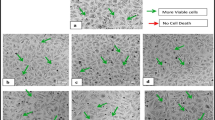
The induction of cell death in the MCF-7 cell line by P. glabra crude leaf extracts was evaluated at 24 h, 48 h, and 72 h of treatments by a morphological analysis using an inverted light microscope as showed in Fig. 4. Figure 4 depicts the observed morphological changes increasing in sequential order with treatment duration in all treated groups. In comparison to the untreated group (Fig. 4A), Fig. 4B–D showed that cells in all treated groups led to a reduction in the number of cells, cell shrinkage, membrane blebbing, and cell rupture as treatment duration increased. These extracts caused the cells to lose their normal shape, with some of the cells detaching after 24 h and the shape of the cells changing more and more as time progressed towards 72 h. On the other hand, in the untreated group, the cells continued to exhibit their original morphological characteristics, such as epithelial cell morphology, while also increasing in number despite the duration of treatment (Fig. 4A). Overall, all treated groups showed cell characteristics such as shrinkage, blebbing, and polygonal shape, which indicate the cells undergo cell death process. Based on these findings, it was determined that crude extracts from P. glabra leaf had the ability to both inhibit the growth of cancer cells and induce cell death as examined through cellular morphological changes in the MCF-7 cell line.
Morphological changes of the MCF-7 cell line in the untreated group as the control A and treated with 500 μg/mL of B LE, C LM, and D LW at 24, 48, and 72 h of treatment. Arrows indicate a membrane blebbing and b lysis of body formation asmorphological changes observed in treated groups. The photographs were taken at 20 × magnification with an inverted light microscope
3.4 LC–MS analysis
The compounds matched via the Metlin database were the predicted compounds for the particular mass-to-charge ratio (m/z). This was due to multiple compounds that may share similar m/z values. To narrow down the data, compounds with Score Db that are close to 100 were chosen, and from all compounds with high scores obtained before, only the compounds with Diff (Db.ppm) that have a value within − 2 to + 2 were chosen. Db is the compound matched against the Metlin database. Figure 5 and Table 2 show the compounds detected in the three extracts that followed the criteria, and Fig. 6 shows the LC–MS chromatograms of P. glabra’s extracts.
Based on Table 2 and Fig. 7, there are 18 identified compounds in LE, nine of which are triterpenoids (quillaic acid, tomentosic acid, ganoderic acid DM, bryononic acid, lucidumol A, emmotin A, ganoderol A, betulinic acid, and dehydroconicasterol) and five of which are flavonoids (silymarin, biochanin A diacetate, 4-o-caffeoyl-3-o-feruloylquinicacid, epigallocatechin5,3′,5′-trimethylether3-o-gallate, and scutellarein4'-methylether7-(2″,6″-diacetylalloside)). Besides, 15 compounds were identified in LM, as shown in Table 2. Seven compounds are triterpenoids (quillaic acid, tomentosic acid, ganoderic acid dm, bryononic acid, ganoderol a, betulinic acid, and dehydroconicasterol) while four compounds are flavonoids (epigallocatechin5,3′,5′-trimethylether3-o-gallate, silymarin, biochanin A diacetate, and neoduleen). Only six chemicals were discovered in LW, five of which are flavonoids (epigallocatechin5,3′,5′-trimethylether3-o-gallate, silymarin, biochanin A diacetate, and scutellarein4′-methylether7-(2″,6″-diacetylalloside) and two of which are triterpenoids (quillaic acid, and ganoderic acid DM). Intriguingly, silymarin, biochanin A diacetate, epigallocatechin 5,3′,5′-trimethyl ether 3-O-gallate, quillaic acid, and ganoderic acid DM were identified in all three extracts (Fig. 7).
4 Discussion
Breast cancer is the most common cause of death in many countries, including Malaysia, where the Malaysian National Cancer Registry Report for 2007–2011 reported the highest incidence rate of 17.7% [48]. In breast cancer treatment, invasive methods such as chemotherapy and surgery have led the majority of patients to turn to complementary and alternative medicine (CAM), which they believe will cause less harm and improve their quality of life. Several of the most prevalent CAMs in Malaysia, including dietary supplements, medicinal herbs, homeopathy, and traditional medicine, were used to treat breast cancer [49]. In a study conducted in Malaysia, approximately 34.8% of breast cancer patients were estimated to use complementary and alternative medicine (CAM), with Malays (43.9%) being the most commonly used, followed by Chinese (41%) and Indians (14.4%) [50]. It is believed that these remedies reduce stress and alleviate symptoms, particularly during conventional and invasive breast cancer treatment [51].
In this study, P. glabra leaf was extracted using ethanol, methanol, and water as the extraction solvents through the maceration technique. According to this finding, different solvents resulted in different percentages of extraction yields. The resulting extraction yield indicated a considerable amount of phytochemical constituents [45]. This is because the concentration of phytochemical constituents in an extract can vary greatly depending on the polarity of the solvents used for extraction. A higher percentage of extraction yield was observed in the aqueous, methanol, and ethanol extracts, indicating that the efficiency of extraction is optimal with highly polar solvents. This may be due to the presence of numerous polar compounds in the plant material that are soluble in highly polar solvents like water, methanol, and ethanol [52]. The solvent used, extraction method, presence of interfering substances, temperature, extraction time, and phytochemical composition all contribute to the efficiency of extraction [53,54,55,56]. In addition to the extraction procedure and plant part used as starting material, the solvent used is also one of the factors that determine the quality of an extract [57]. Based on prior research with Prismatomeris plants, ethanol, methanol, and water were used as solvents for the extraction of the leaves. Root extracts of P. tetrandra and P. connata were extracted with ethanol [37, 58], whereas those of P. sessilifiora and P. malayana were extracted with methanol [59, 60]. P. glabra was extracted utilizing ethanol, methanol, and water as solvents [20, 32, 35]. Due to the paucity of research on Prismatomeris plant crude extracts, similar solvents were used to evaluate the inhibitory activity against cancer cell lines. These solvents were selected not only due to the efficiency of extraction, which favoured the highly polar solvents, but also because the plant material contains many polar chemicals that are soluble in polar solvents, such as water, methanol, and ethanol [52]. Also, the excellent extraction solvent has low toxicity, a preservative effect, is easy to evaporate at low temperatures, accelerates the extract's physiologic absorption, is unable to make the extract to be complex or separate, and ease of evaporation at low temperatures [61].
In our study, the cytotoxic effects of various concentrations of ethanol, methanol, and aqueous extracts of P. glabra leaf were evaluated using the MCF-7 cell line as a breast cancer disease model. The cytotoxicity of these extracts was measured using an MTT assay. In this context, half-maximal inhibitory concentration (IC50) indicates the concentration of the plant extracts needed to inhibit a given biological or biochemical function by half. According to this finding, the aqueous extract of P. glabra leaf consistently exhibited the lowest IC50 across all time points (24 h, 48 h, and 72 h) compared to methanol and ethanol extracts. Meanwhile, ethanol extract consistently manifested the highest IC50 values across all time points. The lower the IC50 values, the higher the anticancer activity, and the higher the IC50 values, the lower the anticancer activity [62]. Based on this finding, we postulate that the polar compounds in P. glabra might be the major contributor to the IC50 parameter. As stated above, the IC50 of the aqueous extract of P. glabra leaf manifested the lowest values across all time points compared to methanol and ethanol. This event might be influenced by the following factors: Firstly, it might be that the major P. glabra composition material is polar compounds. Secondly, the polarity index (P′) of water is 10.2, which is higher than the P′ of ethanol (P′ = 4.3) and methanol (P′ = 5.1) [63]. The composition of the plant material and the polarity of the solvent are the factors that affected the extraction efficiency. The extraction efficiency of plant material increases with increasing solvent polarity [64, 65]. Lastly, these polar compounds might possess specific functional groups that favour the formation of hydrogen bonds with water; thus, they have a higher tendency to dissolve in water compared to ethanol and methanol [66]. According to the American National Cancer Institute (NCI), crude extracts qualify as having cytotoxic activity if their IC50 values < 20 µg/mL or 10 µM after incubation for either 48 h or 72 h [67]. Furthermore, crude extract with IC50 values in the range of 21–200 µg/mL is classified as slightly cytotoxic, whereas the range of 201–500 µg/mL is weakly cytotoxic [68]. Thus, based on this classification, an aqueous extract is slightly cytotoxic, whereas methanol and ethanol extracts are weakly cytotoxic. To the best of our knowledge, this is the first study of P. glabra crude leaf extracts in which ethanol, methanol, and distilled water were used as solvents, so we were unable to make a comparison. The preliminary anticancer activity of P. glabra’s extracts was assessed using an MTT assay. Prior to validating whether an agent has potential anticancer properties using animal studies and further clinical evaluation in humans, it is crucial to conduct preclinical evaluation in vitro using this cell-based assay [69]. Based on this finding, each of these extracts demonstrated an antitumor effect against MCF-7 cells. Interestingly, the aqueous extract of P. glabra demonstrated the most significant antitumor effect against MCF-7 cells. We hypothesized that the active compounds in the aqueous extract have a significant and strong contribution to the antitumor effect compared to the active compounds in the methanol and ethanol extracts. In order to find the active compound that strongly contributed to the antitumor effect against MCF-7 cells through a specific mechanism of action, we did further analyses, such as HPLC, apoptosis assay, cell cycle assay, RNA sequencing, and real-time PCR. However, in this study, we just reported the findings of plant extraction, the cytotoxicity effects of plant extracts (quantitatively and qualitatively), and LC–MS analysis. The subsequent findings showed promising outcomes (unpublished).
However, it was found that few cytotoxicity studies were conducted using P. glabra leaf extract with different solvents. In 2020, Azman and colleagues conducted a cytotoxicity study using the dichloromethane leaf extract of P. glabra, and this extract successfully inhibited the MCF-7 cell line across three different time points [45]. Meanwhile, in 2021, Subramaniam and colleagues used the ethyl acetate leaf extract of P. glabra in the cytotoxicity study, and it was found that this extract inhibited the MCF-7 cell line at different time points [70]. There have been a number of cytotoxicity studies conducted on various plant species using a variety of different types of solvents, and these studies have shown inhibition against a variety of different cell lines. According to Rohin and colleagues, the methanol extract of Duku (Lansium domesticum corr.) showed greater inhibition of human colorectal adenocarcinoma cell lines (HT-29) compared to ethanol and ethyl acetate extracts [71]. In addition, Bismillah leaf (Vernonia amygdalina) extract in ethyl acetate had a greater cytotoxic effect on the U-87 cell line than methanol and ethanol extracts [72]. This study revealed that the active compounds extracted from P. glabra using various solvents may have beneficial effects in the treatment of breast cancer, hence, further research is needed into the identification of the bioactive compounds responsible for these effects at the molecular level. This finding agreed with and was consistent with those from studies on human laryngocarcinoma Hep-2 cell lines and human non-small cell lung cancer cells conducted with other Prismatomeris species, such as P. connata [37, 73]. The effect comes from an active compound in the plant called anthraquinone. This compound caused the cell cycle arrest, which led to apoptosis.
Comparing the IC50 values of crude extracts (ethanol, methanol, and aqueous extracts) and doxorubicin (the most efficacious chemotherapy drug for breast cancer treatment) across three time points, the IC50 values of doxorubicin are too low when compared to the IC50 values of all crude extracts found in this study. This indicates that doxorubicin is a potent drug. Yusuf and his colleagues reported in 2020 that the IC50 value of doxorubicin after 24 h of incubation with the MCF-7 cell line was 5.074 µg/mL [74]. Meanwhile, several studies reported that the IC50 value of doxorubicin on the MCF-7 cell line after 48 h of incubation ranged from 0.417 to 0.68 µg/mL [75,76,77,78]. According to Abdel-Sattar and his colleagues (2023), the IC50 value of doxorubicin on the MCF-7 cell line was 0.2 ± 0.06 µg/mL after 72 h of incubation [79].
This study was conducted not only to determine the cytotoxicity of P. glabra crude leaf extracts but also to examine the ability of P. glabra crude leaf extracts to induce cell death in the MCF-7 cell line by analysing cell morphology. Thus far, P. glabra crude leaf extracts have exerted their antiproliferative activities and induced cell death, with the optimum time being 48 h. This evidence provides a biological mechanism for the morphological changes characterised by blebbing, cell shrinkage, and floating, as well as the appearance of apoptotic cells. However, the cytotoxic activity was slightly lower after 72 h of incubation. It is postulated that the depletion of the active compound occurred after 72 h due to a decrease in antioxidant activity following cellular monolayer absorption [80].
In this study, a total of 40 compounds detected in P. glabra’s extracts, where 18 compounds identified in ethanol extracts, followed by 15 compounds and 7 compounds in methanol and aqueous extracts, respectively. Several identified compounds with masses less than 400 m/z. There are several factors that influence the mass of the identified compound, including in-source fragmentation [81], ion suppression (one form of matrix effect) [82,83,84], collision cell pressure, ion source flow rates, ultimate mass spectrometry vacuum, cleanliness of the ion source, ion optics, and the collision cell [83]. Flavonoids and triterpenoids constitute the preponderance of the 40 compounds identified. Five compounds, including silymarin, biochanin A diacetate, epigallocatechin 5,3′,5′-trimethyl ether 3-O-gallate (EGCG), quillaic acid, and ganoderic acid DM were present in all extracts. EGCG has been selected for the subsequent research as the impact of EGCG on cancer cells, such as MCF-7 cells, is intricate and encompasses diverse molecular processes. EGCG can exert its anticancer efficacy by multiple mechanisms, including inhibiting cell proliferation, arresting the cell cycle, inducing apoptosis, having anti-angiogenic effects, mediating signalling pathways, and modifying the function of estrogen receptors. Zan et al. (2019) identified that EGCG exhibited its anticancer properties by suppressing cell growth, causing cell cycle arrest in the G2/M phase, and stimulating apoptosis in MCF-7 cells [85]. Furthermore, a study conducted by Huang et al. (2017) established that EGCG exhibited its anticancer properties by inhibiting cell proliferation in MCF-7 cells and inducing apoptosis via the P53/Bcl-2 signalling pathway [86]. Luo et al. (2014) postulated that the anticancer properties of EGCG were manifested through its ability to impede the growth and proliferation of MCF-7 cells, potentially by suppressing the protein expression of VEGF and HIF-1α, which are implicated in the process of angiogenesis [87]. We have performed various downstream investigations, such as RNA sequencing, cell cycle analysis, and apoptosis analysis, in order to investigate the mechanism by which EGCG acts against MCF-7 cells. Nevertheless, we are now unable to reveal the results of these studies, as we intend to publish them in our forthcoming publication.
This research establishes the foundation for the investigation of P. glabra's potential active compounds as CAMs. This endeavour is concomitant with or aligned with the support of the World Health Organization (WHO), which promotes the secure application of botanical remedies for therapeutic purposes [88]. Firstly, this research could potentially serve as a viable targeted therapy option for the treatment of breast cancer. Cancer pathogenesis-associated cellular processes, including cell survival and proliferation, are regulated by a multitude of signalling pathways. One method to surmount this limitation is through the application of natural compounds that possess the ability to selectively influence a multitude of signalling pathways, thereby directly affecting the molecular processes of cells [89]. The mTOR/PI3K/Akt signalling pathway is one of the primary mechanisms responsible for the development of resistance to endocrine therapy in breast cancer [90]. Several studies have documented that EGCG inhibits proteins involved in signalling pathways that are responsible for cellular proliferation, survival, differentiation, and growth [91, 92]. Therefore, rather than focusing on the mTOR/PI3K/Akt signalling pathway, researchers can employ natural compounds as CAMs that operate by targeting multiple signalling pathways; this approach can effectively circumvent the development of drug resistance. Additionally, this research establishes a fundamental framework for investigations that centre on the investigation of novel mechanisms to combat breast cancer. Such investigations may utilise EGCG in conjunction with chemotherapy drugs. Jiang et al. (2016) demonstrated that the concurrent use of EGCG and cisplatin effectively reverses cisplatin resistance. This was consistently observed in both NSCLC cell lines and xenografts [93]. Additionally, a study conducted by Wu et al. (2021) unveiled encouraging findings regarding the efficacy of irinotecan and EGCG in the treatment of colorectal cancer [94]. Indirectly, not only can overcome the drug resistance but also enhance the effectiveness of treatments. In essence, this research provides a foundational structure for subsequent investigations that centre on targeted therapy, the investigation of unique mechanisms, the mitigation of drug resistance, and the optimisation of treatment efficacy through the utilisation of natural compounds, specifically EGCG. One of its biomedical applications could be the enhancement of breast cancer treatment through this research concentration.
Furthermore, the methodologies employed in this present study are in accordance with the drug discovery stage of the drug development and discovery pipeline. The process of drug discovery and development is intricate, protracted, laborious, expensive, and involves experts from multiple disciplines. This work represents a crucial advancement in drug discovery, aiming to provide a more reliable alternative to current therapies. Following the completion of the cell viability assay in this investigation, the cytotoxicity was assessed using the dose–response graph. Cell-based phenotypic assays are frequently used in drug discovery stage, as opposed to target-based biochemical assays. Insufficient understanding of complex biological systems and diseases often leads to the failure of target-based biochemical assays to meet projected efficacy levels [95]. Phenotypic tests provide a more pertinent and superior initial stage for drug discovery. Compared to target-based biochemical assays, phenotypic assays not only yield a greater number of first-of-its-kind small-molecule pharmaceuticals [96], but they also effectively identify small molecules with therapeutic effects that are pertinent to their specific molecular mechanisms of action [97]. The cell viability assay is a widely used phenotypic assay by researchers to identify cytotoxic compounds [98]. In addition to providing cost-effective and user-friendly procedures [99], this assay serves as the principal assay for identifying compounds capable of eradicating cancer cells, a counter assay to obviate compounds exhibiting undesired cytotoxicity, and a tool for determining the fate of compounds in the drug discovery and development process based on cytotoxicity endpoint parameters [100].
In addition, chemical profiling analysis was performed using LC–MS QTOF in this study. During the lead identification phase of drug discovery, it is critical to conduct biological activity screenings on thousands of compounds. In addition to high-speed approaches, the primary concern is the utilisation of precise analytical methodologies for the analysis of these compounds; LC–MS is a viable tool for accomplishing this objective [101]. Throughout the drug discovery and development process, LC/MS-based methodologies have been powerful and essential analytical tools, including during the lead identification phase [102]. As an example, its application encompasses the determination of compound molecular weights in order to assess lead candidates [103]. In addition, bioaffinity screening analysis and lead identification during natural product dereplication are both possible with LC–MS [104]. LC–MS can be utilised for an innumerable array of analyses on account of its speed, sensitivity, selectivity, plethora of applications, and minimal sample volume requirements, among other benefits. It is imperative to establish clear objectives for the research in order to capitalise on the superiority of LC–MS [105]. This study therefore signifies progress towards a solid alternative to existing treatments.
Undoubtedly, global attention has been drawn to the trend towards green synthesis of nanomaterial in recent years. The green synthesis of nanomaterials presents numerous benefits, including its simplicity, cost-effectiveness, efficiency, and scalability to accommodate larger operations [106]. Due to these benefits, this technique has been implemented in numerous industries, such as targeted drug or gene delivery of medicine, advanced nanocarriers, cancer immunotherapy, and electronics, and [107, 108], sensors, diagnostics, cosmetics, remediation, and imaging [109]. Thus, this study is a crucial initial phase in the green synthesis of nanomaterials and has the potential to advance biomedical applications through alternative therapeutics utilising green-synthesized nanomaterials.
5 Conclusions
According to the MTT study, the aqueous extract of P. glabra leaf has the lowest IC50 value at all time points compared to the ethanol and methanol extracts. The aqueous extract of P. glabra leaf exhibited promising results as an antiproliferative agent against the MCF-7 cell line when treated optimally after 48 h of treatment. This result is consistent with the results of cell morphology analysis, which revealed that all crude leaf extracts of P. glabra exerted antiproliferative effects and induced cell death, with the optimal time being 48 h. In light of these findings, an aqueous extract of P. glabra leaf could be evaluated as a potential candidate for drug formulation against breast cancer. As far as we are concerned, this is the first study of the cytotoxic and apoptotic effects of extracts of P. glabra leaf extracted with ethanol, methanol, and distilled water. In addition, flavonoids and triterpenoids are hypothesised to contribute to the inhibition of the MCF-7 cell line in this study. Cytotoxicity assays are vital in the basic research of drug discovery and development, specifically for the development of anticancer therapies. The identification of cytotoxicity levels in cancer cells is of utmost importance due to their potential to impede the proliferation of target cells through genetic disruption or the inhibition of essential nutrient uptake necessary for cell survival. It is highly recommended to conduct additional studies on the examination of various breast cancer cell lines, the isolation of bioactive compounds from the extracts, and the implementation of in vivo experiments to assess pharmacokinetic and pharmacodynamic parameters.
Availability of data and materials
The data used to support the findings of this study are included within the article.
Abbreviations
- DMSO:
-
Dimethylsulfoxide
- FBS:
-
Fetal bovine serum
- IC50 :
-
Half-maximal inhibitory concentration
- LC–MS:
-
Liquid chromatography–mass spectrometry
- LE:
-
Ethanol extract
- LM:
-
Methanol extract
- LW:
-
Aqueous extract
- MTT:
-
3-[4, 5-Dimethylthiazaol-2-yl]—2, 5-diphenyltetrazolium bromide
- P. connata :
-
Prismatomeris connata
- P. fragrans :
-
Prismatomeris fragrans
- P. glabra :
-
Prismatomeris glabra
- P. tetrandra :
-
Prismatomeris tetrandra
- PBS:
-
Phosphate-buffered saline
- RPMI:
-
Roswell Park Memorial Institute
- SEM:
-
Standard error of the mean
References
Breast cancer: World Health Organization (2023). https://www.who.int/cancer/prevention/diagnosis-screening/breast-cancer/en/. Accessed 22 Feb 2020
WHO (2018) WHO|Breast cancer. WHO. https://www.who.int/cancer/prevention/diagnosis-screening/breast-cancer/en. Accessed 1 Oct 2019
Lopez D, Sekharam M, Coppola D, Carter WB (2008) Purified human chorionic gonadotropin induces apoptosis in breast cancer. Mol Cancer Ther 7:2837–2844
American Cancer Society (2010) Treating breast cancer: American Cancer Society. https://www.cancer.org/cancer/breast-cancer/treatment.html. Accessed 22 Feb 2020
Mosca L, Ilari A, Fazi F, Assaraf YG, Colotti G (2021) Taxanes in cancer treatment: activity, chemoresistance and its overcoming. Drug Resist Updates 54:100742
Kinnel B, Singh SK, Oprea-Ilies G, Singh R (2023) Targeted therapy and mechanisms of drug resistance in breast cancer. Cancers 15:1320
Luque-Bolivar A, Pérez-Mora E, Villegas VE, Rondón-Lagos M (2020) Resistance and overcoming resistance in breast cancer. Breast Cancer Targets Ther 12:211–229
Nedeljković M, Damjanović A (2019) Mechanisms of chemotherapy resistance in triple-negative breast cancer—how we can rise to the challenge. Cells 8:957
Tokumaru Y, Joyce D, Takabe K (2020) Current status and limitations of immunotherapy for breast cancer. Surgery 167:628–630
Curigliano G, Criscitiello C (2014) Successes and limitations of targeted cancer therapy in breast cancer. In: Successes and limitations of targeted cancer therapy, vol 41, pp 15–35
Wang W, Xu J, Fang H, Li Z, Li M (2020) Advances and challenges in medicinal plant breeding. Plant Sci 298:110573
Narayanan M, Kiran A, Natarajan D, Kandasamy S, Shanmugam S, Alshiekheid M, Almoallim HS, Pugazhendhi A (2022) The pharmaceutical potential of crude ethanol leaf extract of Pedalium murex (L.). Process Biochem 112:234–240
Braun L, Cohen M (2015) Herbs and natural supplements volume 2: an evidence-based guide, 4th edn. Elsevier Health Sciences, Amsterdam
Divya M, Vijayakumar S, Chen J, Vaseeharan B, Duran-Lara EF (2020) South Indian medicinal plants can combat deadly viruses along with COVID-19?-a review. Microb Pathog 148:104277
Sen S, Chakraborty R, De B (2011) Challenges and opportunities in the advancement of herbal medicine: India’s position and role in a global context. J Herb Med 1:67–75
Hayes AW (2008) Principles and methods of toxicology, 5th edn. CRC Press, Boca Raton
Lalitha LJ, Sales TJ, Clarance PP, Agastian P, Kim YO, Mahmoud AH, Mohamed SE, Tack JC, Na SW, Kim HJ (2020) In-vitro phytopharmacological and anticancer activity of Loranthus Longiflorus Desv. Var. Falcatuskurz against the human lung cancer cells. J King Saud Univ-Sci 32:1246–1253
Risinger AL, Giles FJ, Mooberry SL (2009) Microtubule dynamics as a target in oncology. Cancer Treat Rev 35:255–261
Majumdar SH (2012) Antitumor potential of Semecarpus anacardium against Ehrlich ascites carcinoma in nude mice. Int J Pharm Biol Sci 3:820–829
Salleh RM, Hasan MH, Adam A (2015) Phenolic compound and antioxidant levels of Prismatomeris glabra. J Pharmacogn Phytochem 3(5):05–11
Wong K, Turner I, Wang R, Harwood R, Seah W, Ng X, Lim R, Lua H, Mahyuni R (2019) Rubiaceae. In: Middleton D, Leong-Škorničková J, Lindsay S (eds) Flora of Singapore. National Parks Board, Singapore, pp 1–358
Son NT (2017) An overview of the genus Prismatomeris: phytochemistry and biological activity. Bull Fac Pharm Cairo Univ 55:11–18
Wang C, Ding X, Feng SX, Guan Q, Zhang XP, Du C, Di YT, Chen T (2015) Seven new tetrahydroanthraquinones from the root of Prismatomeris connata and their cytotoxicity against lung tumor cell growth. Molecules 20:22565–22577
Tuntiwachwuttikul P, Butsuri Y, Sukkoet P, Prawat U, Taylor WC (2008) Anthraquinones from the roots of Prismatomeris malayana. Nat Prod Res 22:962–968
Krohn K, Gehle D, Dey SK, Nahar N, Mosihuzzaman M, Sultana N, Sohrab MH, Stephens PJ, Pan JJ, Sasse F (2007) Prismatomerin, a new iridoid from Prismatomeris tetrandra. structure elucidation, determination of absolute configuration, and cytotoxicity. J Nat Prod 70:1339–1343
Jing HA, Shi-Xiu FE, Sheng-Xiang Samuel QI, Tao CH (2011) Anthraquinone glycosides from the roots of Prismatomeris connata. Chin J Nat Med 9:42–45
Kanokmedhakul K, Kanokmedhakul S, Phatchana R (2005) Biological activity of Anthraquinones and Triterpenoids from Prismatomeris fragrans. J Ethnopharmacol 100:284–288
Ridley HN (1925) The flora of the Malay Peninsula, vol 5. L. Reeve & co., London
Yun-Zhen R, Yun-Zhen R (1988) Notes on the genus Prismatomeris Thw. (Rubiaceae) of China. J Syst Evol 26:443
Johansson JT (1987) Revision of the genus Prismatomeris Thw. (Rubiaceae, Morindeae). Council for Nordic Publications in Botany
Mohamed Salleh R (2016) Prismatomeris glabra: ergogenic effects and sexual function in mice. Dissertation, Universiti Teknologi MARA
Mohamad TA, Naz H, Jalal RS, Hussin K, Abd Rahman MR, Adam A, Weber JF (2013) Chemical and pharmacognostical characterization of two Malaysian plants both known as Ajisamat. Rev Bras 23:724–730
Abdullah NH (2014) Chemical constituents of Prismatomeris malayana Ridley and quantitative structure activity relationship study on anti-inflammatory agents and their analogues. Dissertation, University of Malaya. http://studentsrepo.um.edu.my/4857/. Accessed 20 Jul 2022
Primus PS, Wu CH, Kao CL, Choo YM (2022) Glabraquinone A and B, new bis anthraquinones from Prismatomeris glabra (Korth.) Valeton. Nat Prod Res 14:1–8
Alkadi KA, Ashraf K, Adam A, Shah SA, Taha M, Hasan MH, John C, Salleh RM, Ahmad W (2021) In vitro cytotoxicity and anti-inflammatory cytokinine activity study of three isolated novel compounds of Prismatomeris glabra. J Pharm Bioallied Sci 13:116
Tikhomirov AS, Shtil AA, Shchekotikhin AE (2018) Advances in the discovery of anthraquinone-based anticancer agents. Recent Patents Anti-Cancer Drug Discov 13:159–183
Feng SX, Hao J, Chen T, Qiu SX (2011) A new anthraquinone and two new tetrahydroanthraquinones from the roots of Prismatomeris connata. Helv Chim Acta 94:1843–1849
Dong X, Fu J, Yin X, Cao S, Li X, Lin L, Huyiligeqi NJ (2016) Emodin: a review of its pharmacology, toxicity and pharmacokinetics. Phytother Res 30:1207–1218
Dave H, Ledwani L (2012) A review on anthraquinones isolated from Cassia species and their applications. Indian J Nat Prod Resour 3:291–319
Feng S, Bai J, Qiu S, Li Y, Chen T (2012) Iridoid and phenolic glycosides from the roots of Prismatomeris connata. Nat Prod Commun 7:1934578X1200700502
Conserva LM, Jesu Costa Ferreira J (2012) Borreria and Spermacoce species (Rubiaceae): a review of their ethnomedicinal properties, chemical constituents, and biological activities. Pharmacogn Rev 6:46
Jiang JS, Feng ZM, Zhang PC (2005) Chemical constituents from root of Prismatomeris tetrandra. China J Chin Mater Med 30:1751–1753
Ayoola GA, Coker HA, Adesegun SA, Adepoju-Bello AA, Obaweya K, Ezennia EC, Atangbayila TO (2008) Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Trop J Pharm Res 7:1019–1024
Beheshti F, Shabani AA, Akbari Eidgahi MR, Kookhaei P, Vazirian M, Safavi M (2021) Anticancer activity of Ipomoea purpurea leaves extracts in monolayer and three-dimensional cell culture. Evid-Based Complement Altern Med 2021:1–4
Azman NA, Ab Rahman SS, Anuar NF, Taib WR, Rawi RI (2020) In vitro cytotoxic effect of dichloromethane extract of Prismatomeris glabra in human breast cancer cells. Asian J Med Biomed 4:59–66
Khazaei S, Abdul Hamid R, Ramachandran V, Mohd Esa N, Pandurangan AK, Danazadeh F, Ismail P (2017) Cytotoxicity and proapoptotic effects of allium atroviolaceum flower extract by modulating cell cycle arrest and caspase-dependent and p53-independent pathway in breast cancer cell lines. Evid-Based Complement Altern Med 2017:1468957
Lawal U, Leong SW, Shaari K, Ismail IS, Khatib A, Abas F (2017) α-glucosidase inhibitory and antioxidant activities of different Ipomoea aquatica cultivars and LC–MS/MS profiling of the active cultivar. J Food Biochem 41:e12303
Azizah Ab M, Nor Saleha IT, Noor Hashimah A, Asmah ZA, Mastulu W (2016) Malaysian National Cancer Registry Report (MNCR) 2007–2011. Ministry of Health, Putrajaya, p 16
Complementary, alternative, or integrative health: What’s in a name?: National Center for Complementary and Integrative Health (NCCIH). https://www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a-name (2021). Accessed 20 Jul 2022
Zulkipli AF, Islam T, Mohd Taib NA, Dahlui M, Bhoo-Pathy N, Al-Sadat N, Abdul Majid H, Hussain S (2018) Use of complementary and alternative medicine among newly diagnosed breast cancer patients in Malaysia: an early report from the MyBCC study. Integr Cancer Ther 17:312–321
Why people use complementary or alternative therapies: cancer research UK (2018). https://www.cancerresearchuk.org/about-cancer/cancer-ingeneral/treatment/complementary-alternative-therapies/about/why-used. Accessed 20 Jul 2022
Truong DH, Nguyen DH, Ta NT, Bui AV, Do TH, Nguyen HC (2019) Evaluation of the use of different solvents for phytochemical constituents, antioxidants, and in vitro anti-inflammatory activities of Severinia buxifolia. J Food Qual 2019:1–9
Ngo TV, Scarlett CJ, Bowyer MC, Ngo PD, Vuong QV (2017) Impact of different extraction solvents on bioactive compounds and antioxidant capacity from the root of Salacia chinensis L. J Food Qual 2017:1–8
Turkmen N, Sari F, Velioglu YS (2006) Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin–Ciocalteu methods. Food Chem 99:835–841
Stalikas CD (2007) Extraction, separation, and detection methods for phenolic acids and flavonoids. J Sep Sci 30:3268–3295
McDonald S, Prenzler PD, Antolovich M, Robards K (2001) Phenolic content and antioxidant activity of olive extracts. Food Chem 73:73–84
Pandey A, Tripathi S (2014) Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. J Pharmacogn Phytochem 2:115–119
Zhang CL, Guan H, Xi PZ, Deng T, Gao JM (2010) Anthraquinones from the roots of Prismatomeris tetrandra. Nat Prod Commun 5:1934578X1000500821
Likhitwitayawuid K, Dej-adisai S, Jongbunprasert V, Krungkrai J (1999) Antimalarials from Stephania venosa, Prismatomeris sessiliflora, Diospyros montana and Murraya siamensis1. Planta Med 65:754–756
Lee HH (1969) Colouring matters from Prismatomeris malayana. Phytochemistry 8:501–503
Stéphane FFY, Jules BKJ, Batiha GE, Ali I, Bruno LN (2021) Extraction of bioactive compounds from medicinal plants and herbs. Nat Med Plants. IntechOpen.
Elufioye TO, Abdul AA, Moody JO (2017) Cytotoxicity studies of the extracts, fractions, and isolated compound of Pseudocedrela kotschyi on cervical cancer (HeLa), breast cancer (MCF-7) and skeletal muscle cancer (RD) cells. Pharmacogn Res 9:46
Ahuja S (2006) High-pressure liquid chromatography. Compr Anal Chem 47:485–559
Do QD, Angkawijaya AE, Tran-Nguyen PL, Huynh LH, Soetaredjo FE, Ismadji S, Ju YH (2014) Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J Food Drug Anal 22:296–302
Lapornik B, Prošek M, Wondra AG (2005) Comparison of extracts prepared from plant by-products using different solvents and extraction time. J Food Eng 71:214–222
Sobiesiak M (2017) Chemical structure of phenols and its consequence for sorption processes. In: Phenolic compounds-natural sources, importance and applications. IntechOpen.
Boik J (2001) Natural compounds in cancer therapy. Oregon Medical Press, Princeton
Srisawat T, Chumkaew P, Heed-Chim W, Sukpondma Y, Kanokwiroon K (2013) Phytochemical screening and cytotoxicity of crude extracts of Vatica diospyroides symington type LS. Trop J Pharm Res 12:71–76
Damia G, D’Incalci M (2009) Contemporary pre-clinical development of anticancer agents—what are the optimal preclinical models? Eur J Cancer 45(16):2768–2781
Subramaniam R, Zahri MK (2021) The cytotoxicity effect on MCF-7 human breast cancer cell line treated with Prismatomeris glabra leaves and roots ethyl acetate extract. Asian J Med Biomed 5:47–53
Khalili RM, Noratiqah JM, Norhaslinda R, Amin BA, Roslan A, Zubaidi AL (2017) Cytotoxicity effect and morphological study of different Duku (Lansium domesticum corr.) extract towards human colorectal adenocarcinoma cells line (HT-29). Pharmacogn J 9:757–761
Bin Rohin MA, Ridzwan N, Jumli MN, Abd Hadi N, Johari SA, Latif AZ (2017) Cytotoxicity study and morphological changes of different extraction for Bismillah leaf (Vernonia amygdalina) in human glioblastoma multiforme cell line (U-87). Int J Med Sci 28:1472–1478
Feng S, Wang Z, Zhang M, Zhu X, Ren Z (2018) HG30, a tetrahydroanthraquinone compound isolated from the roots of Prismatomeris connate, induces apoptosis in human non-small cell lung cancer cells. Biomed Pharmacother 100:124–131
Yusuf H, Satria D, Suryawati S, Fahriani M (2020) Combination therapy of eurycomanone and doxorubicin as anticancer on T47D and MCF-7 Cell Lines. Syst Rev Pharm 11:335–341
Osman AM, Bayoumi HM, Al-Harthi SE, Damanhouri ZA, ElShal MF (2012) Modulation of doxorubicin cytotoxicity by resveratrol in a human breast cancer cell line. Cancer Cell Int 12:1–8
Xu Y, Zheng W, Wang T, Wang P, Zhu L, Ma X (2012) Genetic protein TmSm (T34A) enhances sensitivity of chemotherapy to breast cancer cell lines as a synergistic drug to doxorubicin. Biomed Pharmacother 66:368–372
Razak NA, Abu N, Ho WY, Zamberi NR, Tan SW, Alitheen NB, Long K, Yeap SK (2019) Cytotoxicity of eupatorin in MCF-7 and MDA-MB-231 human breast cancer cells via cell cycle arrest, anti-angiogenesis and induction of apoptosis. Sci Rep 9:1514
Fang XJ, Jiang H, Zhu YQ, Zhang LY, Fan QH, Tian Y (2014) Doxorubicin induces drug resistance and expression of the novel CD44st via NF-κB in human breast cancer MCF-7 cells. Oncol Rep 31:2735–2742
Abdel-Sattar OE, Allam RM, Al-Abd AM, Avula B, Katragunta K, Khan IA, El-Desoky AM, Mohamed SO, El-Halawany A, Abdel-Sattar E, Meselhy MR (2023) Cytotoxic and chemomodulatory effects of Phyllanthus niruri in MCF-7 and MCF-7ADR breast cancer cells. Sci Rep 13:2683
Gonçalves J, Ramos R, Luís A, Rocha S, Rosado T, Gallardo E, Duarte AP (2019) Assessment of the bioaccessibility and bioavailability of the phenolic compounds of Prunus avium L. by in vitro digestion and cell model. ACS Omega 4:7605–7613
He Z, Zhao L, Liu X, Xu Y (2020) The application of in-source fragmentation in ultra-high performance liquid chromatography-electrospray ionization-tandem mass spectrometry for pesticide residue analysis. J Chromatogr A 1633:461637
Zhou W, Yang S, Wang PG (2017) Matrix effects and application of matrix effect factor. Bioanalysis 9:1839–1844
Pitt JJ (2009) Principles and applications of liquid chromatography-mass spectrometry in clinical biochemistry. Clin Biochem Rev 30:19
Volmer D, Jessome LL (2006) Ion suppression: a major concern in mass spectrometry. LCGC N Am 24:498–510
Zan L, Chen Q, Zhang L, Li X (2019) Epigallocatechin gallate (EGCG) suppresses growth and tumorigenicity in breast cancer cells by downregulation of miR-25. Bioengineered 10:374–382
Huang CY, Han Z, Li X, Xie HH, Zhu SS (2017) Mechanism of EGCG promoting apoptosis of MCF-7 cell line in human breast cancer. Oncol Lett 14:3623–3627
Luo HQ, Xu M, Zhong WT, Cui ZY, Liu FM, Zhou KY, Li XY (2014) EGCG decreases the expression of HIF-1α and VEGF and cell growth in MCF-7 breast cancer cells. J BU ON 19:435–459
Brazil (2015) Ministry of Health. Health Care Secretariat. Department of Primary Health Care. National policy for complementary and integrative practices in SUS: access extension attitude/Ministry of Health. Health Care Secretariat. Department of Primary Health Care. 2. Brasília: Ministry of Health
Hashem S, Ali TA, Akhtar S, Nisar S, Sageena G, Ali S, Al-Mannai S, Therachiyil L, Mir R, Elfaki I, Mir MM (2022) Targeting cancer signaling pathways by natural products: exploring promising anti-cancer agents. Biomed Pharmacother 150:113054
Burguin A, Diorio C, Durocher F (2021) Breast cancer treatments: updates and new challenges. J Personal Med 11:808
Oda K, Matsuoka Y, Funahashi A, Kitano H (2005) A comprehensive pathway map of epidermal growth factor receptor signaling. Mol Syst Biol 1:2005–0010
Voldborg BR, Damstrup L, Spang-Thomsen M, Poulsen HS (1997) Epidermal growth factor receptor (EGFR) and EGFR mutations, function and possible role in clinical trials. Ann Oncol 8:1197–1206
Jiang P, Wu X, Wang X, Huang W, Feng Q (2016) NEAT1 upregulates EGCG-induced CTR1 to enhance cisplatin sensitivity in lung cancer cells. Oncotarget 7:43337
Wu W, Dong J, Gou H, Geng R, Yang X, Chen D, Xiang B, Zhang Z, Ren S, Chen L, Liu J (2021) EGCG synergizes the therapeutic effect of irinotecan through enhanced DNA damage in human colorectal cancer cells. J Cell Mol Med 25:7913–7921
Sams-Dodd F (2005) Target-based drug discovery: is something wrong? Drug Discov Today 10:139–147
Swinney DC, Anthony J (2011) How were new medicines discovered? Nat Rev Drug Discov 10:507–519
Moffat JG, Rudolph J, Bailey D (2014) Phenotypic screening in cancer drug discovery—past, present and future. Nat Rev Drug Discov 13:588–602
Zheng W, Thorne N, McKew JC (2013) Phenotypic screens as a renewed approach for drug discovery. Drug Discov Today 18:1067–1073
Bácskay I, Nemes D, Fenyvesi F, Váradi J, Vasvári G, Fehér P, Vecsernyés M, Ujhelyi Z (2018) Role of cytotoxicity experiments in pharmaceutical development. InTech, London
Sun H, Wang Y, Cheff DM, Hall MD, Shen M (2020) Predictive models for estimating cytotoxicity on the basis of chemical structures. Bioorg Med Chem 28:115422
Chen G, Pramanik BN, Liu YH, Mirza UA (2007) Applications of LC/MS in structure identifications of small molecules and proteins in drug discovery. J Mass Spectrom 42:279–287
Espada A, Molina-Martin M, Dage J, Kuo MS (2008) Application of LC/MS and related techniques to high-throughput drug discovery. Drug Discov Today 13(9–10):417–423
Sarvani V, Elisha Raju P, Nama S, Pola LM, Rao CB (2013) Role of LC-MS in drug discovery process. Int J Pharm Ther 4:148–153
Eddershaw PJ, Beresford AP, Bayliss MK (2000) ADME/PK as part of a rational approach to drug discovery. Drug Discov Today 5:409–414
Shibam D, Divya S, Rohit B (2021) Insights of LC-MS in drug discovery, drug development and modern analysis. Nov Appro Drug Des Dev 6:555681
Iravani S, Korbekandi H, Mirmohammadi SV, Zolfaghari B (2014) Synthesis of silver nanoparticles: chemical, physical and biological methods. Res Pharm Sci 9:385
Ashrafizadeh M, Zarrabi A, Bigham A, Taheriazam A, Saghari Y, Mirzaei S, Hashemi M, Hushmandi K, Karimi-Maleh H, Nazarzadeh Zare E, Sharifi E (2023) (Nano) platforms in breast cancer therapy: drug/gene delivery, advanced nanocarriers and immunotherapy. Med Res Rev 43:2115–2176
Ashrafizadeh M, Zarrabi A, Karimi-Maleh H, Taheriazam A, Mirzaei S, Hashemi M, Hushmandi K, Makvandi P, Nazarzadeh Zare E, Sharifi E, Goel A (2023) (Nano) platforms in bladder cancer therapy: challenges and opportunities. Bioeng Transl Med 8:e10353
Khan Y, Sadia H, Ali Shah SZ, Khan MN, Shah AA, Ullah N, Ullah MF, Bibi H, Bafakeeh OT, Khedher NB, Eldin SM (2022) Classification, synthetic, and characterization approaches to nanoparticles, and their applications in various fields of nanotechnology: a review. Catalysts 12:1386
Acknowledgements
The authors would also like to acknowledge the botanist from the School of Marine and Environmental Sciences, University of Malaysia Terengganu, for the assistance and support during the identification stage of P. glabra.
Funding
This research has been funded by Universiti Sultan Zainal Abidin under LabMat Grant (UniSZA/LABMAT/2018/07).
Author information
Authors and Affiliations
Contributions
NNZ contributed to conceptualisation of manuscript, data analysis, and preparing the manuscript; SAB contributed to conceptualisation of manuscript, laboratory experiments, and data collection; WRWT participated in designing the experiments; WRWT, RMR, IS, WANWA and CKDCKD supervised the laboratory work, reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Plant details
P. glabra is a native plant of Malaysia and can be found in Terengganu, Pahang, and Johor. In this study, neither permission nor a license is deemed necessary.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zulkipli, N.N., Rahman, S.A., Taib, W.R.W. et al. The cytotoxicity effect and identification of bioactive compounds of Prismatomeris glabra crude leaf extracts against breast cancer cells. Beni-Suef Univ J Basic Appl Sci 13, 33 (2024). https://doi.org/10.1186/s43088-024-00490-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43088-024-00490-0