Abstract
Background
Excision repair cross-complementing (ERCC) genes are important regulators of DNA repair processes, the aberrant expression of which may lead to treatment failures of breast cancer. The prognostic significance of the ERCC genes in several cancers has been investigated, except for breast cancer; therefore, we explored the ERCC genes, including ERCC1, ERCC2, ERCC3, ERCC4, ERCC5, ERCC6, and ERCC8 in breast cancer, particularly during drug resistance processes.
Results
Using the 2021 provisional study of The Metastatic Breast Cancer Project from cBioPortal, we identified ERCC genetic alterations in 8–36% of patients, where most alterations were considered amplifications followed by deep deletions. Pathway enrichment analyses identified Wnt signaling enrichment which contributed to cell proliferation. ERCC2 had the highest epigenetic alteration levels at 7 DNA methylation sites. Also, the mRNA levels of ERCC1, ERCC2, ERCC4, ERCC6, and ERCC8 were higher in patients with breast cancer when compared to normal breast tissues, with higher ERCC2 but lower ERCC8 levels in metastatic breast tissues. Breast cancer patients with low ERCC6 levels had better overall survival rates than the groups with higher ERCC6 levels. ERCC1, ERCC2, and ERCC4 were identified as endocrine therapy response predictors. ERCC1 was specifically an antihuman epidermal growth factor receptor therapy predictor, and ERCC1, ERCC2, ERCC6, and ERCC8 were chemotherapy response predictors.
Conclusion
We used bioinformatics to investigate and identify the roles of ERCC genes in breast cancer resistant cells, in particular ERCC1, ERCC2, and ERCC6. We also showed how the Wnt pathway and DNA repair processes had a role in drug resistance in breast cancer cells, but further studies are required to validate those results.
Similar content being viewed by others
1 Background
Resistance to therapies, including endocrine, antihuman epidermal growth factor receptor (HER2), and chemo/radiation therapies, is a major hurdle in breast cancer treatment [1]. Therefore, identifying the resistance mechanisms during therapies is the key to successful breast cancer treatment and therapy development [2, 3]. Resistant breast cancer cells may progress to metastatic cells that spread to other tissues and cause patient death; hence, overcoming breast cancer resistance mechanisms can prevent metastasis [4]. Additionally, the discovery of resistance biomarkers can assist in directing treatment decisions and improving the outcomes of patients with breast cancer [5, 6].
During therapy, breast cancer cells may develop resistance mechanisms, such as enhanced DNA repair involving upregulated DNA repair genes [1]. Excision repair cross-complementing (ERCC) genes are essential components of the nucleotide excision repair process and important DNA repair regulators [7]. Sophisticated DNA repair processes that remove DNA damage and maintain chromosome stability are generated by the proteins encoded by the ERCC genes, including ERCC1, ERCC2, ERCC3, ERCC4, ERCC5, ERCC6, and ERCC8 [8]. Genomic instability that leads to genetic and epigenetic alterations, and cancer development of are influenced by ERCC pathway dysregulation [9,10,11,12].
Importantly, the prognostic significance of the ERCC genes in several cancers has been reported. A previous study indicated that ERCC1 mRNA and protein overexpression were correlated with lung cancer cell resistance against platinum-based chemotherapy [13, 14]. Bioinformatics analyses of the ERCC genes in ovarian cancer showed that high ERCC1 and ERCC8 mRNA levels are associated with poor overall survival (OS) rates in patients with ovarian cancer, whereas patients with increased ERCC4 mRNA levels had better OS rates than the patients with low ERCC4 mRNA levels [10]. Another bioinformatic study on the ERCC genes in gastric cancer cells reported that ERCC4, ERCC6, and ERCC8 are candidate prognosis biomarkers and could function as potential therapeutic targets [15]. However, the role of ERCC genes, as well as their genetic and epigenetic regulation in breast cancer cells, remains elusive and requires more investigation.
In this review, the ERCC alterations will be carried out using the data of the provisional study of The Metastatic Breast Cancer Project (MBCP, 2021) after previous reports [16,17,18,19,20,21,22,23,24,25]. Understanding the role of ERCC genes, including epigenetic and genetic modifications, and other factors in drug resistance in breast cancer may suggest mechanisms and help in identifying potential targets for novel therapies. Therefore, it can be helpful for developing new drugs and therapeutic strategies targeting ERCC genes to overcome drug resistance in breast cancer therapy. In this study, using a bioinformatic approach, we explored the ERCC genes, including ERCC1, ERCC2, ERCC3, ERCC4, ERCC5, ERCC6, and ERCC8, in breast cancer, particularly their involvement in drug resistance.
2 Methods
2.1 Genetic alterations
ERCC gene analyses were conducted using cBioPortal (https://www.cbioportal.org/) [26, 27]. Briefly, ERCC gene symbols were entered into cBioPortal, and associated breast cancer studies were selected. Then, studies with the highest number of genetic modifications were selected for additional genetic alteration analyses, including OncoPrint, copy number alterations (CNA), mutations, mutual exclusivity, functional mutant predictions, and pathway enrichment from Pathway Mapper and NDEx. Statistical analyses of CNA were performed using One-Way Analysis of Variance with Tukey’s multiple comparison tests. * indicates p < 0.05.
2.2 Epigenetic alterations
MethSurv (https://biit.cs.ut.ee/methsurv/) was used to analyze epigenetic changes [28]. Briefly, ERCC gene symbols were entered into MethSurv using several criteria, such as breast invasive carcinoma from the Cancer Genome Atlas (TCGA) study, 2017.
2.3 ERCC mRNA and protein expression
We examined ERCC mRNA expression profiles in normal and tumor breast tissue from The Genotype-Tissue Expression (GTEx) and TCGA studies and analyzed data using GEPIA (http://gepia.cancer-pku.cn/) [29, 30]. Briefly, gene symbols were entered into GEPIA and several parameters were selected, including box plot expression, ILog2FC| cutoff = 1, p-value cutoff < 0.01, using a dataset of BRCA, Jitter Size of 0.4, and match TCGA normal and GTEx data. We analyzed ERCC mRNA expression levels in normal, breast tumor, and metastatic breast tumor tissues from TCGA and GTEx studies using TNM Plot (https://tnmplot.com/analysis/) [31]. Briefly, gene symbols were submitted to TNM Plot using different criteria; RNA-sequencing data, and tumor, normal, and metastatic samples from TCGA and GTEx studies, and statistical analyses were conducted using Kruskal–Wallis tests. ERCC protein expression data were analyzed in normal and breast cancer tissue samples using the Human Protein Atlas (https://www.proteinatlas.org/) [32].
2.4 Prognostic values
OS rates related to ERCC mRNA expression levels were analyzed using the Kaplan–Meier (KM) Plotter (https://kmplot.com/analysis/) using the following criteria: mRNA gene chip data and no subtype and cohort restrictions [33].
2.5 Receiver operating characteristic (ROC) plots
Associations between gene expression levels and sensitivity of breast cancer patients to endocrine, anti-HER2, and chemotherapy were examined in ROC Plotter [34]. Estrogen receptor (ER) and HER2 status, pathological complete response (PCR), relapse-free survival (RFS) for 5 years, and patients receiving endocrine anti-HER2 therapy and chemotherapy were selected. Gene symbols were entered into ROC Plotter and p < 0.05 values was selected as statistical significance thresholds.
3 Results
3.1 ERCC genetic alterations
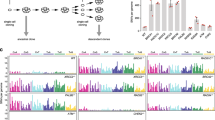
Genetic alteration analyses using cBioPortal showed that ERCC alterations mostly occurred in the 2021 provisional study of The Metastatic Breast Cancer Project by Jain et al. (2023), and therefore was used for further analyses [18] (Fig. 1A). Oncoprint analyses identified genetic alterations in ERCC1 (27%), ERCC2 (28%), ERCC3 (16%), ERCC4 (16%), ERCC5 (8%), ERCC6 (36%), and ERCC8 (13%), where most alterations were amplifications followed by deep deletions, except for ERCC8 which was dominated by deep deletions (Fig. 1B). CNA analyses (Fig. 1C) showed that ERCC4 mRNA expression levels were significantly higher in amplification cases when compared to diploid cases, and ERCC8 mRNA levels were significantly higher in diploid cases when compared to shallow deletion cases. Mutual exclusivity analyses showed that 2 gene pairs exhibited co-occurrence, i.e., ERCC1-ERCC2 and ERCC4-ERCC6 (Table 1). We also detected mutations in the ERCC genes (Fig. 1D, Table 2); ERCC3 (I194M), ERCC5 (L6H), and ERCC6 (R670W) mutations were predicted to be highly impactful, deleterious, and probably damaging. Pathway enrichment analyses related to genetic alterations identified enriched Wnt signaling which contributed to cell proliferation (Pathway Mapper, Fig. 1E) and nucleotide excision repair in Homo sapiens (NDEx, Fig. 1F).
ERCC genetic alterations: ERCC1, ERCC2, ERCC3, ERCC4, ERCC5, ERCC6, and ERCC8 analyses using cBioportal. A Summary of ERCC1, ERCC2, ERCC3, ERCC4, ERCC5, ERCC6, and ERCC8 gene alterations in breast cancer studies in cBioportal. B Oncoprint ERCC1, ERCC2, ERCC3, ERCC4, ERCC5, ERCC6, and ERCC8 gene analyses using samples from the 2021 provisional study of The Metastatic Breast Cancer Project. C Copy number alterations (CNAs) in ERCC1, ERCC2, ERCC3, ERCC4, ERCC5, ERCC6, and ERCC8 genes using samples from the 2021 provisional study of The Metastatic Breast Cancer Project. Statistical analyses were performed using One-Way Analysis of Variance with Tukey’s multiple comparison tests. * indicates p < 0.05. D Mutations in ERCC1, ERCC2, ERCC3, ERCC4, ERCC5, ERCC6, and ERCC8 genes in samples from the 2021 provisional study of The Metastatic Breast Cancer Project. Pathway enrichment analyses of ERCC1, ERCC2, ERCC3, ERCC4, ERCC5, ERCC6, and ERCC8 gene alterations using (E). Pathway Mapper and (F). NDEx data
3.2 Epigenetic alterations
ERCC epigenetic alteration analyses identified 1 alteration in ERCC1 (cg16629408) (Additional file 1: Fig. S1). ERCC2 recorded the highest epigenetic alterations with 7 altered methylation sites, including cg01518138, cg17212420, cg03117793, cg01599094, cg18851932, cg02595770, and cg20674128. Epigenetic alterations were also identified in ERCC3 (cg06373940, cg11957777, and cg26522792), ERCC4 (cg08296903, cg08387426, cg05348793, and cg26493247), ERCC5 (cg23044680, cg23957850, cg10258411, cg14904079, cg00691940, and cg00884663), ERCC6 (cg00025044, cg06437173, cg23926543), and ERCC8 (cg02408480 and cg25966817).
3.3 ERCC mRNA and protein expression
In GEPIA, ERCC1, ERCC2, ERCC4, ERCC6, and ERCC8 mRNA levels were higher in breast cancer samples when compared to normal breast tissues, whereas ERCC3 and ERCC5 mRNA levels were higher in normal breast tissues when compared to breast tumor samples (Fig. 2A). Using TNM Plot, mRNA analyses of normal, tumor, and metastatic breast cancer tissues showed that ERCC2 mRNA levels were significantly higher in breast tumor tissues when compared to adjacent tissues, but lower when compared to metastatic breast tumor tissues (p = 1.86 × 10−10) (Fig. 2B). Additionally, ERCC5 mRNA levels were higher in normal breast tissues when compared to breast tumor samples, but breast tumor tissue levels were still lower when compared to metastatic breast tumor levels (p = 3.93 × 10−7). Also, ERCC8 mRNA levels were higher in normal breast tissue when compared to breast cancer and metastatic breast cancer samples (p = 1.51 × 10−5). From the Human Protein Atlas, ERCC1, ERCC2, ERCC3, ERCC4, and ERCC5 protein levels were increased in breast tumor samples when compared to normal adjacent tissue (Fig. 2C). No data were identified for ERCC6 and ERCC8 protein levels.
A ERCC1, ERCC2, ERCC3, ERCC4, ERCC5, ERCC6, and ERCC8 mRNA levels in breast cancer (TCGA data) and normal adjacent tissues (GTEx data) using GEPIA. B ERCC1, ERCC2, ERCC3, ERCC4, ERCC5, ERCC6, and ERCC8 mRNA levels in normal, breast cancer, and metastatic breast cancer tissues using TNM Plot. Statistical analyses were conducted using Kruskal–Wallis tests. C ERCC, ERCC2, ERCC3, ERCC4, ERCC5, ERCC6, and ERCC8 protein levels in normal and breast cancer tissues as determined by The Human Protein Atlas
3.4 Prognostic values
The prognostic value of the ERCC gene expression across breast cancer samples indicated that only ERCC6 demonstrated significant results; breast cancer patients with low ERCC6 levels had better OS rates than the groups with higher ERCC6 levels Fig. 3). Other gene data were not significant.
3.5 ROC plots
Based on the transcriptome data from patients with breast cancer, we examined relationships between gene expression levels and endocrine/anti-HER2/chemotherapy responses according to PCR and RFS outcomes. Based on the PCR parameters in response to endocrine therapy, 0.689 and 0.743 area under the curve (AUC) values were significantly moderately linked with ERCC1 (p = 5.1 × 10−3) and ERCC2 (p = 1 × 10−4) expression levels, respectively (Fig. 4A). Based on the RFS parameters for endocrine therapy responses, 0.578 (p = 2 × 10−3), 0.578 (p = 1.3 × 10−3), and 0.63 (p = 1.9 × 10−2) AUC values were significantly linked with ERCC1, ERCC2, and ERCC4 mRNA levels, respectively (Fig. 4B). Based on the ROC on anti-HER2, an AUC value of 0.578 (p = 2.3 × 10−2) was moderately significantly linked to ERCC1 expression (Fig. 4C). No significant results were identified for RFS values related to anti-HER2 therapy (Fig. 4D). According to the responses to chemotherapy and based on the PCR parameters, an AUC value of 0.613 (p = 8.3 × 10−6) was significantly linked with ERCC6 levels (Fig. 4E), whereas based on the RFS parameters, 0.598 (p = 9 × 10−5), 0.55 (p = 3.1 × 10−2), 0.618 (p = 8.7 × 10−3), and 0.578 (p = 1.4 × 10−3) AUC values were significantly moderately linked with ERCC1, ERCC2, ERCC6, and ERCC8 expression levels, respectively (Fig. 4F). No data were identified for ERCC5 across all parameters.
ROC Plotter showing correlations between ERCC1, ERCC2, ERCC3, ERCC4, ERCC5, ERCC6, and ERCC8 gene expression levels and endocrine therapy sensitivity using (A). pathological complete response (PCR) and (B). relapse-free survival (RFS) approaches. ROC Plotter showing correlations between ERCC1, ERCC2, ERCC3, ERCC4, ERCC5, ERCC6, and ERCC8 gene expression levels and anti-HER2 therapy sensitivity using (C). PCR and (D). RFS approaches. ROC Plotter showing correlations between ERCC1, ERCC2, ERCC3, ERCC4, ERCC5, ERCC6, and ERCC8 gene expression levels and chemotherapy sensitivity using (E). PCR and (F). RFS approaches
4 Discussion
We explored ERCC gene roles in breast cancer. Genetic alteration analyses (cBioportal) showed that ERCC1, ERCC2, and ERCC6 had the highest alterations of all the ERCC genes. Oncoprint analysis results showed that in the Metastatic Breast Cancer Project Provisional 2021 study, most of genetic alterations are considered as amplification followed by deep deletion. CNA analyses identified significantly altered genes: ERCC4 and ERCC8. Mutual exclusivity analyses showed that 2 gene pairs exhibited co-occurrence traits: ERCC1-ERCC2 and ERCC4-ERCC6. Our findings highlighted the important roles of ERCC1, ERCC2, ERCC4, ERCC6, and ERCC8 in metastatic breast cancer.
Mutations in ERCC3 (I194M), ERCC5 (L6H), and ERCC6 (R670W) genes were predicted to be highly impactful, deleterious, and probably damaging. Previous studies reported that ERCC1, ERCC2, ERCC3, ERCC4, ERCC5, ERCC6, and ERCC8 mutations were associated with the clinical features of some diseases, including xeroderma pigmentosum, and increases the risk of skin cancer, cerebro-oculo-facial-skeletal syndrome, trichothiodystrophy, Cockayne syndrome, and UV-sensitive syndrome [35,36,37,38]. Accordingly, ERCC5 and ERCC6 mutant functions in breast cancer cells must be clarified in future studies.
Our pathway enrichment analyses of ERCC genetic alterations showed enriched Wnt signaling and nucleotide excision repair, although they did not include ERCC genes in the pathway but neighboring genes from ERCC genes that are enriched as genes involved in the Wnt pathway. Previously, Karimaian et al. [39] reported crosstalk between DNA repair and Wnt signaling and highlighted the potential application of Wnt signaling in cancer therapy. Wnt signaling crosstalk with DNA repair pathways plays a role in the genomic stability maintenance due to cisplatin treatment in HeLa, U2OS, and LN229 cells [40]. Additionally, in isogenic triple-negative breast cancer models, Wnt/-catenin inhibition disrupted carboplatin resistance [41]. To date, there has been no study on the relationship between ERCC genes, the Wnt signaling system, and breast cancer resistance. Therefore, we speculate that ERCC might regulate DNA repair and drug resistance in breast cancer by regulating Wnt signaling, but this hypothesis requires experimental confirmation.
Epigenetic ERCC1 results correlated with Oncoprint results where ERCC1 was considerably amplified in metastatic breast cancer cell samples, whereas ERCC2 appeared to have the highest DNA methylation and amplification levels. Epigenetic ERCC6 alterations indicated 3 DNA methylation profiles, and the number of genetic alterations remains high because the majority of genetic alterations are deep deletions, not amplifications. Previous studies reported ERCC1 amplifications in metastatic breast cancer cells [42]. Also, patients with non-small cell lung cancer (NSCLC) with adenocarcinoma had significantly varied ERCC1 mRNA expression levels in main tumors and metastatic sites [43]. Moreover, ERCC1 overexpression inhibited apoptosis in ovarian cancer cells [44]. However, no studies have yet reported other ERCC gene amplifications in cancer, therefore more studies are warranted.
Few studies have reported ERCC genetic variations and alterations in cancer. Genetic polymorphisms in ERCC2 (Asp312Asn) and ERCC4 (Ser835Ser) were correlated with breast cancer risk in Korean women [45]. Glioma susceptibility was influenced by 2 ERCC2 gene polymorphisms, (rs13181 and rs1799793) and the ERCC1 polymorphism (rs3212986) [46]. The rs3212986 polymorphism correlated with higher response and PFS rates in patients with advanced NSCLC receiving anti-PD1 nivolumab [9]. ERCC2 (Lys751Gln) and ERCC5 (His46His) polymorphisms were correlated with good prognosis rates in osteosarcoma [47]. Thus, deregulated DNA repair pathways may encourage genomic instability and enhance DNA lesion and mutation during carcinogenesis as ERCC1 was found to be inversely linked with tumor mutation load and neoantigen expression [48].
Using the TNM Plot, mRNA levels in normal, tumor, and metastatic breast cancer tissues showed increased ERCC2 mRNA expression in breast tumor tissue, ERCC5 mRNA expression in metastatic breast tumor tissue, and decreased ERCC8 mRNA expression in breast and metastatic breast tumors. These results were supported by a previous study which showed that high ERCC1 expression increased metastasis risks in patients with breast cancer [42], although our ERCC1 mRNA overexpression data in metastatic breast cancer were not significant. Using the Human Protein Atlas, ERCC1, ERCC2, ERCC3, ERCC4, and ERCC5 protein levels were increased in breast tumor samples when compared to normal adjacent tissues. No data were identified for ERCC6 and ERCC8 protein levels, therefore future studies must examine the protein levels in patients with breast cancer.
ERCC gene expression analyses across breast cancer samples showed that only ERCC6 had a significant prognostic value; breast cancer patients with low ERCC6 levels had better OS when compared to the opposite group. These results were consistent with previous studies which showed that the higher the mRNA ERCC expression, the greater the capability of DNA repair, drug resistance development, and metastasis. Using PCR and RFS parameters, our ROC plots showed that ERCC1 and ERCC2 were predictive endocrine therapy markers; ERCC1 was a predictive marker for anti-HER2 therapy based on RFS. From PCR, ERCC6 was a predictive marker for chemotherapy, whereas ERCC1, ERCC2, ERCC6, and ERCC8 were predictive markers for chemotherapy (RFS parameters).
Our ROC results were supported by a previous study which reported correlations between high ERCC1 levels and drug resistance. Upregulated ERCC1 was identified in cisplatin-resistant A2780 human ovarian carcinoma cells [49]. Low ERCC1 levels are posited as good prognosis factors for platinum-based chemotherapy at all lung adenocarcinoma stages [50]. In breast cancer, ERCC1 protein levels were correlated with disease resistance to anthracycline therapy, in which samples with high levels of ERCC1 showed poor response to anthracycline therapy, where high elevated ERCC1 levels in samples demonstrated poor responses to anthracycline [51]. The authors concluded that high ERCC1 expression levels were strongly related to poor prognoses in triple-negative breast cancer patients receiving platinum-based chemotherapy [52]. Taken together, ERCC1 is important for predicting chemotherapy responses in breast cancer cells; however, other ERCC gene data are limited and warrant future study.
Our study had some limitations. First, the study was performed using a bioinformatics approach with limited sample numbers; therefore, our data must be validated with other clinical data and in a laboratory setup. Second, ERCC gene functions in breast cancer metastasis, based on subtype, were not examined; therefore, future studies are required. Additionally, protein expression data for several ERCC genes were not identified; therefore, expression data from other databases or protein expression analyses in patients are required. Our study highlighted breast cancer resistance and metastatic mechanisms due to genetic and epigenetic alterations in the ERCC genes, and provided insights on new therapeutic targets, as well as predicted breast cancer patient responses to endocrine, anti-HER2, and chemotherapy.
5 Conclusion
Bioinformatically, we examined and identified roles of ERCC in breast cancer resistance cells. Specifically, ERCC1, ERCC2, and ERCC6 genes had prominent roles in disease resistance and metastasis. We also demonstrated how Wnt pathway and DNA repair processes contributed to drug resistance in breast cancer cells. However, further research is required to confirm our data so that ERCC1, ERCC2, and ERCC6 genes can be used as drug resistance predictors in breast cancer cells.
Availability of data and materials
Study data are available in supplementary files.
Abbreviations
- ER:
-
Estrogen receptor
- ERCC:
-
Excision repair cross-complementing
- HER2:
-
Human epidermal growth factor receptor 2
- KM:
-
Kaplan–Meier
- OS:
-
Overall survival
- PCR:
-
Pathological complete response
- RFS:
-
Relapse-free survival
- ROS:
-
Receiver operator characteristic
References
Kinnel B, Singh SK, Oprea-Ilies G, Singh R (2023) Targeted therapy and mechanisms of drug resistance in breast cancer. Cancers 15(4):1320. https://doi.org/10.3390/cancers15041320
Landeros N, Castillo I, Pérez-Castro R (2023) Preclinical and clinical trials of new treatment strategies targeting cancer stem cells in subtypes of breast cancer. Cells 12(5):720. https://doi.org/10.3390/cells12050720
Yang F, He Q, Dai X, Zhang X, Song D (2023) The potential role of nanomedicine in the treatment of breast cancer to overcome the obstacles of current therapies. Front Pharmacol 14:1143102. https://doi.org/10.3389/fphar.2023.1143102
Pecar G, Liu S, Hooda J, Atkinson JM, Oesterreich S, Lee AV (2023) RET signaling in breast cancer therapeutic resistance and metastasis. Breast Cancer Res 25(1):26. https://doi.org/10.1186/s13058-023-01622-7
Gu G, Dustin D, Fuqua SA (2016) Targeted therapy for breast cancer and molecular mechanisms of resistance to treatment. Curr Opin Pharmacol 31:97–103. https://doi.org/10.1016/j.coph.2016.11.005
Liu S, Xie SM, Liu W, Gagea M, Hanker AB, Nguyen N et al (2023) Targeting CXCR4 abrogates resistance to trastuzumab by blocking cell cycle progression and synergizes with docetaxel in breast cancer treatment. Breast Cancer Res 25(1):62. https://doi.org/10.1186/s13058-023-01665-w
Gavande NS, VanderVere-Carozza PS, Hinshaw HD, Jalal SI, Sears CR, Pawelczak KS et al (2016) DNA repair targeted therapy: The past or future of cancer treatment? Pharmacol Ther 160:65–83. https://doi.org/10.1016/j.pharmthera.2016.02.003
Zhao M, Li S, Zhou L, Shen Q, Zhu H, Zhu X (2018) Prognostic values of excision repair cross-complementing genes mRNA expression in ovarian cancer patients. Life Sci 194:34–39. https://doi.org/10.1016/j.lfs.2017.12.018
Aiello M, Parra HS, Noto L, Restuccia N, Vigneri P, Paratore S (2017) P3.02c–090 The Role of ERCC-1 polymorphisms as predictive biomarker of response to nivolumab in advanced NSCLC: Topic: IT biomarkers. J Thorac Oncol 12(1):S1333. https://doi.org/10.1016/j.jtho.2016.11.1886
Wang H, Wang T, Guo H, Zhu G, Yang S, Hu Q et al (2016) Association analysis of ERCC5 gene polymorphisms with risk of breast cancer in Han women of northwest China. Breast Cancer 23(3):479–485. https://doi.org/10.1007/s12282-015-0590-2
Hu G, Li P, Cui X, Li Y, Zhang J, Zhai X et al (2018) Cr(VI)-induced methylation and down-regulation of DNA repair genes and its association with markers of genetic damage in workers and 16HBE cells. Environ Pollut 238:833–843. https://doi.org/10.1016/j.envpol.2018.03.046
Pasqui A, Boddi A, Campanacci DA, Scoccianti G, Bernini A, Grasso D et al (2022) Alteration of the nucleotide excision repair (NER) pathway in soft tissue sarcoma. Int J Mol Sci 23(15):8360
Altaha R, Liang X, Yu JJ, Reed E (2004) Excision repair cross-complementing-group 1: gene expression and platinum resistance. Int J Mol Med 14(6):959–970
Felip E, Rosell R (2007) Testing for excision repair cross-complementing 1 in patients with non-small-cell lung cancer for chemotherapy response. Expert Rev Mol Diagn 7(3):261–268. https://doi.org/10.1586/14737159.7.3.261
Luo SS, Liao XW, Zhu XD (2018) Prognostic value of excision repair cross-complementing mRNA expression in gastric cancer. Biomed Res Int 2018:6204684. https://doi.org/10.1155/2018/6204684
Barroso-Sousa R, Forman J, Collier K, Weber Z, Jammihal T, Kao K et al (2022) Multidimensional molecular profiling of metastatic triple-negative breast cancer and immune checkpoint inhibitor benefit. JCO Precis Oncol. https://doi.org/10.1200/PO.21.00413
Brett JO, Dubash TD, Johnson GN, Niemierko A, Mariotti V, Kim LSL et al (2023) A gene panel associated with abemaciclib utility in ESR1-mutated breast cancer after prior cyclin-dependent kinase 4/6-inhibitor progression. JCO Precis Oncol 7:e2200532. https://doi.org/10.1200/PO.22.00532
Jain E, Zanudo JGT, McGillicuddy M, Abravanel DL, Thomas BS, Kim D et al (2023) The Metastatic Breast Cancer Project: leveraging patient-partnered research to expand the clinical and genomic landscape of metastatic breast cancer and accelerate discoveries. medRxiv. https://doi.org/10.1101/2023.06.07.23291117
Janeway KA, George S, Painter C, Cibulskis C, Cusher T, Doucette J et al (2023) Abstract 6084: The Osteosarcoma and leiomyosarcoma count me in projects of the cancer moonshot funded PE-CGS network directly engage patient participants in genomics research. Cancer Res 83(7):6084–6084. https://doi.org/10.1158/1538-7445.AM2023-6084
Lamba N, Cagney DN, Catalano PJ, Kim D, Elhalawani H, Haas-Kogan DA et al (2023) A genomic score to predict local control among patients with brain metastases managed with radiation. Neuro Oncol. https://doi.org/10.1093/neuonc/noad098
Li Z, McGinn O, Wu Y, Bahreini SA, Priedigkeit N, Ding K et al (2022) ESR1 mutant breast cancers show elevated basal cytokeratins and immune activation. Nat Commun 13:2011. https://doi.org/10.1038/s41467-022-29498-9
Lipsyc-Sharf M, Jain E, Collins LC, Rosenberg SM, Ruddy KJ, Tamimi RM et al (2023) Genomics of ERBB2-positive breast cancer in young women before and after exposure to chemotherapy plus trastuzumab. JCO Precis Oncol 7:e2300076. https://doi.org/10.1200/PO.23.00076
Parsons HA, Messer C, Santos K, Danysh BP, Hughes ME, Patel A et al (2023) Abstract 3874: Genomic mechanisms of resistance to tyrosine kinase inhibitors (TKIs) in HER2+ metastatic breast cancer (HER2+ MBC). Cancer Res 83(7):3874–2874. https://doi.org/10.1158/1538-7445.AM2023-3874
Waks A, Kim D, Jain E, Snow C, Kirkner G, Rosenberg S et al (2022) Somatic and germline genomic alterations in very young women with breast cancer. Clin Cancer Res 28:2339–2348. https://doi.org/10.1158/1078-0432.CCR-21-2572
Zanudo J, Barroso-Sousa R, Jain E, Jin Q, Li T, Buendia-Buendia J, et al. Genomic and Transcriptomic Determinants of Resistance to CDK4/6 Inhibitors and Response to Combined Exemestane plus Everolimus and Palbociclib in Patients with Metastatic Hormone Receptor Positive Breast Cancer. 2022.
Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA et al (2012) The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2(5):401–404. https://doi.org/10.1158/2159-8290.Cd-12-0095
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO et al (2013) Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6(269):pl1. https://doi.org/10.1126/scisignal.2004088
Modhukur V, Iljasenko T, Metsalu T, Lokk K, Laisk-Podar T, Vilo J (2018) MethSurv: a web tool to perform multivariable survival analysis using DNA methylation data. Epigenomics 10(3):277–288. https://doi.org/10.2217/epi-2017-0118
Li C, Tang Z, Zhang W, Ye Z, Liu F (2021) GEPIA2021: integrating multiple deconvolution-based analysis into GEPIA. Nucleic Acids Res 49(W1):W242–W246. https://doi.org/10.1093/nar/gkab418
Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z (2017) GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res 45(W1):W98-w102. https://doi.org/10.1093/nar/gkx247
Bartha Á, Győrffy B (2021) TNMplot.com: a web tool for the comparison of gene expression in normal, tumor and metastatic tissues. Int J Mol Sci 22(5):2622. https://doi.org/10.3390/ijms22052622
Colwill K, Gräslund S (2011) A roadmap to generate renewable protein binders to the human proteome. Nat Methods 8(7):551–558. https://doi.org/10.1038/nmeth.1607
Győrffy B (2021) Survival analysis across the entire transcriptome identifies biomarkers with the highest prognostic power in breast cancer. Comput Struct Biotechnol J 19:4101–4109. https://doi.org/10.1016/j.csbj.2021.07.014
Fekete JT, Győrffy B (2019) ROCplot.org: Validating predictive biomarkers of chemotherapy/hormonal therapy/anti-HER2 therapy using transcriptomic data of 3,104 breast cancer patients. Int J Cancer 145(11):3140–3151. https://doi.org/10.1002/ijc.32369
DiGiovanna JJ, Kraemer KH (2012) Shining a light on Xeroderma pigmentosum. J Investig Dermatol 132(3, Part 2):785–796. https://doi.org/10.1038/jid.2011.426
Kamileri I, Karakasilioti I, Garinis GA (2012) Nucleotide excision repair: new tricks with old bricks. Trends Genet 28(11):566–573. https://doi.org/10.1016/j.tig.2012.06.004
Menck CF, Munford V (2014) DNA repair diseases: What do they tell us about cancer and aging? Genet Mol Biol 37:220–233
Nouspikel T (2008) Nucleotide excision repair and neurological diseases. DNA Repair 7(7):1155–1167. https://doi.org/10.1016/j.dnarep.2008.03.015
Karimaian A, Majidinia M, Bannazadeh Baghi H, Yousefi B (2017) The crosstalk between Wnt/β-catenin signaling pathway with DNA damage response and oxidative stress: implications in cancer therapy. DNA Repair 51:14–19. https://doi.org/10.1016/j.dnarep.2017.01.003
Pasadi S, Muniyappa K (2020) Evidence for functional and regulatory cross-talk between Wnt/β-catenin signalling and Mre11-Rad50-Nbs1 complex in the repair of cisplatin-induced DNA cross-links. Oncotarget 11(44):4028–4044. https://doi.org/10.18632/oncotarget.27777
Abreu de Oliveira WA, Moens S, El Laithy Y, van der Veer BK, Athanasouli P, Cortesi EE et al (2021) Wnt/β-catenin inhibition disrupts carboplatin resistance in isogenic models of triple-negative breast cancer. Front Oncol 11:705384. https://doi.org/10.3389/fonc.2021.705384
Yang Y, Li X, Hao L, Jiang D, Wu B, He T et al (2020) The diagnostic value of DNA repair gene in breast cancer metastasis. Sci Rep 10(1):19626. https://doi.org/10.1038/s41598-020-76577-2
Zhang W, Guo N, Yu C, Wang H, Zhang Y, Xia H et al (2012) Differential expression of ERCC-1 in the primary tumors and metastatic lymph nodes of patients with non-small cell lung cancer adenocarcinoma. Tumor Biol 33(6):2209–2216. https://doi.org/10.1007/s13277-012-0482-4
Zhang Y, Zhang D, Wang H (2018) Research on correlations of ERCC-1 with proliferation and apoptosis of ovarian cancer cells. JBUON 23:1753–1795
Lee S-A, Lee K-M, Park W-Y, Kim B, Nam J, Yoo K-Y et al (2005) Obesity and genetic polymorphism of ERCC2 and ERCC4 as modifiers of risk of breast cancer. Exp Mol Med 37(2):86–90. https://doi.org/10.1038/emm.2005.12
Qian T, Zhang B, Qian C, He Y, Li Y (2017) Association between common polymorphisms in ERCC gene and glioma risk: a meta-analysis of 15 studies. Medicine 96(20):e6832. https://doi.org/10.1097/md.0000000000006832
Li J, Liu S, Wang W, Zhang K, Liu Z, Zhang C et al (2014) ERCC polymorphisms and prognosis of patients with osteosarcoma. Tumour Biol 35(10):10129–10136. https://doi.org/10.1007/s13277-014-2322-1
Burgess JT, Rose M, Boucher D, Plowman J, Molloy C, Fisher M et al (2020) The Therapeutic potential of DNA damage repair pathways and genomic stability in lung cancer. Front Oncol 10:1256. https://doi.org/10.3389/fonc.2020.01256
Li QQ, Lee RX, Liang H, Wang G, Li JM, Zhong Y et al (2013) β-Elemene enhances susceptibility to cisplatin in resistant ovarian carcinoma cells via downregulation of ERCC-1 and XIAP and inactivation of JNK. Int J Oncol 43(3):721–728. https://doi.org/10.3892/ijo.2013.1996
Piljić Burazer M, Mladinov S, Matana A, Kuret S, Bezić J, Glavina DM (2019) Low ERCC1 expression is a good predictive marker in lung adenocarcinoma patients receiving chemotherapy based on platinum in all TNM stages—a single-center study. Diagn Pathol 14(1):105. https://doi.org/10.1186/s13000-019-0885-2
Wei X, Yang J (2015) Relationship between ERCC1, Ki67, PCNA expression with anthracycline chemo-therapeutic drugs′ sensitivity in breast cancer tissues. Chin J Immunol 12:169–172
Baiomy MAE, El Kashef WF (2017) ERCC1 expression in metastatic triple negative breast cancer patients treated with platinum-based chemotherapy. Asian Pacific J Cancer Prev 18(2):507–513. https://doi.org/10.22034/apjcp.2017.18.2.507
Acknowledgments
The authors thank Badan Penerbit dan Publikasi Universitas Gadjah Mada for their assistance in writing.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
AH was responsible for the design, data curation, formal analysis, original draft writing, review, and editing. HP was responsible for data curation and project administration.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Heatmap of ERCC1, ERCC2, ERCC3, ERCC4, ERCC5, ERCC6, and ERCC8 DNA methylation expression levels in breast cancer cells as determined by the MethSurv database.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hermawan, A., Putri, H. Characterizing excision repair cross-complementing family genes as drug resistance biomarkers in breast cancer. Beni-Suef Univ J Basic Appl Sci 12, 79 (2023). https://doi.org/10.1186/s43088-023-00415-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43088-023-00415-3