Abstract
Dahab is a tourist city located in the Sinai Peninsula, downstream of the Dahab watershed, as a part of the arid coastal region. Groundwater samples have been collected from the Dahab delta and were tested for salinity, major ions, minor elements, and heavy metals to investigate the geochemical processes deteriorating the groundwater quality. The spatial distribution of major, minor, and trace elements integrated with the geochemical interrelationships using the cumulative salinity bases mixing curves have been utilized to investigate the main source(s) of groundwater recharge and salinization origin in Dahab delta aquifer. The groundwater salinity ranges from 339 upstream of Dahab watershed to 53,216 mg/L downstream in the delta area. The groundwater varies from fresh, brackish, and saline water. The spatial distributions of major ions, minor elements (Si, and Br), and heavy metals (B and Sr) confirm that the recharge comes from the upstream watershed. According to the mixing model curves, groundwater quality has declined due to interactions with the aquifer matrix, mixing with seawater, and rejected brine from the reverse osmosis desalination plants. The fresh/brackish groundwater classes have been recorded in the northwestern part of the study area close to the basement rocks, where the Quaternary aquifer receives considerable recharge through the underneath fractures, joints, and faults that enhance the subsurface recharge. The samples with a high saline groundwater class have been recorded in the eastern and southern parts of the delta, demonstrating the effects of seawater intrusion. Based on WHO guideline criteria, the assessment of groundwater for various uses has determined that most groundwater samples from the alluvial aquifer (91%) are unfit for human consumption. The Water Quality Index indicates that the groundwater in the southern part of the delta is not suitable for all uses due to mixing with the seawater, and injection of rejected brine water from the desalination plants. In the north, groundwater is unfit for drinking and aquatics, excellent for recreation, marginal for irrigation, and fair for livestock. The groundwater in coastal arid region aquifers has deteriorated due to seawater intrusion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.1 Background
In most arid region countries, groundwater is the primary source of water [47]. Over the past few decades, groundwater quality in coastal aquifers in arid regions worldwide, including Egypt, North Africa, and Oman has declined due to the expansion of the urban, agricultural, mining, and industrial sectors [53, 62, 80]. In coastal aquifers, excessive withdrawals frequently account for groundwater salinization [36]. Geogenic pollution, including water–rock interaction and seawater intrusion, deteriorates the quality of groundwater resources [52]. In addition, human activities, including residential and agricultural activities, frequently result in a decline in the quality and quantity of groundwater [32, 54]. Water quality monitoring is crucial to ensure the long-term sustainability of freshwater resources [18, 46]. Understanding water quality is essential for the efficient and sustainable management of groundwater resources [14, 62]. Numerous academics have assessed the suitability of water resources in arid regions for various uses, including domestic, agricultural, and industrial operations, using various techniques and methods [28]. Drinking water has attracted more scientific interest than other applications [3, 4, 18, 46]. Identifying processes controlling the water's physical and chemical components, such as water–rock interaction and mixing with other water, is part of groundwater geochemistry [8]. The World Health Organization (WHO) provides information on various boreholes, well selection, implementation and specifies drinking water quality guidelines [59]. However, people in developing countries frequently ignore these regulations, which causes pathogenic, microbiological, and chemical contamination of wells and boreholes [43, 70]. To alleviate water scarcity issues and meet agricultural sector demands, groundwater quality evaluations for agrarian irrigation have been carried out in Egypt [49, 69]. By the end of 2022, Egypt's population will increase by over 100 million, creating considerable difficulties in supplying safe drinking water to rural and small communities. Rural communities are hence practically obliged to drink from existing groundwater sources [2, 5, 32].
The studied region is the delta Dahab watershed, located in the South Sinai governorate, where more than 178,000 people rely primarily on groundwater for agriculture and drinking [76]. The South Sinai Peninsula's Delta Wadi Dahab comprises groundwater, seawater desalination, and local seasonal rainfall as primary water sources. The Quaternary aquifer is the sole aquifer in the Delta Dahab, where limited precipitation results in limited yearly groundwater replenishment and, consequently, groundwater salinization [59]. Additionally, because the upwelling of seawater cannot support the excessive groundwater withdrawals, the groundwater quality of the Quaternary aquifer along the Gulf of Aqaba has worsened [42].
The groundwater quality downstream of the Dahab aquifer has deteriorated due to seawater intrusion and brine water injection deeper into the aquifer [21, 68]. The upwelling of seawater in south Sinai considerably impacted the Quaternary aquifer's salinity because there were no natural groundwater replenishments in such an arid region. A thin layer of brackish water suspended over deep, salty groundwater vulnerable to the stresses of groundwater pumping compensates the groundwater [23]. Groundwater quality has also decreased due to the aquifer's deeper penetration of rejected brine water caused by the widespread use of reverse osmosis desalination. Therefore, assessing how geochemical processes control groundwater salinization is essential for sustainably managing groundwater resources [13, 62, 65]. Contaminated water can impact everyone, which eventually poses a health risk.
Therefore, the main objectives of this research are to (1) identify the primary sources contributing to the groundwater quality decline based on the spatial distribution of major, minor, and trace constituents (2) Investigate the effects of seawater mixing and the rejection of brine deeper into the Quaternary aquifer; and (3) Evaluate the groundwater quality using the Water Quality Index (WQI) model to assess changes and consider the groundwater's chemistry.
2 The study area
2.1 Location
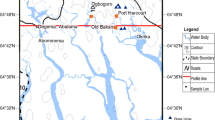
Delta Wadi Dahab is located on the west shore of the Gulf of Aqaba between longitudes 34° 28′ and 34° 32′ E. and latitudes 28° 28 ′ and 29° 32′ N. (Fig. 1). It has an area of 10.9 km2. The average rainfall ranges from 10.3 to 18.9 mm/year, with the majority falling in a handful of days, particularly in the spring and autumn. Temperatures in the summer vary from 31 to 37 °C, while temperatures in the winter range from 2 to 13 °C [20]. The average evaporation rate is between 3976 and 6252 mm/year [1]. The aridity index is 1.3 based on the Emberger equation (1955) [26]. Groundwater is the sole drinking, domestic, and agricultural source in the study area since it can be easily extracted from the Quaternary aquifer. The relative humidity is highest in the winter (45–56%) and lowest in the summer (25–32%). Current coastal overdevelopment, notably in the Delta Wadi Dahab basin on the Gulf of Aqaba, needs increased demand for urban water supply. The tapped water potential is frequently insufficient to meet the excessive growth in water demands required for municipal, agricultural, industrial, and tourism-related activities.
2.2 Geological and hydrogeological setting
The Dahab basin is part of the old Archean Triangle of the Arbo-Nubian Shield. The study area's sedimentary, metamorphic, and igneous rock ages range from the Cambrian to the Quaternary [25, 38, 39, 83]. Most igneous and metamorphic rocks are upstream and primarily composed of fractured granitic rocks that have been intruded by basic and intermediate dykes.
Sedimentary rocks dominate the studied areas in the upstream northern portions. Most of the rocks in the upstream part of the Dahab basin are from the Cambrian, Lower Cretaceous, and Upper Cretaceous periods [27, 74]. Quartz and kaolinite minerals are extremely comparable between the Lower Cretaceous clastic layer and Cambrian rocks [45]. Wadi fills, alluvial deposits, and terraces are common in the downstream delta. Rock fragments the size of cobbles and boulders, gravel, sand, silt, and clays make up most of the wadi fill deposits [41, 66]. Alluvial deposits were considered significant aquifers due to their excellent hydraulic qualities [27]. The stream channel floors are covered with various alluvial deposits of varying thicknesses and textures (Fig. 2) from one meter upstream to more than 50 m in the Dahab downstream delta of the basin [9].
The hydrogeological setting of the Dahab Basin was affected by many factors, including geological, structural, and climatic conditions. The wadi fill deposits distributed in the downstream or upstream parts of Dahab basin contain groundwater [29, 56, 71,72,73]. Surface drainage allowed a significant volume of precipitation to be discharged into the Gulf of Aqaba. The recent rainfall is the main source of groundwater recharge in the basement rocks. Recent rainfall percolation through alluvial stream deposits replenishes the groundwater [19].
3 Methods
In July 2019, thirty-nine groundwater samples were taken from the alluvial aquifer. Additionally, one sample of rainfall, one sample of seawater, four representatives were collected from desalination planets (brine water), and ten samples represented the water–rock extract (Tables 1 and 2). The samples were collected in two bottles: one for major ions determination and the other for heavy metals measurements. The first was preserved in the refrigerator, and the second was preserved by added conc. Nitric acid. The groundwater samples represent the delta aquifer, extending from inland close to the basement mountain to the coast. The exposed rock units and subsurface geological cross sections were used to determine the tapping aquifers and water-carrying formations. The analyses were carried out using the procedures that had been established [31, 63], American Society for Testing and Materials [6] at the Centre laboratories, Desert Research Centre (DRC) in July 2019.
3.1 Determination of major ions
The analysis includes the pH, electrical conductivity (EC), total dissolved solids (TDS) measured during the field trip. Orion 150A + EC meter of Thermo Electron Corporation, USA's was used to measure the electrical conductivity. At 25 °C, the EC was expressed in mohs/cm, and the salinity can be calculated from EC (μmohs/cm) from this equation:
(ke is a constant of proportionality).
Ca2+ and Mg2+ were determined by titration against disodium Ethylenediaminetetraacetic acid (Na2EDTA) using a murexide indicator. At the same time, Mg2+ was estimated by subtracting the calcium value from the total hardness (Ca2+ + Mg2+) using E.B.T (Eriochrome Black T) indicator [10, 40]. Na+ was determined by standard curves using Flame Photometer, PF P7, Jenway, UK. The detailed chemical analysis of such groundwater samples is shown in Table 1.
3.2 Determination of heavy metals
The Inductively Coupled Argon Plasma, ICAP 6500 Duo, from Thermo Scientific, England, was used to measure the dissolved heavy metals (Al, Ba, Cd, Co, Cr, Cu, Fe, Mn, Mo, Ni, Pb, and Zn) in the water samples that were collected. A stock solution for instrument standardization, 1000 mg/L multi-element certified standard solution, Merck, Germany, was used.
3.3 Evaluation of groundwater
Generally, water used for drinking purposes should be colorless, free of turbidity, excessive amounts of dissolved salts, harmful micro-organisms, and unpleasant odor or taste. To evaluate groundwater for human drinking, the groundwater salinity and concentration of major ions and heavy metals have been considered on recommended standards [81]. Water used for household purposes on farms, including that eaten by animals and poultry, is subject to quality restrictions and international standards set by the National Academies of Science (NAS) and Engineering (NAE) [55]. High salinity and toxicity components are two of the most common water quality issues in irrigation. When salts accumulate in soils, the salinization deteriorates its quality. Water contains some elements that can slow down or stop plant growth, including but not limited to salinity, chlorine, and sodium. For a better understanding of whether water is suitable for agricultural use, key factors such as the electrical conductivity (Ec), Na% [82], sodium adsorption ratio (SAR) [77], permeability index (PI) [16], potential salinity (SP), Kelly ratio (KR) [44], and magnesium ratio Paliwal [61] have been considered [34] to understand better whether water is suitable for agricultural use. The following equations have been used for groundwater evaluaton.
The concentrations of anions and cations in Eqs. 3–7 are represented in meq/L.
3.4 Water Quality Index
The code for the Water Quality Index (WQI) version 1.0 [15] has been used to rank categories for different water uses, including human drinking, irrigation, poultry, and recreation. The program contains over 50 predefined parameters to check groundwater availability for drinking and other uses. The criteria for evaluating water quality are based on the Canadian Environmental Guidelines considering the pH, major constituents, dissolved minor and trace elements, and physical parameters, including temperature, turbidity, and color. The model output is a statistical summary of the data used and the rank of the overall water quality.
4 Results
4.1 Groundwater geochemistry
The groundwater is considered the main source of water supply in the middle east as a part of arid and semi-arid regions where the rainfall is scarce [35]. The physical parameters characterizing the groundwater in Wadi Dahab delta show great variations. The pH in the upstream groundwater ranges from 7.1 to 7.8 with a mean value of 7.4, while the downstream groundwater in the delta ranges from 7.0 to 8.6 with a mean value of 7.8, indicating alkaline media. The electrical conductivity of upstream groundwater ranges from 641 to 5790 µmhos/cm with a mean value of 1764 µmhos/cm, while the downstream delta groundwater ranges from 1793 to 78,100 µmhos/cm, with a mean value of 17,287 µmhos/cm, indicating elevated groundwater salinization due to mixing with the seawater. Salinity is the term used to describe the dissolved concentrations of major ions in water. The concentration of the groundwater constituents varies according to how mineral-rich the aquifer matrix through which the groundwater flows. The average salinity of the groundwater located upstream ranges from 339 to 3842 mg/L while the groundwater in the delta alluvial aquifer downstream ranges from 895 to 53,216 mg/L (Table 1). The Na+ ion predominates, explaining the significance of saltwater through and the seawater intrusion [60]. The dissolved Na+ ion concentration in the upstream watershed ranges between 52 and 660 mg/L with a mean average of 2545 mg/L, while in the delta groundwater it ranges between 250 and 17,600 mg/L with a mean value of 3156 mg/L. HCO3− and Ca2+ predominance reveals the impact of rock water interaction with limestone boulders embedded in the aquifer matrix [37]. Calcium concentration ranges between 66.5 mg/L and 874 mg/L with a median value of 420 mg/L. The chloride ion concentration in the upstream watershed ranges between 58 and 833 mg/L with a mean value of 217 mg/L, while downstream in the delta ranges between 333 and 25,906 mg/L, with a mean value of 5062 mg/L (Table 1). Based on the values of groundwater salinity, water can be classified as fresh, brackish, and saline water classes [11]. The bulk of groundwater samples located in the delta area are classified as saline (68%) and brackish (22%) water classes, with a minority classified as freshwater (10%).
4.2 Dissolved minor elements in groundwater
The concentration of minor elements (Si, Sr, B, and Br) dissolved in groundwater is displayed in Table 1. The silica concentration in the upstream groundwater samples ranges between 7.69 and 15.59 mg/L, with a mean value of 10.1 mg/L. In the downstream delta, silica ranges between 7.77 and 34.82 mg/L, with a mean value of 17.3 mg/L. The strontium concentrations in the upstream watershed range from 0.58 to 9.57 mg/L with a mean value of 2.2 mg/L. In the downstream delta, the Sr ranges between 0.8 and 16.8 mg/L with a mean value of 7.5 mg/L. The boron concentration in the upstream ranges between 0.16 and 1.27 mg/L with a mean value of 0.6 mg/L, in the downstream delta, it ranges from 1.1 to 9.1 mg/L with a mean value of 2.8 mg/L. The bromide in the delta ranges between 8.8 and 144 mg/L with a mean value of 46.8 mg/L. The rejected brine water samples possess very high concentrations of minor elements, especially bromide ions, while the water extract samples have lower concentrations of minor elements (Table 1).
4.3 Dissolved trace and heavy elements
The main source of dissolved elements in groundwater in rural desert areas mainly comes from the geogenic source due to leaching, dissolution processes, and interaction with the aquifer matrix [51]. The concentration of heavy and trace elements records high variation in the groundwater samples. The dissolved aluminum, iron, manganese, and zinc concentration in groundwater record higher variations, while the Cobalt, chromium, and copper show lower variations (Fig. 3). The aluminum concentration in groundwater ranges between 0.02 and 0.75 mg/L, with a median value of 0.1 mg/L and an average value of 0.16 mg/L. The concentration of dissolved iron ranges between 0.034 and 4.6 mg/L, with a median value of 0.24 mg/L and an average value of 0.51 mg/L. The manganese concentration ranges between 0.003 and 0.09 mg/L, with a median value of 0.015 mg/L and an average value of 0.0 mg/L. The concentration of zinc ranges between 0.001 and 1.19 mg/L, with a median value of 0.026 and an average value of 0.089 mg/L.
5 Discussion
5.1 Origin of groundwater salinization
The fresh groundwater class has been recorded in the northern part of the study area close to the basement rocks, where the Quaternary aquifer receives considerable recharge through the underneath fractures, joints, and faults that enable surface and groundwater percolation and enhance the subsurface recharge [59]. The samples with a high saline groundwater class have been recorded in the eastern and southern parts of the delta, demonstrating the effects of seawater incursion. The seawater intrusion is mainly due to withdrawals and the impact of injecting hypersaline water from desalination plants [24]. Figure 4 shows the spatial distribution of groundwater salinity, major cations and anions using kriging interpolation method. In Fig. 4a, the groundwater salinity increases from northwest to southeast, which coincides with the groundwater flow path due to leaching and dissolution with the flow direction that reported by Shabana [68]. Groundwater salinity is made up of dissolved major ions. In all collected groundwater samples, the Ca2+, Mg2+, Na+, HCO3−, SO42−, and Cl− ions typically make up most of the dissolved solid's load. The primary components of carbonate rocks (limestone and dolomite) in the aquifer matrix are calcium and magnesium, readily dissolved in water as alkaline earth metals. They are mostly accused of water hardness and are typically present in natural water in dissociated form as bivalent ions. In the delta Dahab area, the calcium concentration ranges from 67 to 874 mg/L with a mean value of 420 mg/L, while magnesium concentration ranges from 5 to 1453 mg/L with a mean value of 264 mg/L (Fig. 4b, c). The illustration depicts the interaction of water and rock in an aquifer with a carbonate-rich substrate and seawater mixing [7, 57, 79].
The alkali-metal group of the periodic table is dominated by sodium. The primary source of sodium in the fresh groundwater class is the leaching of sedimentary rocks like clay minerals. Due to the great solubility of sodium salts and their weakly-bonding nature to clay minerals and other adsorbents, the sea becomes enriched, and deposits eventually evaporate [50]. Natural freshwater typically has a sodium concentration of less than 200 mg/L, whereas seawater and brines have sodium concentrations of roughly 10,000 mg/L and 25,000 mg/L, respectively [40]. The sodium levels in the investigated aquifer range from 250 to 17,600 mg/L, with a mean value of 3156 mg/L. The northwestern region, near the mountain’s granitic rock, has a lower sodium concentration, indicating a geogenic source of sodium (Fig. 4d). Due to weathering, mineral dissolution, and atmospheric CO2 gas dissolution, bicarbonates and carbonates are typically present in natural groundwater [63]. Groundwater's bicarbonate concentrations range from 49 to 201 mg/L, with a typical value of 100 mg/L (Fig. 4e). The main contributors to sulfate in natural water are gypsum and anhydrite, which are contained in the aquifer matrix of the Quaternary aquifer [40]. The decomposition of organic materials in the soil and leachable sulfates in fertilizers lead to further sulfate addition to groundwater [58].
The sulfate content in the alluvial aquifer ranges from 179 to 7266 mg/L, with a mean value of 1689 mg/L (Fig. 4f). The aquifer's comparatively low sulfate contents (less than 2000 mg/L) result from water–rock interaction mechanisms that cause leaching and dissolution. However, mixing with saltwater is primarily responsible for the greater sulfate amounts observed. Chloride in the groundwater is mostly produced by the dissolution of evaporite and halite found in the delta deposits [22]. Chloride levels in groundwater samples taken from the alluvial aquifer range from 333 to 25,906 mg/L, with a mean value of 5062 mg/L (Fig. 4g). The concentrations of major cations (Ca2+, Mg2+, Na+) and major anions (HCO3−, SO42−, and Cl−) decrease at the northeast while increasing at the southeast of the delta area (Fig. 4b–g). The lower ions concentrations recorded at the northwestern side indicate subsurface recharge from the fractured granitic rocks located upstream of Dahab watershed.
The Durov diagram [17] (Fig. 5) was employed to understand the groundwater system's hydrochemical process. The groundwater samples of various hydrochemical types illustrated most of the groundwater samples located in box 8, showing that the samples are (Cl-Mg) related to reverse ion exchange. Still, the rest of the sample located in box 9 shows that the groundwater samples (Cl-Na) indicate mixing of fresh and saline waters, possibly influencing reverse ion exchange or halite dissolution. Otherwise, some samples are forced to boxes 4 and 5, meaning that SO4 and Ca water types indicate a gypsum-bearing sedimentary aquifer. This confirms the interaction of water with the rock. In Fig. 6, the Sulin diagram [75] shows that most of the upstream groundwater (31, 32, 34–47, and 44) have a Na2SO4 water indicating meteoric water origin, while most of groundwater samples in the delta area have a CaCl2 water type indicating old marine water due to the impact of the upwelling of deep saline water because of over pumping. The groundwater samples Nos. 1, 4, 19, 25, 27, 29, 40, and 42 have a MgCl2 water type as well as most of the reverse osmosis brine water samples indicating recent marine water.
Durov plot for groundwater in alluvial aquifer indicating the hydrochemical processes involved [48]
5.2 Implication of seawater intrusion using minor elements
The result in Fig. 7 reveals that the higher silica concentration appears in the main channel region due to the weathering of granitic basement rocks. However, the lower concentrations recorded are close to seawater due to seawater mixing as silica records lower concentration in the sea. In groundwater, the higher concentration of Sr, B, and Br in the South-East of the study area are associated with the rejected brine water, and seawater intrusion as these elements are used as a fingerprint of marine deposits. The reject brine water samples possess very high concentrations of minor elements, especially bromide ions, while the water extract samples have lower concentrations of minor elements (Tables 1, 2).
Figure 8 shows the relationship between the groundwater salinity and the Si, Sr, B, and Br (in mg/L). To illustrate the mixing breakthrough curves, the equation derived by Faure [30] has been used to demonstrate the extent of the average concentration of these minor elements in the rejecting brine water, water–rock extract, and seawater samples. In Fig. 8a–d, the groundwater samples plotted on the lower left sides close to the extract samples and samples collected from the upstream watershed indicate meteoric recharge comes from upstream and the water–rock interaction is the dominating process affecting the groundwater quality. However, groundwater samples plotted on the upper right displayed mixed with the rejected brine and seawater.
5.3 Evaluation of groundwater quality for human drinking
To evaluate the groundwater suitability for human drinking, the World Health Organization [81] guideline has been used which indicates the permissible limits for salinity, major, minor and trace elements. The groundwater in the study area has high salinity, only six samples are suitable for drinking, where the recorded salinity is less than 1000 mg/L. One sample is located downstream in the delta (No. 20) and eight samples are in the upstream watershed (31–37, inclusive and 44). The dissolved major ions including Ca, Mg and Na record higher concentrations, where most of the groundwater samples are exceeding the permissible limits recommended for drinking (Table 3). Because heavy metals cannot biodegrade, they tend to accumulate in living organisms. In significant doses, heavy metals are known to be toxic or carcinogenic. The heavy metals Al, Cd, Co, Cr, Cu, Fe, Mn, Mo, Ni, Pb, and Zn are particularly important in groundwater. The dissolved amounts of chromium, copper, and zinc in groundwater samples drawn from the alluvial aquifer in this research are lower than the World Health Organization’s allowed levels. In the alluvial aquifer, 10% of aluminum, 38% of cadmium, 23% of iron, 8% of manganese, 36% of nickel, and 62% of lead concentrations exceed the acceptable limit, according to the WHO [81].
5.4 Evaluation of groundwater quality for livestock and domestic
The chemical analysis data reported in (Table 1) are compared to the standards' limits (Table 4 and Fig. 9a) to determine the acceptability of groundwater for livestock and poultry. It is obvious that 3% of total groundwater samples in the alluvial have an excellent class (No. 20), 19% are regarded as very satisfactory (Nos. 15, 19, 22, 26, 28, and 30), 55% are satisfactory (Nos. 6, 7, 8, 9, 14, 16, 17, 18, 24, 25, 26, 28, 29, 38, 39, 40 and 41), and 23% are deemed water with a risk class for livestock and poultry (1, 3, 4, 11, 12 and 42) in the alluvial groundwater tests.
Evaluation of groundwater in delta Dahab watershed for different purposes a Suitability of groundwater for livestock and poultry. b Suitability of groundwater for domestic and laundry uses based on hardness values. c Evaluation of groundwater based on salinity for irrigation. d Evaluation of groundwater based on sodium percent (Na%) for irrigation
Water for laundry and domestic applications should be soft or hard. In the alluvial aquifer, total groundwater hardness ranges from 20.8 to 7602.8 mg/L, with an average value of 1745.7 mg/L (Table 5). According to the permitted limit of hardness indicated by Sawyer and Mc Carty [67], 87% of the collected groundwater samples have a hardness level above 300 mg/L. So, they are unfit for residential and laundry usage (Fig. 9b). No. 20 is the only groundwater sample collected from the study area evaluated as hard water; while the other groundwater samples consist of very hard water and not suitable for domestic and laundry uses.
5.5 Evaluation of groundwater for irrigation
5.5.1 Sodium percentage (Na%)
The salt content in irrigation water is generally expressed as a percentage (%). According to [82], the Na% is a standard criterion used to determine the appropriateness of natural waters for irrigation. Sodium percent in groundwater samples determines its suitability to irrigation purposes (Table 6). Based on the Na% of the collected groundwater sample it is clear that 84% of groundwater samples in the alluvial aquifer are outside the diagram because their electrical conductivity (EC) exceeds 5000 μmohs/cm and are unsuitable for irrigation (Figs. 9c, d and 10). While 3% fall into permissible to doubtful (No.20), 3% go into the doubtful to unsuitable category (No. 15), and 10% fall into unsuitable category (22, 27 and 28).
5.5.2 Sodium adsorption ratio (SAR)
The SAR is a measurement of the suitability of water for use in agricultural irrigation purposes. High sodium ions concentration decreases the permeability of the soil and soil structure [77]. When the SAR value is high, salt in irrigation water may replace Ca2+ and Mg2+ ions in the soil, potentially inflicting significant harm. To evaluate waters for irrigation, a monogram is frequently utilized. The specific conductivity (mhos/cm), a function of the groundwater salinity against SAR, is plotted in this monogram. The water is divided into sixteen different quality classes (C1-S1, C1-S2, C1-S3, etc.) based on four salinity classes (C1 to C4) and four SAR classes (S1 to S4) (Table 7, Fig. 11).
84 percent of the groundwater samples from the alluvial aquifer are not represented on the graph because their Electrical conductivity (EC) values, calculated using US standards, are greater than 5000 mhos/cm. The classification of groundwater includes a salinity hazard graph. The groundwater samples plotted outside of the diagram are deemed unfit for irrigation, and 9% of samples in the alluvial aquifer are categorized as intermediate water class (C4-S2), 7% as moderate water class (C3-S2) and (C4-S1) (Fig. 12).
5.5.3 Permeability index (PI)
Since its creation in 1964 [16], the permeability index (PI) has been primarily used to assess a water's suitability for irrigation. Three categories for PI were established,Class I and II waters are regarded as appropriate for irrigation; however, class III waters are not.
The classification system based on the PI has been used to determine the suitability of groundwater for irrigation. The alluvial aquifer's documented PI values range from 51.76 to 101.40, with an average of roughly 73.4. According to the values for the permeability index, 69% of groundwater samples from the alluvial aquifer are out of curve because their Electrical conductivity (EC) is greater than 5000 mhos/cm, 3% are classified as class 2, and 13% are classified as class 1 (Fig. 12).
5.5.4 Potential salinity (PS)
The increase of potential salinity (PS) is a function of the dissolved chloride and sulfate (meq/L) in irrigation water. The higher SP value increases the osmosis soil pressure, greatly affects the plant roots soil water uptake, and damages the root system [12, 16, 64, 78]. The estimated SP values for the groundwater tapping the Quaternary aquifer range between 11.2 and 805 meq/L, with a mean value of 148.9 meq/L and a median of 56.6 meq/L. Based on 42 classifications, all the Quaternary groundwater samples consist of poor water class (Table 8).
5.5.5 The Kelly ratio (KR)
The Kelly’s ratio [44] indicates the effect of an overabundance of dissolved sodium ions in irrigation water. With a mean value of 2.2 meq/L and a median of 1.6 meq/L, the predicted KR ratio ranges from 0.8 to 8.9 meq/L. 9.6% of the samples are appropriate for irrigation, according to the estimated KR values for the Quaternary groundwater, whereas the remaining samples (90.4) are deemed unsuitable (Table 9).
5.5.6 Magnesium ratio
Since calcium and magnesium are often in equilibrium in water, the magnesium hazard index (MH) was created by Paliwal [61]. The crop production suffers due to the high magnesium hazard value (> 50%) when the soil becomes more alkaline. Magnesium hazard values for the alluvial aquifer range from 5.57 to 82.19%. 16% of groundwater samples in the alluvial aquifer are appropriate for irrigation, according to the computed MH values in Table 1, whereas 84% are unsuitable and could reduce agricultural productivity.
5.6 Water Quality Index for all uses using Water Quality Index (WQI)
Water Quality Index integrated with statistics have been used to evaluate groundwater aquifers in arid regions for drinking and irrigation [7, 33, 34]. For various purposes, the groundwater quality in Delta Wadi Dahab has been rated using the Water Quality Index (WQI) program version 1.0. Three groups of groundwater in the study area have been identified (Figs. 13, 14). Group I for coastal saltwater is situated near desalination plants and is mostly impacted by the infusion of reject water. Group II describes the brackish groundwater samples located at the main channel of the flooding path and the upstream groundwater samples. Group III defined groundwater samples near granitic rocks at the uphill mountainous terrain in the northern regions of the study area. The model's output demonstrates that the groundwater in Group I is marginal and unfit for cattle irrigation, human drinking, aquatic activities, or enjoyment. Although good for recreation, Group II groundwater samples are marginal for irrigation and cattle. They are not acceptable for drinking or aquatic use. Group III groundwater is great for recreation, mediocre for irrigation, inappropriate for drinking and aquatic use, and acceptable for cattle. Group III has relatively acceptable groundwater, and these groundwater samples receive significant recharge from the basement-fractured mountainous igneous racks.
6 Conclusion
Groundwater is the main source of human potable uses and agriculture in the Delta Wadi Dahab, southeast Sinai. The results show that the groundwater salinity exceeds the recommended international standard limit for human drinking. The major and minor elements (Si, B, Br, and Sr) give good insights into delineating the recharge sources and investigate the geochemical processes affecting groundwater quality in coastal arid groundwater aquifers. The main recharge for the delta alluvial aquifers comes mainly from the northeastern side of the fractured basement granitic rocks in the upstream watershed. The water–rock interaction, seawater mixing, and implications of brine reject water decline the groundwater quality in the delta. According to the World Health Organization, standard limits, most groundwater samples in the alluvial aquifer (91%) are unsuitable for drinking. 10% of aluminum, 38% of Cadmium, 23% of iron, 8% of manganese, 36% of nickel, and 62% of lead concentrations in the alluvial aquifer exceed the permissible limit. Chromium, copper, and zinc concentrations in all groundwater samples tapped in the alluvial aquifer are below the allowable limit. To evaluate the suitability of groundwater for livestock and poultry, 15% of the alluvial aquifer groundwater samples have an excellent class, 18% are considered very satisfactory, 49% are satisfactory, and 18% are considered as water having a risk class for livestock and poultry. Based on SAR, Na %, MH, and PI, 82% of total samples are unsuitable for irrigation. The reverse osmosis brackish groundwater desalination is crucial for providing sustainable freshwater and cost-effective for human drinking and other purposes.
Availability of data and materials
The authors confirm that; all data submitted in this manuscript are intended to be public. The data and materials are open to the research community interested in developing best practices in managing materials data.
References
Abd El Samie S, Sadek M (2001) Groundwater recharge and flow in the lower cretaceous nubian sandstone aquifer in the Sinai Peninsula, using isotopic techniques and hydrochemistry. Hydrogeol J 9(4):378–389
ACSAD (1990) Water resources in Arab countries. Regional office for Science and Technology in the Arab states, UNESCO Report, p 168
Adimalla N (2019) Groundwater quality for drinking and irrigation purposes and potential health risks assessment: a case study from semi-arid region of South India. Exposure Health 11(2):109–123
Adimalla N (2020) Controlling factors and mechanism of groundwater quality variation in semiarid region of South India: an approach of Water Quality Index (WQI) and health risk assessment (HRA). Environ Geochem Health 42(6):1725–1752
Allam A, Helal E, Mansour M (2019) Retarding contaminant migration through porous media using inclined barrier walls. J Hydrol Hydromech 67(4):339–348
ASTM (American Society for Testing and Materials) (2012) Annual book of ASTM standards, Baltimore, vol 11.01, p 939
Athamena A, Gaagai A, Aouissi HA, Burlakovs J, Bencedira S, Zekker I, Krauklis AE (2023) Chemometrics of the environment: hydrochemical characterization of groundwater in Lioua plain (North Africa) using time series and multivariate statistical analysis. Sustainability 15(1):20
Bartram J, Ballance R (1996) Water quality monitoring: a practical guide to the design and implementation of freshwater quality studies and monitoring programmes. CRC Press
Barseem M (2002) Geophysical study on the groundwater occurrence in delta of some wadies in the Gulf of Aqaba, Sinai. M.Sc thesis, Menoufiya University
Baird RB, Eaton AD, Clesceri LS (2012) Standard methods for the examination of water and wastewater. In: Rice EW (ed), vol 10. American Public Health Association, Washington, DC
Chebotarev II (1955) Metamorphism of natural waters in the crust of weathering—1. Geochim Cosmochim Acta 8(1–2):22–48
Couture I (2006) Principaux critères pour évaluer la qualité de l’eau en micro-irrigation. In: Colloque sur l’irrigation, L’eau, Source de Qualité et de Rendement, p 13
Dahiphale P, Kasal Y, Madane D, Darandale V (2018) Groundwater quality assessment using different techniques
Datta B, Chakrabarty D, Dhar A (2011) Identification of unknown groundwater pollution sources using classical optimization with linked simulation. J Hydro Environ Res 5(1):25–36
Davies JM (2006) Application and tests of the Canadian Water Quality Index for assessing changes in water quality in lakes and rivers of central North America. Lake Reserv Manag 22(4):308–320
Doneen LD (1964) Notes on water quality in agriculture, published as a water sciences and engineering. Department of Water Sciences and Engineering, University of California, p 4001
Durov SA (1948) Natural waters and graphic representation of their composition. In: DoklAkadNauk SSSR, vol 59, No. 3, pp 87–90
Egbueri JC (2020) Heavy metals pollution source identification and probabilistic health risk assessment of shallow groundwater in Onitsha, Nigeria. Anal Lett 53(10):1620–1638
El Kiki MF, Eweida EA, El-Refeai AA (1992) Hydrogeology of the Aqaba rift border province. In: Proceeding of the 3rd conference on geology sinai development, Ismailia, Egypt, pp 91–100
El-Wahab A, Zayed AEM, Moustafa AER, Klopatek J, Helmy M (2018) Landforms, vegetation, and soil quality in south Sinai, Egypt. Catrina Int J Environ Sci 1(2):127–138
Eissa M, Thomas JM, Hershey RL, Pohll GM, Dawoud MI, Dahab KA, Gomaa MA, Shabana AR (2013) Geochemical and isotopic evolution of groundwater in the Wadi Watir watershed, Sinai Peninsula, Egypt. Environ Earth Sci 71(4):1855–1869
Eissa M, Thomas JM, Pohll GM, Hershey RL, Dahab KA, Dawoud MI, Gomaa MA, El Shiekh A (2013) Groundwater resource sustainability in the Wadi Watir delta, gulf of Aqaba. Hydrogeol J 21(8):1833–1851
Eissa M, Thomas JM, Pohll GM, Shouakar-Stash O, Hershey RL, Dawoud MI (2016) Groundwater recharge and salinization in the arid coastal plain aquifer of the Wadi Watir delta, Sinai, Egypt. Appl Geochem 71:48–62
Eissa MA (2018) Application of multi-isotopes and geochemical modeling for delineating recharge and salinization sources in Dahab Basin aquifers (South Sinai, Egypt). Hydrology 5(3):41
El-Masry NN, Hassen SI, Hegazi MA, (2003) A newly recognized example of a late Precambrian subsurface cauldron subsidence intrusion in southern Sinai: Jabal Laiq ring dike. In: Proceedings 5th international conference on the geology of the middle east, pp 549–558
Sabri MA (2016) Evaluation of water resources and its impact on the future development in Wadi Dahab, gulf of Aqaba, Sinai, Egypt. Egypt J Geol 60:1–16
El-Shafei MK, Khawasik, SM, EI-Ghawaby MA (1992) Deformational styles in the tectonites of Wadi Sa’al, South Sinai. In: Proceedings of the 3rd conference on geology sinai development, Ismailia, Egypt, pp 1–8
Elshalakany M, Ahmed M, Abou Elmagd A (2022) Groundwater resources in Sinai’s basement terrains: optimal recharge and extraction locations. In: SEG/AAPG international meeting for applied geoscience and energy
El-Rayes AE (1992) Hydrogeological studies of Saint Katherine area, South Sinai, Egypt. Doctoral dissertation, Suez Canal University
Faure G (1986) Principles of isotope geology, 2nd edn. Wiley, New York
Fishman MJ, Friedman LC (1985) Methods for determination of inorganic substances in water and fluvial sediments. US Department of the Interior, Geological Survey
Foster S, Hirata R, Gomes D, D’Elia M, Paris M (2002) Groundwater quality protection: a guide for water utilities, municipal authorities, and environment agencies. World Bank, Washington, DC
Gaagai A, Boudoukha A, Boumezbeur A, Benaabidate L (2017) Hydrochemical characterization of surface water in the Babar watershed (Algeria) using environmetric techniques and time series analysis. Int J River Basin Manag 15(3):361–372
Gaagai A, Aouissi HA, Bencedira S, Hinge G, Athamena A, Haddam S, Ibrahim H (2023) Application of water quality indices, machine learning approaches, and GIS to identify groundwater quality for irrigation purposes: a case study of Sahara Aquifer, Doucen Plain, Algeria. Water 15(2):289
Ghodbane M, Benaabidate L, Boudoukha A, Gaagai A, Adjissi O, Chaib W, Aouissi HA (2022) Analysis of groundwater quality in the lower Soummam Valley, North-East of Algeria. J Water Land Dev 8:1–12
Gnanachandrasamy G, Dushiyanthan C, Jeyavel Rajakumar T, Zhou Y (2020) Assessment of hydrogeochemical characteristics of groundwater in the lower Vellar river basin: using geographical information system (GIS) and Water Quality Index (WQI). Environ Dev Sustain 22(2):759–789
Gopinath S, Srinivasamoorthy K, Saravanan K, Prakash R (2019) Discriminating groundwater salinization processes in coastal aquifers of southeastern India: geophysical, hydrogeochemical and numerical modeling approach. Environ Dev Sustain 21(5):2443–2458
Hassen IS, El-Shafei MK, Stüwe K (2007) Late proterozoic crustal evolution in the Arabian-Nubian shield of Wadi Zaghra tectonites, south Sinai, Egypt. Annal Geol Surv Egypt 29:77–93
Hegazi AM (2006) Tectonic evolution of the polydeformed Sa’al Belt, South Sinai, Egypt. Acta Geol Hung 49(3):271–284
Hem JD (1985) Study and interpretation of the chemical characteristics of natural water, vol 2254. Department of the Interior, US Geological Survey
Hume WF (1906) The topography and geology of the Peninsula of Sinai (south-eastern portion). National Print Department
Isawi H, El-Sayed MH, Eissa M, Shouakar-Stash O, Shawky H, Abdel Mottaleb MS (2016) Integrated geochemistry, isotopes, and geostatistical techniques to investigate groundwater sources and salinization origin in the Sharm EL-Shiekh Area, South Sinia, Egypt. Water Air Soil Pollut 227:1–23
Ismail E, El-Rawy M (2018) Assessment of groundwater quality in West Sohag, Egypt. Desal Water Treat 123:101–108
Kelly WP (1963) Use of saline irrigation water. Soil Sci 95(6):385–391
Kora M, Genedi A (1995) Lithostratigraphy and facies development of Upper Cretaceous carbonates in east central Sinai, Egypt. Facies 32(1):223–235
Li P, Wu J (2019) Drinking water quality and public health. Expos Health 11(2):73–79
Li R, Merchant JW (2013) Modeling vulnerability of groundwater to pollution under future scenarios of climate change and biofuels-related land use change: a case study in North Dakota, USA. Sci Total Environ 447:32–45
Lloyd JW, Heathcote JAA (1985) Natural inorganic hydrochemistry in relation to ground water
Mansour MM, Ellayn AF, Helal E, Rashwan IM, Sobieh MF (2018) Delaying solute transport through the soil using unequal double sheet piles with a surface floor. Ain Shams Eng J 9(4):3399–3409
Matthess G (1982) The properties of ground-water (No. 551.49 M38)
Megahed HA, Farrag AEHA (2019) Groundwater potentiality and evaluation in the Egyptian Nile Valley: case study from Assiut Governorate using hydrochemical, bacteriological approach, and GIS techniques. Bull Natl Res Centre 43(1):1–20
Moneim AAA, Zaki S, Diab M (2014) Groundwater conditions and the geoenvironmental impacts of the recent development in the south eastern part of the Western Desert of Egypt. J Water Resour Protect
Mosaad S, Eissa M, Alezabawy A (2022) Geochemical modeling and geostatistical categorization of groundwater in Nubian Sandstone Aquifer, El Bahariya Oasis, Egypt. Environ Earth Sci 81:421
Nair IS, Rajaveni SP, Schneider M, Elango L (2015) Geochemical and isotopic signatures for the identification of seawater intrusion in an alluvial aquifer. J Earth Syst Sci 124(6):1281–1291
National Academy of Science (NAS) and National Academy of Engineering, (NAE) (1973) Nutrients and toxic substances in water for livestock and poultry. US Environmental Protection Agency, Washington, DC
Oada K (1995) Hydrogeological studies on East Saint Katherine environ, South Central Sinai, Egypt
Oliva P, Dupré B, Martin F, Viers J (2004) The role of trace minerals in chemical weathering in a high-elevation granitic watershed (Estibere, France): chemical and mineralogical evidence. Geochim Cosmochim Acta 68(10):2223–2243
Omar GA (2008) Hydrochemistry of the groundwater in the area between El Dabaa and Mersa Matruh, North Western coast, Egypt. M.Sc. Thesis, Faculty of Science, Menoufia Univ., Egypt
Omran ESE (2017) Land and groundwater assessment for agricultural development in the Sinai Peninsula, Egypt. Conventional water resources and agriculture in Egypt, pp 165–209
Park SC, Yun ST, Chae GT, Yoo IS, Shin KS, Heo CH, Lee SK (2005) Regional hydrochemical study on salinization of coastal aquifers, Western coastal area of South Korea. J Hydrol 313:182–194
Paliwal KV (1972) Irrigation with saline water
Qian H, Chen J, Howard KW (2020) Assessing groundwater pollution and potential remediation processes in a multi-layer aquifer system. Environ Pollut 263:114669
Rainwater FH, Thatcher LL (1960) Methods for collection and analysis of water samples (No. 1454-1458). US Government Printing Office
Ramesh K, Elango L (2012) Groundwater quality and its suitability for domestic and agricultural use in Tondiar river basin, Tamil Nadu, India. Environ Monit Assess 184(6):3887–3899
Reda A, Eissa M, El Shamy I, Dotsika E, Saied M, Mosaad S (2022) Using geochemical and environmental isotopic tracers to evaluate groundwater recharge and mineralization processes in Qena Basin, Eastern Nile Valley, Egypt. Appl Sci 12:8391. https://doi.org/10.3390/app12178391
Said R (1962) The geology of Egypt. Elsevier, Amsterdam, p 377
Sawyer CN, Mc Carty PL (1967) Chemistry for sanitary engineers, and classification of naturally soft and naturally hard waters to sources and hardness of their water supplies. J Hyg
Shabana AR (1998) Geology of water resources in some catchment areas draining in the Gulf of Aqaba, Sinai-Egypt. Doctoral dissertation, Faculty of Science Ain Shams University
Sharaky AM, El Abd ESA, Shanab EF (2018) Groundwater assessment for agricultural irrigation in Toshka area, Western Desert, Egypt. In: Conventional water resources and agriculture in Egypt, pp 347–387
Sharaky AM, El Hasanein AS, Atta SA, Khallaf KM (2016) Nile and groundwater interaction in the western Nile Delta, Egypt. The Nile Delta, pp 33–62
Shendi EH, El-Rayes A (1992) Geophysical prospecting for groundwater in Wadi Sibaiya-Wadi El-Sheikh area, south Sinai. MERC Ain Shams Univ Earth Sci Ser 6:55–61
Shendi EH, Geriesh MH, Mousa MM (1997) Geophysical and hydrogeological studies on Wadi Saal Basin, Southern Sinai, Egypt. Egypt J Geol 41(2B):871–908
Shendi EH, Oada K (1999) Groundwater possibilities of wadi El-Nasb basin, southeastern Sinai, Egypt. Ann Geol Surv 403–418
Soliman FA (1986) Geology of Wadi Sa’al area with special emphasis of metamorphism and tectonics, Central Sinai of Egypt. Faculty of Science, Suez Canal University, p 240
Sulin VA (1946) Oil water in the system of natural groundwater, vol 30. USSR, Gostopichezdat, pp 37–45
Tam VT, Nga TTV (2018) Assessment of urbanization impact on groundwater resources in Hanoi, Vietnam. J Environ Manag 227:107–116
Todd DK, Mays LW (2004) Groundwater hydrology. Wiley
Udom EA, Ekpo AE, Oluka SI (2019) Assessment of irrigation water qualities of Abak River, Abak, Akwa Ibom State, Nigeria. Global J Pure Appl Sci 25(2):135–144
U.S. Salinity Laboratory Staff (1954) Diagnosis and improvement of saline and alkali soils. Agric Handbook 60:83–100
Vengosh A, Rosenthal E (1994) Saline groundwater in Israel: it’s bearing on the water crisis in the country. J Hydrology 156(1–4):389–430
Vetrimurugan E, Brindha K, Elango L, Ndwandwe OM (2017) Human exposure risk to heavy metals through groundwater used for drinking in an intensively irrigated river delta. Appl Water Sci 7(6):3267–3280
Who G (2017) Guidelines for drinking-water quality, vol 216. World Health Organization, pp 303–304
Wilcox L (1955) Classification and use of irrigation waters (No. 969). US Department of Agriculture
Zalata AA, El Metwally AA, El Aassy IE, El Sayed AA (1997) Evolution and geochemistry of the basement rocks of west Dahab area, Southeastern Sinai. In: Third conference on geochemistry, Alexandria University, pp 1–15
Acknowledgements
No acknowledgments are included in the article.
Funding
“The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.”
Author information
Authors and Affiliations
Contributions
AS: Developed the theoretical formalism, performed the geospatial analysis, and conceived and planned the geochemical field measurements and data interpretation for the geochemical groundwater characteristics. In addition, he wrote part of the result and discussion in the manuscript. ME: Carried out the geochemical analyses for groundwater samples and wrote part of the geochemical methodology and results of the manuscript as took the lead in writing the whole manuscript with input from all authors. SS: Carried out measurements for geochemical analyses, contributed to interpreting its results and wrote part of the methodology of the manuscript. MMS: Gives all the facilities of the water analyses laboratory for the Ph.D. student Amira Samy and provided all the chemical and field work instruments, including pH, Ec, GPS…etc. Also, he helps interpret geochemical results and water–rock interaction. RMA: She reviewed all the manuscripts before submission and was responsible for interpreting groundwater quality. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Samy, A., Eissa, M., Shahen, S. et al. Geochemistry and assessment of groundwater resource in coastal arid region aquifer (Dahab delta, South Sinai, Egypt). Beni-Suef Univ J Basic Appl Sci 12, 54 (2023). https://doi.org/10.1186/s43088-023-00393-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43088-023-00393-6