Abstract
Background
Ovarian cancer (OC) is the leading cause of death associated with gynecologic cancer. IL-10 plays an important role in tumorigenesis. We investigated IL-10 gene polymorphisms in OC patients. The current case–control study screened forty-eight women with OC and forty-eight healthy women who did not have OC. The genotyping of SNPs (− 1082 G > A; rs1800896 and − 819 C > T; rs1800871) of the IL-10 gene was done by tetra primers sequence-specific primer polymerase chain reaction (SSP-PCR) technique. The plasma levels of IL-10 were measured using an enzyme-linked immunosorbent assay (ELISA).
Results
For IL-10 (− 1082 G/A) polymorphism, the G (wild allele) was significantly associated with increasing the risk of OC (OR = 2.054 with CI = 1.154–3.657; P < 0.05), while the A (variant allele) and AA genotype was significantly associated with decreasing the risk of OC (OR = 0.487 with CI = 0.273–0.867; P < 0.05) and (OR = 0.15; 95% CI = 0.04–0.63; P < 0.05), respectively. For IL-10 (− 819C/T) polymorphisms, the T allele (variant allele) and (TT, CT genotypes) were significantly associated with increasing the risk of OC (OR = 2.800 with 95% CI = 1.577–5.037; P < 0.05), (OR = 18.33 with 95% CI = 3.46–97.20; P < 0.001), and (OR = 9.44 with 95% CI = 2.52–35.40; P < 0.001), respectively, while the C (wild allele) was significantly associated with decreasing the risk of OC (OR = 0.357 with 95% CI = 0.199–0.642; P < 0.05). The haplotype analysis for (− 1082 G > A and − 819 C > T shows the GT haplotype was significantly associated with increasing the risk of OC (OR = 50.09 with CI = 6.34–395.92; P < 0.001). OC was substantially correlated with IL-10 level (r = 0.457; p < 0.001). There is no linkage disequilibrium (LD) between IL 10 − 1082 G/A and IL 10 − 819 C/T (D′ = 0.1315, r2 = 0.016; P = NS). A statistically significant positive relationship existed between IL-10 and CA125 and ALT (P < 0.05). IL-10 and albumin showed a strong negative association (P < 0.05), whereas the correlation of IL10 plasma level with BUN, AST, T. Bil., TLC, PLT, Cr., and HB has not any significant value (P > 0.05).
Conclusions
Overall, this study supports an association of IL-10 (− 1082 G/A and − 819C/T) polymorphisms with the risk of ovarian cancer.
Similar content being viewed by others
1 Background
Ovarian cancer (OC) is the primary reason for women’s gynecologic cancer-related deaths [1,2,3]. Worldwide, around 3.4% incidence and 4.4% mortality rates of OC in females per year [4]. In more developed regions, 9.2 per 100,000 women are affected by ASR, compared to 5 per 100,000 in less developed areas [5]. The absence of early symptoms in OC resulted in an advanced stage prior to diagnosis which accounts for the high incidence and mortality rate of OC [1, 6]. The majority of ovarian neoplasms (about 80%) are epithelial ovarian cancers (EOC) [7]. Four major histological kinds of EOC have been identified: serous, mucinous, clear cell, and endometrioid carcinoma [1, 8].
The pathogenesis of OC may be significantly influenced by immune dysregulation [9]. Multifunctional cytokines are small glycoproteins included in the development of immunity and inflammation, as well as the pathogenesis of a range of malignancies as OC [10]. Multiple cytokines are released by OC cells, and their changes may have prognostic and diagnostic relevance [11]. Because inflammatory cytokines in a microenvironment promote the rapid proliferation of malignant cells, inflammation has a significant relationship to the development of cancer [12]. Numerous diseases are largely influenced by cytokines that promote and inhibit inflammation [13]. One of the fundamental causes of OC’s underlying mechanisms may be chronic inflammation [9].
Many different cell types, including CD4 + Th2, monocytes/macrophages, activated B cells, and even some human malignancies, produce the immunoregulatory cytokine interleukin 10 (IL-10) [14, 15]. Interleukin 10 (IL-10) downregulates the cytokine production by Th1 cells and macrophages [15], and it has anti-angiogenic and anti-inflammatory properties, making it both tumor-inhibiting and tumor-promoting [17, 18]. IL-10 may assist tumor cells in evading immune surveillance and accelerate tumor progression [19]. IL10 has been reported to exhibit anti-tumorigenic properties in numerous malignancies by activation of natural killer (NK) cells [20]. IL-10’s anti-angiogenesis impact, on the other hand, has the potential to prevent the growth and metastasis of malignant tumors [21, 22] and represses of IL-1b, TNF-α, IL-6, MMP-9, and VEGF, all of which are needed for angiogenesis[15, 16]. The concentrations of IL-10 are elevated in OC patients’ ascites and plasma, and they are linked to progressed illness and destitute quiet forecast in OC [24–26].
So as to improve the long permanence of OC patients, chemotherapy and surgery had already been thoroughly researched [27], but, recently, the rate of survival for 5-years does not have a considerable rise [27, 28]. So, novel pharmacologic therapies with an acceptable adverse effect profile are urgently needed, and specialized treatment is a viable method [29]. Antibody-based therapy for hematologic cancers and solid tumors has become a well-established therapeutic method over the past fifteen years. OC patients are increasingly being treated with antibody therapy, which is a promising field of research [30]. Rituximab (Rituxan) is a chimeric monoclonal anti-human CD20 antibody that is located largely on the B cells’ surface of immune system and causes B cell depletion [31, 32]. It causes cell death when it attaches to this protein [33]. The usage of this antibody reduced the average of lymphotoxins (LTs) in an OC patient population [34]. In the same way, rituximab treatment would reduce the rate of LT development in hepatopancreatobiliary (HPB) and gastrointestinal (GI) cancers [35]. Rituximab’s sensitization may be attributed in part to a change in cytokine production. Cytotoxicity experiments employing exogenous IL-10 and antibodies that neutralize it have shown that IL-10 acts as an anti-apoptotic/protective factor in tumor cells against cytotoxic agents. The FDA has confirmed Rituximab for medicament of chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphoma (NHL) [31, 36]. It is included among the essential medications by the World Health Organization (WHO) [37].
The IL-10 gene, which has five exons and four introns, which can be found on chromosome 1’s long arm at 1q31-1q32. In the promoter area, several polymorphic sites have been discovered [38]. The most frequently investigated single nucleotide polymorphisms (SNPs) in various diseases are the three SNPs in the IL-10 promoter region, rs1800896 (− 1082G/A), rs1800871 (− 819C/T), and rs1800872 (− 592A/C) [38, 39]. It was also indicated that IL-10 SNPs regulate transcriptional activity and produce gene expression changes, which affect IL-10 level, severity, and progression of the disease [15, 40]. The goal of this investigation was to look into the correlation of ovarian cancer risk with genetic polymorphisms within the promoter of the IL10 gene (− 1082 G/A, − 819 C/T) which is involved in inflammation, and we inspected the concentrations of IL-10 within the plasma of typical and cancerous ovarian women.
2 Methods
2.1 Population
Recruit 48 Egyptian women who had primary epithelial OC (histologically verified) from the Gynecology and Obstetrics’s Department, Medicine’s Faculty, Egypt, in period (2017 to 2019). Our final clinical diagnosis based on the international gynecology and obstetrics federation (www.figo.org) categorization divided the OC stages into (stage I, II, III, or IV), grade of tumor (G1: tumor with good differentiation, G2: tumor with quite modest differentiation, and G3: tumor with no differentiation), and histological kinds (mucinous, endometrioid, serous-papillary, clear cell, and others). As healthy controls, 48 females from Egypt women participated in the research, and they were cancer-free adults who were age-matched to the OC patients. The size of sample was counted utilizing open EPI software (www.openepi.com) and according to ovarian cancer incidence in Egypt.
Two groups were created from the recruited patients and healthy volunteers.
-
1.
Group 1: consists of 48 healthy controls with a mean age of 45.27 years.
-
2.
Group 2: consists of 48 patients with primary ovarian cancer, comprising a range of subtypes (endometrioid, mucinous, serous, and clear cell carcinoma), found to have a mean age of 50.60 years.
The current study’s protocol received approval as per the announcement of (1964 Declaration of Helsinki, revised in 2013) and good clinical researches guidelines, and the ethics committee authorized all of the study subjects’ verbal informed consent.
The inclusion criteria for this study included: women who are 45 to 80 years old and different subtypes of primary EOC (clear cell, mucinous, serous, endometrioid carcinoma). The exclusion criteria included women who under the age of 45 or older than 80, endometriosis, inflammatory illnesses, pregnancy, lactation, smoking, autoimmune disease, pneumonia, type I diabetes, thyroid disease, or borderline tumors.
2.2 Genotyping of IL-10-1082G/A and IL-10-819C/T
For groups 1 and 2, Genomic DNA was extracted from whole blood in EDTA K3 (tri-potassium ethylenediaminetetraacetic acid) tubes using a genomic DNA’s purification kit (Promega, Madison, USA) and stored at − 80 °C until the genotyping. The genotyping of two SNPs in the study was tetra primers SSP-PCR technique as described previously [41]. The four different primer combinations are illustrated in Table 1. In summary, the outer primers of the SNPs − 1082G/A and − 819C/T produce the confirmative amplicon for the SNP’s 5′ UTR and promoter region. By mismatching the primer’s 3′ end, the inner reverse primers were created for the mutant allele. In order to improve specificity, the third nucleotide of the 3′ end was mismatched. Two tubes were employed for the reaction, one for each allele, and each tube contains generic primer, sense primer ((G or A (− 1082G/A), C or T (− 819 C/T)), and internal control (reverse and forward primer). There was a 25-μl final volume for each process of PCR. 10 pmol of allele’s specific primer, 10 pmol of reverse primer, 3.5 pmol of each of the control primers, and 0.1 μg total amount of DNA were used. DreamTaq Green PCR Master Mix (2) (Fermentas) was used in the PCR preparations.
The PCR steps were as follows: 1 cycle at 94 °C for 2 min,
then 5 cycles at 96 °C for 25 s, 70 °C for 45 s, and 72 °C for 20 s;
then 11 cycles at 96 °C for 25 s, 65 °C for 50 s, and 72 °C for 45 s;
and in the end 15 cycles at 96 °C for 25 s, 55 °C for 60 s, and 72 °C for 2 min.
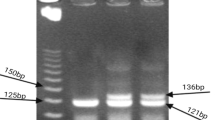
After amplification, the PCR products of the control primer produced an amplicon of 429 bp, and the amplicons of the − 1082 and − 819 primers were 258 bp (Fig. 1a) and 233 bp (Fig. 1b), respectively. The size of PCR products was assessed in relation to the migration of a 100 bp step ladder (Fermentas) using a 2% agarose gel in 0.5X TAE buffer stained with ethidium bromide.
a IL-10 (− 1082G/A) PCR product. Lanes (2 and 3) and (6 and 7) are GG genotype; lanes (4 and 5) and (8 and 9) are GA genotype, lanes (10 and 11) are AA genotype, and lane (1) represents 100 bp DNA ladder. b IL-10 (− 819 C/T) PCR product. Lanes (4 and 5) and (8 and 9) are TT genotype, lanes (6 and 7) are CC genotype, lanes (10 and 11) have CT genotype, and lanes (1 and 12) represent 100 bp DNA ladder
2.3 Measurement of IL-10’s plasma levels.
Plasma was collected from all patients and controls and was separated by centrifugation at 2000 rpm for 15 min at 4 °C, aliquoted, and stored at − 80 °C. The total concentrations of IL-10 in plasma samples were measured using a commercial ELISA kit (R&D System, Inc., Minneapolis, MN), according to the manufacturer’s instructions.
The plasma of all patients and controls was collected and separated by centrifugation at 1500 rpm for 10 min at 4 °C, aliquoted, and kept at − 80 °C. Total IL-10 concentrations in plasma samples were determined using a commercial ELISA kit (R&D System, Inc., Minneapolis, USA) and the manufacturer’s instructions.
2.4 Statistical analysis
The Statistical Package for the Social Sciences (SPSS) version 25 (SPSS, IBM, USA) was used for all statistical analyses. The independent t test was employed for the comparison of groups of healthy control and OC. Data were given as means with associated standard deviations (SDs). To compare the same group’s ELISA results and genotypes/haplotypes, a one-way ANOVA test was employed. The post hoc analysis was used by LSD when the substantial difference between groups was present. To determine whether two variables were correlated, we used the spearman’s correlation test. The genotype distribution was analyzed using a single-degree-of-freedom chi-square test and checked for Hardy–Weinberg equilibrium using DeFinetti software (http://ihg.gsf.de/cgi-bin/hw/hwa1.pl).
The haplotype analyses and LD parameters (D0 and r2) were determined using the web application SNP stats (http://bioinfo.iconcologia.net/SNPstats). The odds ratio (OR), which represents the probability of possessing a given allele if there is an incessant OC, as the p values and 95% confidence interval, was determined. P values below 0.05 were statistically critical, and all p values were 2-tailed.
3 Results
3.1 Clinical and pathological characteristics of the patients
A total of 48 females were diagnosed with OC, and 48 women found to be cancer-free were enrolled in this study. There is no substantial variation in age between the two groups (P > 0.05) which indicates that the matching based on this variable was satisfactory. OC had a statistically strong positive association with age (r = 0.238; P < 0.05).
The specific clinicopathologic traits of OC patients are shown in Fig. 2. All of the malignant lesions were caused by surface epithelial carcinomas. Advanced FIGO stages III and IV made up 34 (70.83%) of the patients’ diagnoses, followed by stages I (18.75%), II (10.41%). The most frequent malignant tumor was papillary serous cystadenocarcinoma, which accounted for 56.25% (27/48) of patients.
In ovarian cancer, there was a substantial rise in ALT and AST, as well as BUN and CA125 levels. While the count of white blood cells and albumin concentrations was substantially reduced in OC cases than in healthy females, there was no significant variation in Hb, PLT, Cr, or TB between OC and healthy women (P > 0.05) (Fig. 3). When biochemical markers and OC were compared using Pearson correlation analysis, OC showed a statistically significant positive association with CA125 (r = 0.430; p = 0.000), AST (r = 0.391; p = 0.000), ALT (r = 0.507; p = 0.000), and BUN (r = 0.286, p = 0.005). OC demonstrated a statistically substantial inverse association with albumin production (r = − 0.737; p = 0.000) and TLC (r = − 0.311; P = 0.002). We demonstrated no substantial association of T. Bil., PLT, HB, or Creat. with OC (P > 0.05).
3.2 Association between promoter polymorphisms of IL-10 gene and ovarian cancer
The genotype distributions of IL-10 gene polymorphisms among groups (1) and (2) were all according to the expected Hardy–Weinberg equilibrium, since there was no significance in testing their genotypes’ divergence from equilibrium, in the controls (− 1082A > G, χ2 = 0.904, P = 0.44; − 819C > T, χ2 = 0.884, P = 0.739), in the OC (− 1082A > G, χ2 = 2.868, P = 0.625; − 819C > T, χ2 = 10.03, P = 0.416). As a result, we used the two polymorphisms to compare cases to controls.
IL-10 (− 1082 and − 819) allele and genotype distributions of both OC patients and controls are illustrated in Table 2. To calculate the risk of the dominant model (GA + AA vs. GG), the recessive (AA vs. GG + GA), and the models 1, 2 (codominant) (GA vs. GG and AA vs. GG), the frequencies of persons homozygous for the wild-type allele of IL10-1082 G > A (GG) were used as a reference.
As seen in the codominant pattern, there are GA (mutant kind that is heterozygous) vs GG (wild kind that is homozygous) (P > 0.05; OR = 0.52; 95% CI = 0.19–1.41) and AA (mutant kind that is homozygous) vs GG (P < 0.05; OR = 0.15; 95% CI = 0.04–0.63).
There was a substantial drop (p < 0.05) in the AA genotype’s frequency of OC’s patients in comparison with control. It was thought that the substitution might guard against OC in its homozygous form. On the other hand, it showed that both OC and controls had higher rates of the GA genotype (Table 2).
As seen in the dominant pattern (GA + AA vs GG), IL10 (− 1082G > A) substitution was insignificantly decreased in OC (P > 0.05; OR = 0.40; 95% CI = 0.15–1.05) (Table 2). Additional research utilizing the recessive pattern (AA vs GG + GA), (P < 0.05; OR = 0.24; 95% CI = 0.07–0.82) reported that G > A substitution may be a protective factor for OC in the homozygous form and that the best-fitting model of inheritance is the recessive pattern (based on BIC and AIC) (Table 2).
Furthermore, G (wild allele)’s frequency elevated considerably (p < 0.05), and A (mutant allele)’s frequency was considerably decreased (p < 0.05) in OC cases in comparison with controls. On the other hand, the controls had a higher prevalence of A (mutant allele) than the OC group did and considered a preventative factor (OR = 0.487 with CI = 0.273–0.867), whereas in comparison with the controls, the OC group had a higher prevalence of the G (wild allele) and was considered a risk factor (OR = 2.054 with CI = 1.154–3.657) (Table 2).
In IL10-819 C > T, to evaluate the risk of dominant inheritance (CT + TT vs. CC), the frequencies of subjects homozygous for the wild-type allele of IL10 − 819C > T (CC) were used as a reference, recessive (TT vs. CT + CC), and patterns 1, 2 (codominant) (CT vs. CC, TT vs. CC). The codominant pattern, CT (mutant kind that is heterozygous) vs CC (wild kind that is homozygous) (P < 0.001; OR = 9.44; 95% CI = 2.52–35.40) and TT (mutant kind that is homozygous) vs CC (P < 0.001; OR = 18.33; 95% CI = 3.46–97.20), suggested a substantial rise (P < 0.001) in the genotype’s distribution of TT and CT in OC in comparison with controls. The substitution was thought to increase the risk of OC in the heterozygous and homozygous forms.
Furthermore, it showed that both OC and controls had a higher prevalence of the CT genotype.
The dominant model (CT + TT vs CC) was found to have significantly higher levels of IL10 (− 819C > T) substitution in OC (P < 0.001; OR = 10.71; 95% CI = 2.91–39.39), suggesting that C > T substitution perhaps a hazard factor for OC and the dominant pattern is the most-fitting pattern of inheritance (BIC and AIC results). Another analysis utilizing the recessive pattern (TT vs CC + CT), (P < 0.05; OR = 3.27; 95% CI = 0.96–11.13) (Table 2). Furthermore, it was discovered that T (mutant allele)’s frequency was significantly more common (P < 0.05) in OC cases in comparison with controls, and it is possible that this variant may be a hazard factor for the illness (OR = 2.800, 95% CI = 1.577–5.037). C (wild allele), on the other hand, may act as a protective factor for OC as it was significantly less common (P < 0.05) in OC’s patients in comparison with controls (OR = 0.357, 95% CI = 0.199–0.642) (Table 2).
3.3 IL-10 polymorphisms (− 1082 and − 819) haplotype frequencies in healthy individuals and people with ovarian cancer
By estimating the IL-10 haplotypes’s frequencies of (− 1082G/A) and (− 819C/T), 4 haplotypes have produced: GC, AC, AT, and GT. GC was the most permanent haplotype in healthy women, while GT was the highly prevalent haplotype in OC. When OC cases were compared to controls, the GT haplotype frequency increased substantially (p < 0.001), and this finding could be seen as a hazard factor for OC (OR = 50.09 with CI = 6.34–395.92) (Table 3).
3.4 Correlations between IL 10 SNPs in OC
Spearman correlation analyses were performed for IL 10 (− 819C/T and − 1082 G/A) alleles and genotypes among OC’s patients. The AA genotype has a poor disease correlation in IL 10-1082 G/A (r = − 0.246; P < 0.05).
The association between the OC disease and the TT and CT genotypes, and T allele in IL 10–819 C/T is positive (r = 0.213; P < 0.05, r = 0.201; P < 0.05, and r = 0.415; P < 0.001, respectively), while the disease has a negative correlation with the CC genotype and C allele (r = − 0.415; P < 0.001 and r = − 0.201; P < 0.05, respectively).
There was not any significant correlation between all genotypes or alleles of two SNPs of IL 10. This result might be supported by the result of the linkage disequilibrium (LD) pattern (D′ = 0.1315, r2 = 0.016; p = NS) which indicates the absence of any level of LD between IL 10 − 1082 G/A and IL 10 − 819 C/T.
3.5 Correlation between promoter polymorphisms of IL-10 gene and IL-10 plasma levels
Compared to healthy controls, OC patients’ mean plasma concentrations of IL-10 were substantially greater (P < 0.001) than those of the controls (49.17 ± 18.01 versus 34.23 ± 10.34 pg/ml for OC and controls, respectively) (Fig. 4A). OC was substantially correlated with IL-10 level (r = 0.457; p < 0.001).
IL10 levels (pg/ml) in connection to A control and OC groups, B IL10 (− 1082 G/A) genotypes and alleles in healthy and OC groups, C IL10 (− 819 C/T) genotypes and alleles in healthy and OC groups, and D IL10 haplotypes in healthy and OC groups. Results are given as the mean ± the standard error. ***p < 0.001; **p < 0.01; *p < 0.05. Box plots are used to display the data, with the 10th and 90th percentiles indicated by the lines outside the boxes, the medians by the lines inside the boxes, and the 25th and 75th percentiles by the boxes
In both controls and OC patients, comparisons between IL-10’s plasma concentrations in various genotypes and haplotypes are shown in Table 4 and Fig. 4. In IL-10 (− 1082 G/A), the mean concentrations of IL10 plasma in persons with AA and GA genotypes were increased substantially (P < 0.05 and P < 0.001, respectively) in OC cases in comparison with controls. Additionally, persons with the G (wild allele) and A (mutant allele) had plasma concentrations of IL-10 that were considerably higher (P < 0.001) in OC cases than in healthy women. Plasma levels of IL-10 were higher in OC cases with the GG genotype, but there was no substantial difference (Fig. 4B).
In IL-10 (− 819 C/T), the IL-10 level was significantly higher in OC cases with CT genotype (P < 0.001). C (wild allele) and T (mutant allele) were also elevated substantially (P < 0.001) in OC cases. The IL-10’s plasma concentration was elevated in OC cases with CC and TT genotypes but without a statistical significance (Fig. 4C).
When IL-10 levels were compared to different IL-10 haplotypes, it was discovered that the AC, AT, and GT haplotypes had significantly higher levels (P < 0.001, P < 0.001, and P < 0.01, respectively) in OC cases. OC patients with the GC haplotype had greater plasma levels of IL-10, but there was no statistically substantially variance (Fig. 4D).
Between the haplotypes or genotypes of IL-10 gene polymorphisms, the plasma level of IL-10 showed no discernible variation for two SNPs (P > 0.05). These findings demonstrated that no one gene, allele, or haplotype might be the cause of the increased levels of IL-10 in OC patients’ plasma.
3.6 Plasma IL-10 with biochemical characteristics of patients and controls correlations
Following investigation to see whether the biochemical traits of healthy controls and OC’s patients were correlated with the levels of IL-10 plasma, a statistically significant positive relationship existed between IL-10 and CA125 and ALT. IL-10 and albumin showed a strong negative association (Fig. 5), whereas the correlation of IL10 plasma level with BUN, AST, T. Bil., TLC, PLT, Cr., and HB has not any significant value (P > 0.05).
Correlation between IL10 (pg/ml) with A CA125, B albumin, C ALT, D AST, E BUN, F creatinine, G HB, H PLT, I T. Bil, and J TLC among OC and control groups, Pearson’s correlation coefficient. *P < 0.05, **P < 0.01, and ***P < 0.001. Abbreviations: IL, interleukin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BUN, blood urea nitrogen; CA125, cancer antigen 125; and TLC, total leucocytic count
3.7 Differential manifestation of biochemical characteristics in patients according to the IL-10 genotypes
In OC patients with different genotypes of − 819 C/T and IL10-1082 G/A, the mean concentrations of CA125, AST, ALT, BUN, TLC, and ALB were comparable, as indicated in Table 5. Two SNPs had no discernible impact on the plasma level of any biochemical parameter (P > 0.05). These findings found that the elevation of these parameters in the OC cases’ plasma could not be attributed to any particular genotype, allele, or haplotype.
4 Discussion
The most fatal kind of gynecologic cancer in the world is OC [3]. The majority of OC patients have incurable, advanced-stage diseases when they first present, so there is a high case fatality rate for OC. Only 25% of OC patients were a stage I diagnosis [42]. Only 70.83% (34 females) of the OC cases in the current study had FIGO (III and IV) stages. Braicu EI. [43] reported FIGO (III and IV) stages were encountered in 82.6% of OC cases. Considerable efforts have been devoted to the search for novel OC markers. Because of their influence on cell activation, proliferation, differentiation, and migration, cytokines found in the tumor microenvironment have obtained a lot of attention, and they are becoming more well acknowledged as possible cancer-modifying genes [44]. IL-10 may aid tumor cell survival by allowing them to evade immune monitoring and promote tumor development [19] and may also protect malignant cells by inhibiting tumor-specific cell lysis mediated by cytotoxic T lymphocytes [45]. IL-10’s anti-angiogenesis impact, on the other hand, could prevent malignant tumors from growing and spreading [21]. The control of the immune system by IL-10’s pleiotropic effects, combined with polymorphic expression modulation, adds to the difficulty of interpretation, particularly in the context of disease pathogenesis [46]. Some studies have reported that the upregulation of IL-10 is related to tumorigenesis, autoimmunity, and resistance of transplantation [10, 47]. IL-10 protects the antigen absorption function of antigen-presenting cells (APCs) by inhibiting the traditional maturation process [48]. IL-10 suppresses the expression of several genes, including pro-inflammatory cytokines, chemokines, and chemokines receptors [49]. Concerning its suppressive activities on T lymphocytes, IL-10 helps stimulating the survival, differentiation, and proliferation of B lymphocytes. It promotes the synthesis of IgG4 by plasma cells [50,51,52].
IL10’s expression is elevated in the tumor microenvironment (TME) of OC patients as well as patients with other cancers [53,54,55], it can also act on most immune cells and suppress their effector responses [56], and it can increase programmed cell death-1 (PD-1) surface expression in a signal transducer and activator of transcription 3 (STAT-3)-dependent manner. T cells, B cells, monocytes, macrophages, natural killer (NK) cells, and dendritic cells (DC) all express the inhibitory PD-1 receptor, which inhibits antitumor effector responses in the tumor microenvironment [57, 58].
Different endogenous and external stimuli drive the synthesis of IL-10, but some allelic variations of the IL-10 gene are linked with changes in IL-10 levels. To see if a genetic variant is linked to an illness, researchers performed genetic association studies. Because it modulates both humoral and cellular immunity, in the pathologic mechanisms of autoimmune disorder, inflammatory disorders, and several cancers, IL-10 has been identified as a potential gene [46, 56, 57].
The most significant bi-allelic SNPs, located at locations − 1082G/A, − 592 C/A, and − 819 C/T, have been found to be present in the proximal promoter region of IL-10 [58]. In the IL-10 promoter gene, two SNPs (− 592 C/A and − 819 C/T) were previously discovered to be in linkage disequilibrium [59]. As a result, only − 1082 and − 819, two of the three polymorphism sites, were investigated, and IL-10 promoter haplotypes and genotyping of IL-10-592C/A were discarded in this study. Our study included 48 OC cases and 48 controls. Our study at the -1082G/A position determined distribution of the genotypes in OC which was as follows: The GG, GA, and AA were encountered in 33.3%, 58.3%, and 8.3%, respectively; in the − 819 C/T, the TT, CT, and CC genotypes were shown in 22.9%, 70.8%, and 6.2%, respectively. Braicu EI. [43] demonstrated the polymorphism of the IL-10 promoter gene in the − 1082 G/A, and the distribution of the genotypes was as follows: The GG, GA, and AA were encountered in 17%, 55.8%, and 27.2%, respectively; in the − 819 C/T, the TT, TC, and CC genotypes were present in 5.4%, 43.5%, and 51%, respectively. The lymphoid or myeloid cells that invade or are linked to ovarian tumors may be the most frequent source of IL-10 found in OC patients, or it may be expressed in the malignant tissues of EOC [60]. The concentration of plasma IL-10 in OC cases was considerably higher than in healthy females in our investigation. These results were in agreement with previously published data [43, 61,62,63,64]. Several studies have also found that people with specific malignancies had significantly higher levels of IL-10 expression such as gastric cancer (GC), [65, 66], hepatocellular carcinoma (HCC) [17], and non-Hodgkin lymphoma (NHL) [67]. These findings suggest that IL-10 may influence tumor evasion of the immunological response functions by suppressing Th1-type cytokine production, particularly IL-2, as a result of its un intended immunosuppressive properties. Individual genetic variations have been found to have an impact on the host’s response to malignant tumors [68]. It has been discovered that the 1082G > A alleles in the promoter region of the human IL-10 gene physically interact with a nuclear protein known as poly (ADP-ribose) polymerase1 (PARP-1) in an allele-specific manner, resulting in different levels of IL-10 transcription. They discovered that PARP-1 functions as a transcription repressor [69].
Similar to the research of Braicu EI, our investigation on the genotyping of IL-10-1082G/A found that GA was more common in both OC and controls [43]. According to our findings, the distribution of the AA genotype and the A (mutant allele) decreased substantially and the G (wild allele) substantially elevated in OC cases in comparison with healthy women. Around 55% of healthy women have the A (mutant allele), it could imply that it plays a protective role, while the G (wild allele) was considered a hazard factor for OC.
The studies of Makni et al. [70] in head and neck cancer (HNC) and nasopharyngeal carcinoma (NPC), Sabet et al. [20] in breast cancer, Kuo et al. [71] in gastric cancer (GC), and Tsai et al. [72] in nasopharyngeal carcinoma (NPC) reported that the IL10-1082GA genotype was linked to a rise of cancer risk, that is, different from the current study, whereas we found an insignificant increase in GA genotype frequency in OC compared with controls, while the study of Goud et al. [73] suggested that GA genotype may have not influence with oral squamous cell carcinoma (OSCC) susceptibility, consistent with our study.
In accordance with our findings, Wang et al.’s meta-analysis [45] revealed that in Asians, lung cancer, and non-lymphoma, Hodgkin’s participants with the GG genotype and G (wild allele) had a greater chance of developing cancer than those with the AA genotype. A research by Kuo et al. [71], in the Taiwanese population, reported that AA genotype and A (mutant allele) carriers demonstrated an increased risk for gastric cancer (GC) which is contradictory to our findings. Shamran et al. [74] established that the G (wild allele) plays a preventive effect in glioma which is inconsistent with our findings.
Contrary to our findings, the allelic and genotypic distributions of the IL-10 (− 1082) polymorphisms were not substantially different between the healthy group and OC cases in the study by Braicu EI. [43, 75].
Also, several findings were reporting no (significant difference) association of IL10 − 1082G/A SNP with various types of cancers as a non-Hodgkin lymphoma [76], cervical cancer [77], gastric cancer [78], colorectal cancer [79], prostate cancer [80], breast cancer [23], and diffuse large B cell lymphoma DLBCL [67].
In our study, the average plasma levels of IL10 in the participants with GA, AA genotypes, and G (wild allele), A (mutant allele) were elevated substantially but were insignificantly higher in OC with GG genotype. In line with the current findings, the study by Braicu EI. [43] discovered that OC persons with the AA genotype had plasma concentrations of IL-10 that were considerably greater than controls. Braicu EI. [43] found that plasma levels of IL-10 were substantially elevated in OC individuals with GG genotypes compared to controls which were inconsistent with our results. Braicu EI. [43] found that plasma concentration of IL-10 was substantially elevated in controls with GA genotype which is inconsistent with the current results. Also, Braicu EI. [43] found that the highest expression was linked to the − 1082 AA genotype in the OC group and with the lowest expression in the controls which is similar to our findings. Braicu EI. [43] found that the highest expression was associated with the − 1082 GA genotype in the control group which is different from our results which reported that the highest expression was associated with the − 1082 GG genotype in the healthy control. In contrast to our findings, Trifunovi et al. [46] revealed that non-small cell lung cancer, diffuse large B cell lymphoma, cervical cancer, and oral cancer are all connected with and promoted the development of pathological processes by high expression of − 1082 G (wild allele).
Our research found that both OC and controls had an increased frequency of the IL-10-819 CT genotype. The study of Braicu EI. [43] showed that CC was more frequent in OC and controls which are different from our study. According to our findings, the distribution of CC genotype and C (wild allele) was substantially reduced, and the frequencies of TT, CT genotypes, and allele T were substantially higher, in OC patients in comparison with healthy women, indicating that the substitution was thought to be a hazard factor for OC in both heterozygous and homozygous forms. Contrary to our findings, the investigation by Braicu EI. [43] revealed that the control group and OC showed no statistically substantial changes in the distributions of genotypic and allelic frequencies at the IL-10 (− 819C/T) polymorphisms. Our results are consistent with a meta-analysis of 73 studies involving 15,942 cancer cases and 22,336 controls published in 2013 by Yu et al. [38] that discovered that people with CT genotypes had a greater risk of developing OC, but they demonstrated that Asians’ − 819TT genotype was more preventive against stomach cancer, inconsistent with our results. Consistent with our results in ovarian cancer, Sabet et al. [20] and Yu et al. [38] reported that the CT genotype seems to take on a significant role in inflammatory breast cancer (IBC) and cervical cancer, respectively.
The studies of Martínez-Camposet al. [78] in Mexican population and meta-analyses (Yu et al. and Xue et al. [38, 81]) indicated a substantial drop in the TT genotype frequency in gastric cancer patients in comparison with controls and regarded as a stomach cancer preventive factor, which is inconsistent with the current results, while Makni et al. [70] suggested a positive correlation with HNC, NPC, and laryngeal cancer (LC) was observed for -819 TT genotype, similar to our results. On contrary, IL10 − 819C/T SNP was not connected to prostate cancer, hepatocellular carcinoma, breast cancer, colorectal cancer, lymphoma, or melanoma according to Yu et al. [38].’s meta-analysis [38].
According to our findings, OC patients with the CT genotype, C (wild allele), and T (mutant allele) had plasma concentrations of IL-10 that were substantially elevated than those in OC cases with the CC and TT genotypes, but not by a statistically significant amount. We reported the lowest expression was associated with the − 819 CC genotypes and the highest expression was associated with the − 819CT in the OC group.
Consistent with the current results, the study of Braicu EI. [43] demonstrated that IL-10’s plasma level was substantially elevated in OC individuals with CT genotype compared with controls, while plasma concentration of IL-10 was substantially elevated in OC individuals with TT genotypes compared to controls which were inconsistent with our results. Also, Braicu EI. [43] reported the lowest expression was associated with the − 819 CC genotypes in the OC group which is similar to our findings. Braicu EI. [43] reported that high levels of circulatory IL-10 are linked to the CC genotype in the healthy control, inconsistent with current results, whereas we reported CT genotype is connected to increased circulating IL-10 levels in the group of controls. The − 819TT genotype is linked to a lower level of IL-10 in individuals with colon cancer [46], which is different from our results.
The goal of our research was to see if polymorphisms are linked to IL-10 production differences. The rise in IL-10 production concentration in OC cases is irrespective of the patient’s genotypes in the IL-10 (− 819) or IL-10 (− 1082) loci, according to our findings, which are similar to those of Braicu EI [43].
Pairwise haplotypes were rare in previous findings, and the results were contradictory. In the present study, several haplotypes have found: GC, AC, GT, and AT. The most prevalent haplotype in controls was GC, but the most prevalent haplotype in OC was GT. In the Egyptian population, Talaat et al. [67] showed that the GC was the most prevalent haplotype in diffuse large B cell lymphoma (DLBCL) patients, whereas the AC was the most popular haplotype in healthy women. According to Mohammadi et al.’s study [82] in the Iranian population, both controls and SLE patients have the AT as their most common haplotype.
Similar to what we found, Talaat et al. [67] showed that the GT is the lower one in controls. Our study found AT is the lower one in OC patients, while Talaat et al. [67] found that the GT is the lower one in DLBCL patients. Mohammadi et al. [82] reported that the AC is the lower one in both groups.
In our results, the frequency of GT haplotype exhibited a substantial rise in OC in comparison with healthy women and can be viewed as an OC risk factor. Talaat et al. [67] study in DLBCL patients and Makni et al. [70] in HNC and NPC, similar to our study, found that the frequency of GT haplotype demonstrated a substantial rise in patients when compared to healthy women and may be a hazard factor for disease. Mohammadi et al. [82] reported that frequencies of GC and AC haplotypes showed a significant increase in systemic lupus erythematosus (SLE) compared to controls and can be viewed as an OC hazard factor for SLE in the Iranian population, inconsistent with our findings.
Furthermore, when IL-10 levels were compared to different IL-10 haplotypes in the current study, it was discovered that IL10 levels are substantially elevated in OC cases with the AC, AT, and GT haplotypes. Inconsistent with our study, Mohammadi et al. [82] found that there were no discernible changes in IL10 plasma expression between the paired haplotypes.
In the current study, the levels of AST, ALT, CA125, and BUN all significantly increased, while the count of white blood cells and albumin concentration was substantially reduced in OC cases than in healthy women, and no discernible differences existed in age, Hb, PLT, or T. Bil., and Cr between OC and healthy women. Celik et al. [83] in OC and Ma et al. [84] in lymph node enlargement patients suggested a substantial rise of CA125 in comparison with healthy women; similar to the current study, they reported insignificant difference in AST, ALT, and TLC between OC and healthy control, different from our results. Celik et al. showed a significant decrease of BUN and a substantial rise in PLT in OC cases when compared to healthy women, inconsistent with our study, and they did not find any significant difference of Hb between OC patients and control, similar to the current results. Inconsistence with the current study, Ma et al. [84] reported a significant increase in T. Bil. and creatinine in lymph node enlargement patients. Ahmet et al. [76] in NHL and Ma et al. [84] in lymph node enlargement patients found a significant decrease in Hb level in patients versus the control group which is different from our study. Inconsistent with the current study, Talaat et al. [67] reported a significant increase in ALT and AST compared to control, similar to our study. Talaat et al. [67] and Ahmed et al. [76] demonstrated a significant decrease of ALB level in non-Hodgkin lymphoma (NHL) compared to control. Talaat et al. [67] and Ma et al. [84] did not find any significant difference of PLT between patients and control, similar to our results. Talaat et al. [67] suggested a significant increase in TLC, in contrast to our study.
Our results found that the gene polymorphism of IL10 in OC cases does not affect on all biochemical parameters plasma level (P > 0.05). Similar to our study, Vural et al. [16] and Ahmed et al. [76] stated that IL10 (− 1082) genotypes had no impact on the laboratory outcomes in polycystic ovary syndrome (PCOS) and NHL patients, respectively. Karadeniz et al. [85] also found that the gene polymorphism of IL10 (− 1082G/A and − 819C/T) in PCOS cases does not affect on laboratory parameters.
Although it is difficult to confirm the causes of these conflicting results, it is possible that one of the key aspects is the various genetic backgrounds. These findings suggested that various ethnic cultures may have different functions for the same gene variation in cancer risk. Different racial and cultural backgrounds, contextual circumstances, and sample size are some variables that could affect the association detection.
5 Conclusion
The current research revealed an increase in the IL-10-1082 G (wild allele) frequency that is significant, which might be a genetic hazard factor for OC progress. We suggested a significant decrease in IL-10-1082 AA and IL-10-1082 A (mutant allele) frequencies which can be considered as protective factors for OC. Additionally, the genetic risk factors for OC susceptibility may include the IL-10-819 TT, CT genotypes, and the IL-10-819 T (mutant allele), whereas the IL-10-819 C (wild allele) may act as a protective factor. GT haplotype could be considered a risk factor for OC. The differences that have been observed in the current study from earlier researches in other populations could be traced back to ethnic distinctions. To corroborate our findings, larger prospective investigations are required. The sample size is a significant concern in the current investigation because the genetic variants are population-specific. Based on these findings, only speculative generalizations can be made because of the sample size’s modest size. The findings ought to be reproduced in a greater population with various polymorphic locations of the IL-10 gene in order to properly comprehend the probable link between cytokine gene polymorphisms and OC as well as how it interacts with additional genetical and environmental variables. Finally, ovarian cancer cells secrete IL10 to help tumor cells avoid immune surveillance and to hasten tumor progression and metastasis. We conclude that therapeutic IL10 blockade warrants further investigation in the development of immunotherapeutic protocols for the treatment of ovarian cancer patients. As a result, novel antibody therapy with acceptable side effects is urgently required and regarded as a promising field of OC diagnosis and treatment research.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- CLL:
-
Chronic lymphocytic leukemia
- DLBCL:
-
Diffuse large B cell lymphoma
- EOC:
-
Epithelial ovarian cancer
- EDTA.K3:
-
Tri-potassium ethylenediaminetetraacetic acid
- ELISA:
-
Enzyme-linked immunosorbent measure
- FIGO:
-
International Federation of Gynecology and Obstetrics
- ALT:
-
Alanine aminotransferase
- GC:
-
Gastric cancer
- BUN:
-
Blood urea nitrogen
- CA125:
-
Cancer antigen 125
- GI:
-
Gastrointestinal
- HNC:
-
Head and neck cancer
- HCC:
-
Hepatocellular carcinoma
- HPB:
-
Hepatopancreatobiliary
- IHC:
-
Immunohistochemistry
- IBC:
-
Inflammatory breast cancer
- IL-10:
-
Interleukin 10
- LC:
-
Laryngeal cancer
- OR:
-
Odds ratio
- LTs:
-
Lymphotoxins
- LD:
-
Linkage disequilibrium
- NK:
-
Natural killer
- ANOVA:
-
Analysis of variance
- NPC:
-
Nasopharyngeal carcinoma
- SPSS:
-
Statistical Package for the Social Sciences
- NS:
-
Not significant
- OSCC:
-
Oral squamous cell carcinoma
- NHL:
-
Non-Hodgkin lymphoma
- AST:
-
Aspartate aminotransferase
- OC:
-
Ovarian cancer
- SNPs:
-
Single nucleotide polymorphisms
- SD:
-
Standard deviation
- PCR-SSP:
-
Polymerase chain reaction sequence-specific primer
- APC:
-
Antigen-presenting cell
- TLC:
-
Total leucocytic count
- SLE:
-
Systemic lupus erythematosus
- PARP-1:
-
Poly (ADP-ribose) polymerase1
- APCs:
-
Antigen-presenting cells
- TME:
-
Tumor microenvironment
- PD-1:
-
Programmed cell death-1
- STAT-3:
-
Signal transducer and activator of transcription 3
- DC:
-
Dendritic cells
- PCOS:
-
Polycystic ovary syndrome
References
Missaoui N, Salhi S, Bdioui A, Mestiri S, Abdessayed N, Mokni M, Yacoubi MT (2018) Immunohistochemical characterization improves the reproducibility of the histological diagnosis of ovarian carcinoma. Asian Pac J Cancer Prev APJCP 19:2545
Chai H, Ren Q, Fan Q, Ye L, Du G, Du H, Xu W, Li Y, Zhang L, Cheng Z (2017) PAX8 is a potential marker for the diagnosis of primary epithelial ovarian cancer. Oncol Lett 14:5871–5875
Sopik V, Iqbal J, Rosen B, Narod SA (2015) Why have ovarian cancer mortality rates declined? Part I Incid Gynecol Oncol 138:741–749
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Zhang Y, Luo G, Li M, Guo P, Xiao Y, Ji H, Hao Y (2019) Global patterns and trends in ovarian cancer incidence: age, period and birth cohort analysis. BMC Cancer 19:1–14
Hatzipetros I, Gocze P, Koszegi T, Jaray A, Szereday L, Polgar B, Farkas N, Farkas B (2013) Investigating the clinical potential for 14-3-3 zeta protein to serve as a biomarker for epithelial ovarian cancer. J Ovarian Res 6:1–11
Aziz KKA, Shehata MA, Ghany AEA, El Khouly EAB, Aziz RAA (2014) Retrospective study of epithelial ovarian cancer in the oncology department Menoufia University. Menoufia Med J 27:650
Bian J, Li B, Kou X-J, Wang X-N, Sun X-X, Ming L (2014) Clinical applicability of multi-tumor marker protein chips for diagnosing ovarian cancer. Asian Pac J Cancer Prev 15:8409–8411
Charbonneau B, Goode EL, Kalli KR, Knutson KL, DeRycke MS (2013) The immune system in the pathogenesis of ovarian cancer. Crit Rev Immunol 33(2):137–164
Briukhovetska D, Dörr J, Endres S, Libby P, Dinarello CA, Kobold S (2021) Interleukins in cancer: from biology to therapy. Nat Rev Cancer 21(8):481–499
Arango Duque G, Descoteaux A (2014) Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol 5:491
Greten FR, Grivennikov SI (2019) Inflammation and cancer: triggers, mechanisms, and consequences. Immunity 51:27–41
Kany S, Vollrath JT, Relja B (2019) Cytokines in inflammatory disease. Int J Mol Sci 20:6008
Yang Y, Fa X (2015) Role of IL-10 gene polymorphisms on the susceptibility for esophageal cancer and its association with environmental factors. Int J Clin Exp Pathol 8:9580
Tian K, Zhang R, Wang X (2017) Association of interleukin-10 polymorphisms and haplotypes with the risk of breast cancer in northern China. Int J Clin Exp Pathol 10:6989–6996
Vural P, Değirmencioğlu S, Saral NY, Akgül C (2010) Tumor necrosis factor α (− 308), interleukin-6 (− 174) and interleukin-10 (− 1082) gene polymorphisms in polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol 150:61–65
Othman MS, Aref AM, Mohamed AA, Ibrahim WA (2013) Serum levels of interleukin-6 and interleukin-10 as biomarkers for hepatocellular carcinoma in Egyptian patients. Int Sch Res Not. https://doi.org/10.1155/2013/412317
Ding Q, Fan B, Fan Z, Ding L, Li F, Tu W, Jin X, Shi Y, Wang J (2013) Interleukin-10-819C> T polymorphism contributed to cancer risk: evidence from 29 studies. Cytokine 61:139–145
Lee SJ, Borrello I (2016) Role of the immune response in disease progression and therapy in multiple myeloma. In: Plasma cell dyscrasias. Springer, pp 207–225
Sabet S, El-Sayed SK, Mohamed HT, El-Shinawi M, Mohamed MM (2017) Inflammatory breast cancer: High incidence of GCC haplotypes (− 1082A/G,− 819T/C, and− 592A/C) in the interleukin-10 gene promoter correlates with over-expression of interleukin-10 in patients’ carcinoma tissues. Tumor Biol 39:1010428317713393
Zhen Z, Guo X, Liao R, Yang K, Ye L, You Z (2016) Involvement of IL-10 and TGF-β in HLA-E-mediated neuroblastoma migration and invasion. Oncotarget 7:44340
Sato T, Terai M, Tamura Y, Alexeev V, Mastrangelo MJ, Selvan SR (2011) Interleukin 10 in the tumor microenvironment: a target for anticancer immunotherapy. Immunol Res 51:170–182
AlSuhaibani ES, Kizilbash NA, Malik S, Dasti JI, Al Beladi F, El-Morshedi N (2016) Polymorphisms in promoter regions of IL-6 and IL-10 genes in breast cancer: a case-control study. Genet Mol Res 15:56
Vinod C, Jyothy A (2015) A common SNP of IL-10 (-1082A/G) is associated with increased risk of premenopausal breast cancer in South Indian women. Iran J Cancer Prev 8:e3434
Rezaeifard S, Razmkhah M, Robati M, Momtahan M, Ghaderi A (2015) Cytokines, chemokines, and chemokine receptors quantitative expressions in patients with ovarian cancer. Iran J Med Sci 40:225
Usui Y, Wakabayashi Y, Okunuki Y, Kimura K, Tajima K, Matsuda R, Ueda S, Ma J, Nagai T, Mori H (2012) Immune mediators in vitreous fluids from patients with vitreoretinal B-cell lymphoma. Invest Ophthalmol Vis Sci 53:5395–5402
Browning L, Patel MR, Horvath EB, Tawara K, Jorcyk CL (2018) IL-6 and ovarian cancer: inflammatory cytokines in promotion of metastasis. Cancer Manag Res 10:6685
Coleman RL, Monk BJ, Sood AK, Herzog TJ (2013) Latest research and treatment of advanced-stage epithelial ovarian cancer. Nat Rev Clin Oncol 10:211–224
Walsh C (2017) Targeted therapy for ovarian cancer: the rapidly evolving landscape of PARP inhibitor use. Minerva Ginecol 70:150–170
Chester C, Dorigo O, Berek JS, Kohrt H (2015) Immunotherapeutic approaches to ovarian cancer treatment. J Immunother cancer 3:1–10
Cang S, Mukhi N, Wang K, Liu D (2012) Novel CD20 monoclonal antibodies for lymphoma therapy. J Hematol Oncol 5:1–9
Bosch X, Ramos-Casals M, Khamashta MA (2014) Drugs targeting B-cells in autoimmune diseases. Springer
Beard EL Jr (2001) The american society of health system pharmacists. JONAS Healthc Law Ethics Regul 3:78–79
Butler KA, Hou X, Becker MA, Zanfagnin V, Enderica-Gonzalez S, Visscher D, Kalli KR, Tienchaianada P, Haluska P, Weroha SJ (2017) Prevention of human lymphoproliferative tumor formation in ovarian cancer patient-derived xenografts. Neoplasia 19:628–636
Leiting JL, Hernandez MC, Yang L, Bergquist JR, Ivanics T, Graham RP, Truty MJ (2019) Rituximab decreases lymphoproliferative tumor formation in hepatopancreaticobiliary and gastrointestinal cancer patient-derived xenografts. Sci Rep 9:1–10
Panayi GS (2005) B cell-directed therapy in rheumatoid arthritis–clinical experience. J Rheumatol Suppl 73:19–24
Organization WH (2019) World Health Organization model list of essential medicines: 21st list 2019. World Health Organization
Yu Z, Liu Q, Huang C, Wu M, Li G (2013) The interleukin 10–819C/T polymorphism and cancer risk: a HuGE review and meta-analysis of 73 studies including 15,942 cases and 22,336 controls. Omi a J Integr Biol 17:200–214
Liang L, Zhao Y-L, Yue J, Liu J-F, Han M, Wang H, Xiao H (2011) Interleukin-10 gene promoter polymorphisms and their protein production in pleural fluid in patients with tuberculosis. FEMS Immunol Med Microbiol 62:84–90
Chenjiao Y, Zili F, Haibin C, Ying L, Sheng X, Lihua H, Wei D (2013) IL-10 promoter polymorphisms affect IL-10 production and associate with susceptibility to acute myeloid leukemia. Die Pharm Int J Pharm Sci 68:201–206
Talaat RM, Ashour ME, Bassyouni IH, Raouf AA (2014) Polymorphisms of interleukin 6 and interleukin 10 in Egyptian people with Behcet’s disease. Immunobiology 219:573–582
Wang Z, Liu Q-L, Sun W, Yang C-J, Tang L, Zhang X, Zhong X-M (2014) Genetic polymorphisms in inflammatory response genes and their associations with breast cancer risk. Croat Med J 55:638
Braicu EI (2011) Polymorphism and expression of IL-10 in serum and ascites from patients with advanced ovarian cancer
Henke E, Nandigama R, Ergün S (2020) Extracellular matrix in the tumor microenvironment and its impact on cancer therapy. Front Mol Biosci 6:160
Wang J, Ding Q, Shi Y, Cao Q, Qin C, Zhu J, Chen J, Yin C (2011) The interleukin-10-1082 promoter polymorphism and cancer risk: a meta-analysis. Mutagenesis 27:305–312
Trifunović J, Miller L, Debeljak Ž, Horvat V (2015) Pathologic patterns of interleukin 10 expression–a review. Biochem medica 25:36–48
Heo G, Lee Y, Im E (2021) Interplay between the gut microbiota and inflammatory mediators in the development of colorectal cancer. Cancers 13:734
Krupa A, Kowalska I (2021) The Kynurenine pathway—new linkage between innate and adaptive immunity in autoimmune endocrinopathies. Int J Mol Sci 22:9879
Akdis M, Aab A, Altunbulakli C, Azkur K, Costa RA, Crameri R, Duan S, Eiwegger T, Eljaszewicz A, Ferstl R (2016) Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: receptors, functions, and roles in diseases. J Allergy Clin Immunol 138:984–1010
Akdis CA, Akdis M (2014) Mechanisms of immune tolerance to allergens: role of IL-10 and Tregs. J Clin Invest 124:4678–4680
Stanic B, Van De Veen W, Wirz OF, Rückert B, Morita H, Söllner S, Akdis CA, Akdis M (2015) IL-10–overexpressing B cells regulate innate and adaptive immune responses. J Allergy Clin Immunol 135:771–780
van de Veen W, Stanic B, Yaman G, Wawrzyniak M, Söllner S, Akdis DG, Rückert B, Akdis CA, Akdis M (2013) IgG4 production is confined to human IL-10–producing regulatory B cells that suppress antigen-specific immune responses. J Allergy Clin Immunol 131:1204–1212
Block MS, Maurer MJ, Goergen K, Kalli KR, Erskine CL, Behrens MD, Oberg AL, Knutson KL (2015) Plasma immune analytes in patients with epithelial ovarian cancer. Cytokine 73:108–113
Coosemans A, Decoene J, Baert T, Laenen A, Kasran A, Verschuere T, Seys S, Vergote I (2016) Immunosuppressive parameters in serum of ovarian cancer patients change during the disease course. Oncoimmunology 5:e1111505
Napoletano C, Bellati F, Landi R, Pauselli S, Marchetti C, Visconti V, Sale P, Liberati M, Rughetti A, Frati L (2010) Ovarian cancer cytoreduction induces changes in T cell population subsets reducing immunosuppression. J Cell Mol Med 14:2748–2759
Lamichhane P, Karyampudi L, Shreeder B, Krempski J, Bahr D, Daum J, Kalli KR, Goode EL, Block MS, Cannon MJ (2017) IL10 release upon PD-1 blockade sustains immunosuppression in ovarian cancer. Cancer Res 77:6667–6678
Hart KM, Byrne KT, Molloy MJ, Usherwood EM, Berwin B (2011) IL-10 immunomodulation of myeloid cells regulates a murine model of ovarian cancer. Front Immunol 2:29
Castelli EC, Ramalho J, Porto IOP, Lima THA, Felício LP, Sabbagh A, Donadi EA, Mendes-Junior CT (2014) Insights into HLA-G genetics provided by worldwide haplotype diversity. Front Immunol 5:476
Birlea SA, Jin Y, Bennett DC, Herbstman DM, Wallace MR, McCormack WT, Kemp EH, Gawkrodger DJ, Weetman AP, Picardo M (2011) Comprehensive association analysis of candidate genes for generalized vitiligo supports XBP1, FOXP3, and TSLP. J Invest Dermatol 131:371–381
Jammal MP, Araújo da Silva A, Martins Filho A, de Castro CE, Adad SJ, Murta EFC, Nomelini RS (2015) Immunohistochemical staining of tumor necrosis factor-α and interleukin-10 in benign and malignant ovarian neoplasms. Oncol Lett 9:979–983
Nishio H, Yaguchi T, Sugiyama J, Sumimoto H, Umezawa K, Iwata T, Susumu N, Fujii T, Kawamura N, Kobayashi A (2014) Immunosuppression through constitutively activated NF-κB signalling in human ovarian cancer and its reversal by an NF-κB inhibitor. Br J Cancer 110:2965–2974
Sipak-Szmigiel O, Włodarski P, Ronin-Walknowska E, Niedzielski A, Karakiewicz B, Słuczanowska-Głąbowska S, Laszczyńska M, Malinowski W (2017) Serum and peritoneal fluid concentrations of soluble human leukocyte antigen, tumor necrosis factor alpha and interleukin 10 in patients with selected ovarian pathologies. J Ovarian Res 10:25
Tarkowski R, Polak G, Wertel I, Kotarska M (2011) Evaluation of IL-10 and TGF-beta levels and myeloid and lymphoid dendritic cells in ovarian cancer patients. Ginekol Pol 82(6):414–420
Zhang L, Liu W, Wang X, Wang X, Sun H (2019) Prognostic value of serum IL-8 and IL-10 in patients with ovarian cancer undergoing chemotherapy. Oncol Lett 17:2365–2369
Cui X, Huang Q, Li X, Liu F, Wang D, Yan D, Wang B, Yang C, Mi J, Tian G (2016) Relationship between interleukin-10 gene C-819T polymorphism and gastric cancer risk: insights from a meta-analysis. Med Sci Monit Int Med J Exp Clin Res 22:2839
Shokrzadeh M, Mohammadpour A, Hoseini V, Abediankenari S, Ghassemi-Barghi N, Tabari YS (2018) Serum cytokine of IL-2, IL-10 and IL-12 levels in patients with stomach adenocarcinoma. Arq Gastroenterol 55:385–389
Talaat RM, Abdel-Aziz AM, El-Maadawy EA, Abdel-Bary N (2014) Interleukin 10 gene promoter polymorphism and risk of diffuse large B cell lymphoma (DLBCL). Egypt J Med Hum Genet 15:7–14
Pandya PH, Murray ME, Pollok KE, Renbarger JL (2016) The immune system in cancer pathogenesis: potential therapeutic approaches. J Immunol Res. https://doi.org/10.1155/2016/4273943
Kang X, Kim H-J, Ramirez M, Salameh S, Ma X (2010) The septic shock-associated IL-10− 1082 A> G polymorphism mediates allele-specific transcription via poly (ADP-Ribose) polymerase 1 in macrophages engulfing apoptotic cells. J Immunol 184:3718–3724
Makni L, Hamda BC, Ansari AA, Souaiai O, Gazouani E, Mezlini A, Almawi WY, Yacoubi-Loueslati B (2019) Association of common IL-10 promoter gene variants with the susceptibility to head and neck cancer in Tunisia. Turkish J Med Sci 49:123–128
Kuo W-H, Huang C-Y, Fu C-K, Hsieh Y-H, Liao C-H, Hsu C-M, Huang Y-K, Tsai C-W, Chang W-S, Bau D-T (2014) Effects of interleukin-10 polymorphisms and smoking on the risk of gastric cancer in Taiwan. In Vivo 28:967–971
Tsai C-W, Tsai M-H, Shih L-C, Chang W-S, Lin C-C, Bau D-T (2013) Association of interleukin-10 (IL10) promoter genotypes with nasopharyngeal carcinoma risk in Taiwan. Anticancer Res 33:3391–3396
Goud EVSS, Malleedi S, Ramanathan A, Wong GR, Ern BTH, Yean GY, Ann HH, Syan TY, Zain RM (2019) Association of interleukin-10 genotypes and oral cancer susceptibility in selected Malaysian population: a case-control study. Asian Pac J Cancer Prev APJCP 20:935
Shamran HA, Ghazi HF, Ahmed A-S, Al-Juboory AA, Taub DD, Price RL, Nagarkatti M, Nagarkatti PS, Singh UP (2015) Single nucleotide polymorphisms in IL-10, IL-12p40, and IL-13 genes and susceptibility to glioma. Int J Med Sci 12:790
He X, Wang L, Zhao H, Wu D, Tang H, Cao F (2008) Association of interleukin-10 polymorphisms with susceptibility to ovarian cancer in Chinese Han population. Chin J Birth Heal Hered. https://doi.org/10.1111/IGC.0b013e3181a130ab
Ahmed MIS, Hashad DI, Hassen AE (2014) Interleukin-10 (− 1082G/A) gene promotor polymorphism in Egyptian non-Hodgkin lymphoma patients: relation to other prognostic factors. Egypt J Haematol 39:156
Zidi S, Benothmen Y, Sghaier I, Ghazoueni E, Mezlini A, Slimen B, Yacoubi-Loueslati B (2014) Association of IL10-1082 and IFN-γ+ 874 polymorphisms with cervical cancer among Tunisian women. ISRN Otolaryngol
Martínez-Campos C, Torres-Poveda K, Camorlinga-Ponce M, Flores-Luna L, Maldonado-Bernal C, Madrid-Marina V, Torres J (2019) Polymorphisms in IL-10 and TGF-β gene promoter are associated with lower risk to gastric cancer in a Mexican population. BMC Cancer 19:1–8
Mirjalili SA, Moghimi M, Aghili K, Jafari M, Abolbaghaei SM, Neamatzadeh H, Mazaheri M, Zare-Shehneh M (2018) Association of promoter region polymorphisms of interleukin-10 gene with susceptibility to colorectal cancer: a systematic review and meta-analysis. Arq Gastroenterol 55:306–313
Ruan X-L, Li S, He Z-Q, Huang J-Y, Zhang C, Weng H (2017) Meta-analysis on the possible role of interluekin-10 rs1800896 polymorphism in association with prostate cancer susceptibility. Int J Clin Exp Med 10:1967–1975
Xue H, Lin B, An J, Zhu Y, Huang G (2012) Interleukin-10-819 promoter polymorphism in association with gastric cancer risk. BMC Cancer 12:1–11
Mohammadi S, Saghaeian Jazi M, Zare Ebrahimabad M, Eghbalpour F, Abdolahi N, Tabarraei A, Yazdani Y (2019) Interleukin 10 gene promoter polymorphisms (rs1800896, rs1800871 and rs1800872) and haplotypes are associated with the activity of systemic lupus erythematosus and IL10 levels in an Iranian population. Int J Immunogenet 46:20–30
Celik B, Yalcin AD, Genc GE, Gumuslu S (2015) Proteomics pattern of peritoneal sApo-2L but not CD200 (OX-2) as a possible screening biomarker for metastatic ovarian, endometrial and breast carcinoma. Age 45:48–76
Ma S, Guo J, Lu D, Zhu L, Zhou M, Zhou D, Li L, Zhu J, Yang X, Zheng Y (2017) Blood biomarkers in adults with lymph node enlargement contribute to diagnostic significance of malignancy. Oncotarget 8:98533
Karadeniz M, Erdogan M, Zengi A, Tamsel S, Berdeli A, Saygili F, Yilmaz C (2008) Polymorphism of the interleukin-10 gene in polycystic ovary syndrome. Int J Immunogenet 35:119–123
Acknowledgements
Not applicable.
Funding
This study is self-funded and did not receive any support from funding agencies.
Author information
Authors and Affiliations
Contributions
ZMA and AN designed the research. ZMA, KAE, and EHM performed the research. ZMA analyzed data. ZMA and RE designed the figures. ZMA, AN, EHM, KAE, and RE wrote the text. All authors discussed the data and contributed to the final version. All authors read and edited the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the ethical committee of Department of Biotechnology and Life Sciences, Faculty of Postgraduate Studies for Advanced Sciences, Beni-Suef University, Beni-Suef, Egypt, and the Informed consent was approved by the ethical committee from all the study subjects.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing interests/conflicts of interest or personal relationships with other people or organizations that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Almolakab, Z.M., El-Nesr, K.A., Mohamad, E.H. et al. Gene polymorphisms of interleukin 10 (− 819 C/T and − 1082 G/A) in women with ovarian cancer. Beni-Suef Univ J Basic Appl Sci 11, 141 (2022). https://doi.org/10.1186/s43088-022-00321-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43088-022-00321-0