Abstract
Background
The tumorigenesis and development in a variety of cancers is reportedly encouraged via Annexin A5. The levels of Annexin A5 were tested in patients with or without HCC who were affected by liver cirrhosis. The objective of our study was to detect Annexin A5 levels in such patients in order to assess their function as an HCC marker. The longitudinal study comprised 91 cirrhotic HCV patients with and without HCC, and 20 healthy volunteers in the control group approved by the National Hepatology and Tropical Medicine Research Institute (NHTMRI) between March 2017 and August 2018.The serum levels Annexin A5 were found in all groups with ELISA. ANOVA, Mann-Whitney, and χ2 tests had been applied.
Results
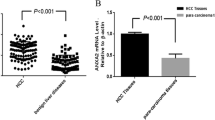
High scales of Annexin A5 (3.89 + 0.85) were recorded for cirrhosis with HCC, either than cirrhotic patients without HCC (3.06 ± 0.88) (P = 0.041), and either than the control group (0.54 ± 0.11) (P < 0.001).
Conclusion
In HCV cirrhotic patients with and without HCC, AnxA5 can be used as HCC marker.
Similar content being viewed by others
Background
One of the world’s most common cancers is hepatocellular carcinomas (HCC). Epidemiologic studies have shown that HCC is the fifth and seventh most diagnosed cancer among men and women and the world’s second and sixth cancer cause for death [1]. The main cause of cancer-related death is hepatocellular carcinoma (HCC), which causes about 800,000 deaths worldwide in 2018 [2]. There is a definite need for non-invasive quantifiable biomarkers to classify the early stages of HCC in order to minimize HCC morbidity and mortality [3]. Annexins are water soluble proteins, usually present in many animals, tissues, and cells. These proteins bind calcium and phospholipids and form ion voltage channels which are calcium-dependent. They are inscribed in cell signals, apoptosis, and control of various inflammatory elements, such as cytokines. Annexin A5 (Annex A5) is a member of the 36 kDa molecular weight annexin family which is based on the Ca2+ membrane binding [4]. Inhibition of pro-Inflammatory actions during apoptosis, Annexin-A5 appears to be of significance. Annexin-A5 was suggested to act as an active and selective anti-inflammatory agent to minimize inflammation during apoptosis [5]. The circulating AnxA5 can be emitted from wounded myocardial tissue, vascular endothelial and smooth muscular cells, liver secretary cells, and spleen; once it is in the plasma, it binds to blood cells (platelets and erythrocytes) or to endothelial cells [6] or also can be released from apoptotic particles derived from circulating blood cells, and the level of which has been shown to reflect severity of cell damage [7]. The levels of annexin gene expression inside human organs vary from universal to selective (for example A1, A2, A4, A5, A6, A7, and A11 annexes), including neutrophil annexin A3, placental and skin annexin A8, tongue A9, stomach annexin A10, and small intestine annexin A13 [8]. It is well known that ANXA5 can be liberated from damaged or dying cells not explicitly [9]. In understanding how the presence of HCC can affect the weather equilibrium, it is important to consider the sense in which HCC is present, which is basically often a complication of cirrhosis to the liver [10], with the prevalence of cirrhosis among patients with HCC estimated of 85–95 [11]. By superimposed conditions, like HCC, the rebalanced and unstable hemostasis of hepatic cirrhosis can easily be tipped to thrombotic complications. In non-cirrhotic liver, HCC can develop, according to Kulik L.et al.; in a most recent analysis, the occurrence of HCC without prior cirrhosis is unusual and specifically about 15% of HBV-related cases [12]. The objective of our study was to detect Annexin A5 levels in such patients in order to assess their function as an HCC marker.
Methods
The longitudinal study comprised 44 patients with HCC cirrhosis (23 were male and 21 were female), 47 patients without HCC cirrhosis (27 were male and 20 are female), and 20 control healthy (10 were male and 10 were female) without evidence for the diseases of the liver approved by the National Hepatology and Tropical Medicine Research Institute (NHTMRI) between March 2017 and August 2018. The formal consent was given to all studied citizens. The NHTMRI Ethics Committee has approved this analysis under serial No. 15/2016. The study has been organized in accordance with the Declaration of Research on Human Subject in Helsinki for human subject’s research. Both of the patients were diagnosed with hepatic biopsy histology, simple lab changes, and ultrasound from Doppler. The magnitude of liver disease was calculated in accordance with the MELD score and the Child-Pugh classification. Cirrhosis with and without HCC impacted hepatitis C and laboratory and imagery studies based on guidelines for AASLD practice have been clinically conformed. The exclusion criteria were patients of different malignancies that could raise the level of Annexin A5 such as colorectal cancer and pancreatic cancer. The amount of the serum anxA5 was calculated on Stat Fax 4700 Microstrip Reader1 ELISA Microstrip Reader by means of a commercial enzyme immunoassay (SinoGeneClon Biotech Co., Ltd).
Statistical analysis
All statistical analyses were evaluated in SPSS Version 19. Quantitative variables are listed as mean + SD or median, while qualitative variables are listed as frequencies. In order to differentiate the three classes, one way analysis of variance (ANOVA) was used. A non-parametrical analysis was performed by Mann-Whitney, and a parametric student evaluation was performed when a categorical test of χ2 had been applied. Statistically important was a P value below 0.05.
Results
Ninety-one cirrhotic patients, 44 HCC, and 47 HCC-free were recruited. Twenty healthy people were also hired (control group). Table 1 displays demographic, clinical, and biochemical properties of all classes.
No statistically significant differences in sex, age, albumin, ALKB, ALT, AST, GGT, T-bilirubin, PT, INR, platelet count, APRI score, and FIB-4 score were discovered between cirrhotic group with HCC and cirrhotic group without HCC (Table 1). There were statistically important variations between the two groups regarding AFP (P < 0.001) (Table 1). There were statistically relevant differences in Annexin A5 values (P = 0.041) between the two groups (Table 1). ROC curve reveals that in the cirrhotic HCC group, Annexin A5 has AUC 0.566 and in the cirrhotic group without HCC has AUC 0.571 (Tables 2 and 3; Figs. 1 and 2).
Discussion
Annexin A5 has been shown to facilitate tumorigenesis and cancers such as hepatocarcinoma, breast, colorectal, pancreas, bladder, and prostate cancer [13, 14]. In most different types of tumors, including liver cancer, ANXA5 exhibits tumor promoter activity. Two-dimension gel electrophoresis was used by Guo et al. to analyze protein expression in five pairs of tumor and tumor thrombal matched samples, which showed an upregulation of ANXA5 in tumor thrombus samples [15]. Sun and others reported that ANXA5 expression was positive for liver cancer progression and metastasis and that ANXA5 promoted cancer through the integrin and mitogen-activated protein kinase-extracellular signal-regulated kinase pathways [16]. Anxa5 could be a predictive biomarker for tumor development, metastasis, and invasion and be of diagnostic, prognostic, and therapeutic significance in cancer [14]. In addition, Sun and Zhuang et al. showed that HCC expression in ANXA5 was higher than normal liver tissue [16, 17]. The findings of the present study indicate, as compared the cirrhotic patients with and without HCC, that the expression of ANXA5 was increased in HCC cirrhotic HCV patients. In addition, the pronounced role of ANXA5 in liver cancer has not been documented to the best of our knowledge. In another review, high ANXA5 expression has been shown to be correlated significantly with poor overall survival [17]. AnxA5 has proved to participate in a wide range of intra- and extracellular processes including blood clotting, signal translation, anti-inflammatory processes, membrane trafficking, and ion channel activity, but it has not yet established the exact biological function of anxA5. However, it is assumed that the biological functions of anxA5 rely primarily on the interaction between them and lipids of the membranes [18], and at least two crucial physiological mechanisms are regulated: the cascade of coagulation, which is known to involve anticoagulant activity, and apoptosis [19]. Various research models have used annexin A5 as a probe to measure apoptosis in vitro and in vivo. A non-invasive imaging protocol using annexin A5 has been developed and applied successfully to measure programmed cell death in patients [20]. In addition, our study match to previous results showing that HCC hepatitis C patients have greater levels of microparticles than HCC-free hepatitis C patients, and these levels of microparticles dynamically alter after surgery [21]. In contrast to healthy controls, the explanation of the presence of microparticles in cirrhotic liver is possibly due to systemic inflammation and liver cell damage [22], asserting microparticulate roles that are included in hepatic fibrosty process in mediation of anti-inflammatory reactions, endothelial damage, and coagulation activation.
Conclusion
In HCC prediction in cirrhotic HCV patients, serum Annexin A5 values were found to be a forecasting factor. Larger scale tests are important to promote the use of an inexpensive and easily accessible Annexin A5 biomarker as HCC marker in HCV cirrhotic patients.
Limitation of study
In our analysis, the sample size of the control group compared to the disease group is relatively small. Finally, it would have been more fitting if the control group number was at least equal to the diseased group.
Recommendation
Wide sample sizes are suggested to validate this result.
Availability of data and materials
The published article [and its supplementary information files] contains all the data produced or analyzed during this research.
Abbreviations
- AnxA5:
-
Annexin A5
- HCV:
-
Hepatitis C virus
- HCC:
-
Hepatocellular carcinoma
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E et al (2011) Global cancer statistics. CA: Cancer J. Clin 61:69–90. https://doi.org/10.3322/caac.201072
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J. Clin 68(394):424–424. https://doi.org/10.3322/caac.21492
Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK (2018) Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 68:723–750. https://doi.org/10.1002/hep.299132
Bouter A, Carmeille R, Gounou C, Bouvet F, Degrelle S et al (2015) Annexin-A5 and cell membrane repair. Placenta 36:S43–SS9. https://doi.org/10.1016/j.placenta.2015.01.193
Francesconi L, Ceresér K, Mascarenhas R, Stertz L, Gama C et al (2011) Increased annexin-V and decreased TNF-alpha serum levels in chronic-medicated patients with schizophrenia. Neurosci. Lett. 502:143–146. https://doi.org/10.1016/j.neulet.2011.06.0423
Roldán V, Marn F, Pineda J, Marco P, Corral J, Climent V, García A, Martínez JG, Sogorb F (2002) nexina V en pacientes supervivientes de un infarto de miocardio prematuro. Rev Esp Cardiol. 55:1230–1234. https://doi.org/10.1016/S0300-8932(02)76794-812
Lai C, Pc C, Wong S, Kw C, Yf C. (2012). Circulating Annexin A5 levels after atrial switch for transposition of the great arteries.
Mirsaeidi M, Gidfar S, Vu A, Schraufnagel D (2016) Annexins family: insights into their functions and potential role in pathogenesis of sarcoidosis. J Transl Med 14:1–9. https://doi.org/10.1186/s12967-016-0843-71
Kaneko N, Matsuda R, Hosoda S, Kajita T, Ohta Y (1996) Measurement of plasma annexin V by ELISA in the early detection of acute myocardial infarction. Clin. Chim. Acta 251:65–80. https://doi.org/10.1016/0009-8981(96)06294-81
Velázquez RF, Rodríguez M, Navascués CA, Linares A, Pérez R et al (2003) Prospective analysis of risk factors for hepatocellular carcinoma in patients with liver cirrhosis. Hepatology 37:520–527. https://doi.org/10.1053/jhep.2003.500933
Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA (2018) (2018). AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 67:358–380. https://doi.org/10.1002/hep.290861
Kulik L, El-Serag HB (2019) Epidemiology and management of hepatocellular carcinoma. Gastroenterology 156:477-91. e1.https://doi.org/10.1053/j.gastro.2018.08.065. 156(2):477–491.e1
Wang J, Zhang Y, Liu X, Ma J, Liu P et al (2014) Annexin A5 inhibits diffuse large B-cell lymphoma cell invasion and chemoresistance through phosphatidylinositol 3-kinase signaling. Oncol. Rep. 32:2557–2563. https://doi.org/10.3892/or.2014.35476
Peng B, Guo C, Guan H, Liu S, Sun M-Z (2014) Annexin A5 as a potential marker in tumors. Clin. Chim. Acta 427:42–48 https://doi.org/10.1016/j.cca.2013.09.048
Guo W-X, Man X-B, Yuan H-X, Shi J, Xue J, Wu MC, Cheng SQ (2007). Proteomic analysis on portal vein tumor thrombus-associated proteins for hepatocellular carcinoma. Zhonghua yi xue za zhi 87:2094-2097. PMID: 17988525, 30
Sun X, Liu S, Wang J, Wei B, Guo C, Chen C, Sun MZ et al (2018) Annexin A5 regulates hepatocarcinoma malignancy via CRKI/II-DOCK180-RAC1 integrin and MEK-ERK pathways. Cell Death Dis. 9:1–16. https://doi.org/10.1038/s41419-018-0685-86
Zhuang C, Wang P, Sun T, Zheng L, Ming L (2019) Expression levels and prognostic values of annexins in liver cancer. Oncol. Lett. 18:6657–6669 https://doi.org/10.3892/ol.2019.11025
Ravassa S, Bennaghmouch A, Kenis H, Lindhout T, Hackeng T, Narula J, Hofstra L, Reutelingsperger C (2005) Annexin A5 down-regulates surface expression of tissue factor a novel mechanism of regulating the membrane receptor repertoir. J. Biol. Chem. 280:6028–6035. https://doi.org/10.1074/jbc.M4117102007
Baleva M, Hristova M, Nikolov K (2010) Diagnostic significance of anti-annexin-A5 antibody determination. Open Med. 5:6–11. https://doi.org/10.2478/s11536-009-0132-41
Corsten MF, Hofstra L, Narula J, Reutelingsperger CP (2006) Counting heads in the war against cancer: defining the role of annexin A5 imaging in cancer treatment and surveillance. Cancer Res 66(3):1255–1260. https://doi.org/10.1158/0008-5472.CAN-05-3000
Brodsky SV, Facciuto ME, Heydt D, Chen J, Islam HK, et al.(2008). Dynamics of circulating microparticles in liver transplant patients. J Gastrointestin Liver Dis 17:261-268. PMID: 18836617
Rautou PE, Bresson J, Sainte-Marie Y, Vion AC, Paradis V et al (2012) Abnormal plasma microparticles impair vasoconstrictor responses in patients with cirrhosis. Gastroenterology 143:166–76.e6. https://doi.org/10.1053/j.gastro.2012.03.040
Acknowledgements
Great confession of collaboration for all NHTMRI doctors.
Funding
No particular support from publicly supported, financially or non-profit organizations has been granted in this research.
Author information
Authors and Affiliations
Contributions
WS was responsible for the practical section, analysis of data, and manuscript preparation. BE was responsible for the selection and classification of the samples and the manuscript revision. The final manuscript was read and accepted by all contributors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Informative approval from all study participants, with the number 15/2016 of the National Institute of Hepatology and Tropical Medicine Research, was obtained written and validated by the Ethical Committee.
Consent for publication
Not applicable
Competing interests
The authors note that the work reported in this paper was not known for its conflict of financial interests or personal relationships.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Serag, W.M., Elsayed, B.E. Annexin A5 as a marker for hepatocellular carcinoma in cirrhotic hepatitis C virus patients. Egypt Liver Journal 11, 32 (2021). https://doi.org/10.1186/s43066-021-00101-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43066-021-00101-y