Abstract
Background
Hypertension is the most common cardiovascular disease in Nigeria and contributes to a large non-communicable disease burden. Our aim was to implement and evaluate a large-scale hypertension treatment and control program, adapted from the Kaiser Permanent Northern California and World Health Organization HEARTS models, within public primary healthcare centers in the Federal Capital Territory, Nigeria.
Methods
A type 2 hybrid, interrupted time series design was used to generate novel information on large-scale implementation and effectiveness of a multi-level hypertension control program within 60 primary healthcare centers in the Federal Capital Territory, Nigeria. During the formative phase, baseline qualitative assessments were held with patients, health workers, and administrators to inform implementation package adaptation. The package includes a hypertension patient registry with empanelment, performance and quality reporting, simplified treatment guideline emphasizing fixed-dose combination therapy, reliable access to quality essential medicines and technology, team-based care, and health coaching and home blood pressure monitoring. Strategies to implement and adapt the package were identified based on barriers and facilitators mapped in the formative phase, previous implementation experience, mid-term qualitative evaluation, and ongoing stakeholder and site feedback. The control phase included 11 months of sequential registration of hypertensive patients at participating primary healthcare centers, followed by implementation of the remainder of the package components and evaluation over 37 subsequent, consecutive months of the intervention phase.
The formative phase was completed between April 2019 and August 2019, followed by initiation of the control phase in January 2020. The control phase included 11 months (January 2020 to November 2020) of sequential registration and empanelment of hypertensive patients at participating primary healthcare centers. After completion of the control phase in November 2020, the intervention phase commenced in December 2020 and will be completed in December 2023.
Discussion
This trial will provide robust evidence for implementation and effectiveness of a multi-level implementation package more broadly throughout the Federal Capital Territory, which may inform hypertension systems of care throughout Nigeria and in other low- and middle-income countries. Implementation outcome results will be important to understand what system-, site-, personnel-, and patient-level factors are necessary for successful implementation of this intervention.
Trial registration
ClinicalTrials.gov NCT04158154. The trial was prospectively registered on November 8, 2019.
Similar content being viewed by others
Background
In response to the global burden of cardiovascular disease, the World Health Organization (WHO) developed the HEARTS technical package, which is a strategy aimed at assisting ministries of health to manage cardiovascular diseases with a focus on primary health care settings in resource-limited contexts [1]. The WHO HEARTS technical package includes six modules which are informed by the Kaiser Permanente Northern California (KPNC) hypertension control program (Fig. 1) [2]. Several large-scale hypertension programs have adopted or adapted the HEARTS or KPNC models for implementation at the national and sub-national levels [3,4,5,6]. Though intended for use within resource-limited settings, implementation of the WHO HEARTS model within low- and middle-income countries requires systematic formative work to evaluate and address barriers to implementation, as evidenced by efforts of the Pan American Health Organization to introduce the model within 12 countries in the Americas [7].
Alignment of the KPNC and WHO HEARTS technical packages with the Hypertension Treatment in Nigeria Program Implementation Package. The HTN Program implementation package is developed from the Kaiser Permanente Northern California and WHO HEARTS technical packages. Each component of the HTN Program implementation package is developed and deployed at different levels of the health system in the Federal Capital Territory, Nigeria. These levels include the patient, health worker, health clinic, health system, and national policy. All images are from Flaticon.com
Hypertension is the most common cardiovascular disease (CVD) in Nigeria, found in 86.4% of CVD patients, and is prevalent in an estimated 29–38% adult Nigerians [8, 9]. Multiple efforts are underway to address the burden of hypertension in Nigeria by increasing awareness, treatment, and control rates. For example, the National Hypertension Control Initiative (NHCI) is being piloted in 12 facilities in Ogun and Kano states to increase hypertension awareness, diagnosis, treatment, and control [10, 11]. The Hypertension Treatment in Nigeria (HTN) Program described in this report represents separate, yet related work. Both programs aim to lower hypertension-related morbidity and mortality, as well as strengthen hypertension diagnosis and management at the primary healthcare level through team-based care. More robust primary healthcare services will alleviate time and resource burdens on both patients and healthcare providers at secondary and tertiary care centers where hypertensive services have historically been provided in Nigeria.
Our aim was to implement and evaluate a large-scale hypertension treatment and control program within 60 public primary healthcare centers in the Federal Capital Territory, Nigeria through a type 2 hybrid effectiveness-implementation, interrupted time series design with outcomes measured using the RE-AIM framework [12].
Methods/design
The methods described are based on the Standardized Reporting Items: Recommendations for Intervention Trials (SPIRIT) statement and checklist (Additional file 1) [13]. Details of the intervention and implementation strategies are described based on the Standards for Reporting Implementation Studies (StaRI) statement and checklist (Additional file 2) [14].
Study design
The type 2 hybrid design was selected to generate novel information on large-scale implementation and effectiveness of a multi-level hypertension control program adapted from the KPNC intervention and WHO HEARTS technical package within primary healthcare centers in the Federal Capital Territory of Nigeria, and to inform subsequent plans for regional and national scale-up (Fig. 1). The interrupted time series design was selected as a pragmatic trial design which allows for the incorporation of contextual factors and underlying trends in treatment and control of hypertension [15]. Our study design allows for evaluation of health system, community, and individual-level characteristics that may affect the adaptation, effectiveness, and implementation of the HTN Program implementation package. The interrupted time series evaluation is accompanied by formative, interim, and follow-up qualitative assessments to simultaneously evaluate effectiveness and implementation. We chose not to use a randomized trial design given the high-quality evidence for our intervention components. A community health extension worker- (CHEW) led home blood pressure monitoring and health coaching sub-study was also included to evaluate implementation and effectiveness among randomly selected patients with persistently uncontrolled hypertension in 10 primary healthcare centers compared with propensity-matched controls. These individual-level strategies were selected because they have the largest effect sizes based on previous research [16].
Timeline
During the formative phase of the study (April 2019 to August 2019), baseline qualitative assessments (i.e., key informant interviews and focus group discussions) were held with patients, health workers, and administrators to inform adaptation of the implementation package [17]. The control phase of the study included 11 months (January 2020 to November 2020) of sequential registration and empanelment of hypertensive patients at participating primary healthcare centers (Fig. 2). The intervention phase includes implementation of the remainder of the package components and evaluation over the subsequent 37 months (December 2020 to December 2023). Analysis and dissemination of baseline results occurred following conclusion of the control period [18]. Midway through the intervention phase, an additional component of CHEW-led home blood pressure monitoring and health coaching was introduced within a subset of 10 participating primary healthcare centers. Recruitment and follow-up of these patients will be carried out from September 2022 to February 2023.
Timeline for the Hypertension Treatment in Nigeria Program. The HTN Program is implemented over 5 years, including a baseline formative phase, an 11-month control phase, and a 37-month intervention phase. Concurrent to the control and intervention phases, community advocacy and awareness campaigns are conducted within the Federal Capital Territory to increase awareness and demand for services.
Participants
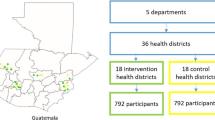
Prior to study initiation, we identified and assessed all public primary healthcare centers within the Federal Capital Territory of Nigeria (N=243) based on staffing, patient volumes, and functionality. To be eligible, primary healthcare centers had to have at least two full-time staff, be accessible to the study team by road and in all seasons, and be functional (i.e., defined as open at least between the hours of 8 AM and 4 PM and providing the minimum service package for primary healthcare centers). Sites were randomly selected through a multi-stage sampling frame including stratification by caseload and geography. Selected primary healthcare centers were invited to participate in the program, and all 60 (100%) of invited sites agreed to participate. Each selected primary healthcare center participated in a hypertension-adapted Service Availability and Readiness Assessment (SARA) based on the WHO instrument prior to initiation of the control phase to evaluate and confirm capacity and readiness for the study [19].
During the control and intervention phases, all adults (≥ 18 years) who presented at a participating site with persistently elevated blood pressure (systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥90 mmHg, measured twice) or a history of hypertension were eligible for inclusion. Patients were registered in the study database through entry in a site-based patient registry log and empaneled in the hypertension cohort (Additional file 3). Informed consent was not collected per the Common Rule [20].
We collected data on paper case report forms, which were designed to be included in the patients’ medical files at each primary healthcare center (Additional file 4). Paper case report forms are completed and maintained by the CHEW at registration and each subsequent patient visit. After the patient visit, a record information officer at the site abstracted information from the patient case report form into the electronic REDCap database [21]. The REDCap database was hosted at the University of Abuja, and each participating primary healthcare center was provided with a tablet upon which the REDCap mobile application was installed. Data capture included, but was not limited to socio-demographics, medical history, anthropometrics, blood pressure values, relevant laboratory studies when available (e.g., serum potassium, serum creatinine), medications, side effects, adverse events, counseling, and patient vital status.
The CHEW-led home blood pressure monitoring and health coaching sub-study will focus on a subset of patients in the HTN Program. This strategy focuses on high-risk adults with uncontrolled hypertension despite participating in facility-based care among 10 selected primary healthcare centers. High-risk patients are defined as patients with elevated blood pressure (systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg), who are 35 years and above and did not complete secondary school, the last characteristic serving as a marker of social disadvantage to prioritize this intervention among individuals with greater relative need for support.
Intervention
The implementation package components were informed by the KPNC intervention toolkit and WHO HEARTS technical package (Fig. 1). To adapt the implementation package, we performed formative in-depth interviews and focus group discussions with patients, healthcare workers, and administrative staff to identify facilitators, barriers, and context to implementation of hypertension services within primary healthcare centers in the Federal Capital Territory, Nigeria [17]. The final implementation package includes a hypertension patient registry (health system level), performance and quality reporting (health clinic level), simplified treatment guidelines (national policy level), fixed-dose combination therapy (health system level), access to essential medicines and technology (health system level), team-based care and non-physician follow-up (health worker level), and health coaching and home blood pressure monitoring (patient level), all of which have demonstrated improved population-level hypertension control in other settings (Table 1) [3, 22, 23].
During the first 11 months (January 2020 through November 2020) of the program, all sites were in the control phase of the program during which time the only implementation package component used was the paper- and electronic-based patient registry. At initiation of the control phase, a 2-day, in-person training was provided to a minimum of two CHEWs from each participating site, which was focused on measurement of blood pressure, diagnosis of hypertension, and record management (Additional file 5) [24]. Pre- and post-workshop tests were conducted to evaluate changes in knowledge and practice, including evaluation of accurate blood pressure measurement. Record officers at each site were also trained through an in-person half-day training on electronic data management.
In December 2020, all sites transitioned to the intervention phase of the program. Healthcare workers participated in a full-day in-person training focused on a simplified treatment guideline following the Nigeria 2019 Hypertension Protocol, appropriate case management and patient treatment, and use of performance and quality reports [10]. The team trained six supervising Pharmacists/Pharmacy Technicians, one from each area council, and one pharmacy focal health worker from each of the 60 HTN primary healthcare centers on drug dispensing and logistics management before commencing the intervention phase. Primary healthcare center personnel were also retrained centrally at periodic intervals as well as quarterly, on-site supervision visits. Training was informed by feedback from audit and supervision reports. Moreover, all other full-time staff of the participating primary healthcare centers who were not among the healthcare workers initially selected for HTN Program were trained to build their capacity and to assist in the Program based on future needs. All training provided to staff at the participating primary healthcare centers was facilitated by supervisors who are members of the study team at University of Abuja Teaching Hospital.
In November 2021, a 2-day in-person training was provided to 30 CHEWs and 10 facility managers from 10 participating sites on how to carry out the home blood pressure and health coaching sub-study. The training focused on health coaching and motivational interviewing, screening consent at the participating sites, medication management, behavioral change related to hypertension, accurately measuring clinic and home blood pressure, health coaching visits, and role-playing. Pre-and post-training tests were conducted with CHEWs to understand changes in current knowledge and experience in health coaching and blood pressure monitoring. A second training session in April 2022 served as a refresher.
Implementation strategies
Strategies to implement the package were identified based on barriers and facilitators mapping during the formative phase, previous implementation experience, mid-term qualitative evaluation, and ongoing stakeholder and site feedback (Table 2).
Community awareness campaign
A community awareness campaign was prospectively planned to accompany both baseline and intervention phases of the HTN Program. The purpose of the community awareness campaigns was to address misconceptions about hypertension, increase awareness at the community level, and to generate uptake of treatment of hypertension at the primary healthcare level. The campaign is led by members of the study team and implemented by health educators who are part of the primary healthcare workforce and local residents of the communities in which the campaigns are held. Within each area council, campaigns are held and reviewed quarterly.
Outcomes
Primary outcomes
The study’s co-primary implementation outcomes include reach, effectiveness, adoption, implementation, maintenance, acceptability, and cost of the multi-level hypertension control program. The study's co-primary effectiveness outcomes are the change in slope from baseline slope of monthly hypertension treatment rates (defined as using any blood pressure-lowering drug) and monthly hypertension control rates (defined as systolic blood pressure <140 mmHg and diastolic blood pressure <90 mm Hg) among participating primary healthcare centers. We chose this definition of control based on the 2019 Nigeria Hypertension Protocol.
Secondary effectiveness
The study’s secondary effectiveness outcomes include (1) mean systolic blood pressure and diastolic blood pressure among eligible clinic patients with hypertension, and (2) rates of single versus two- or three-drug blood pressure-lowering medication use (including use of fixed-dose combination therapies).
Safety
The study’s safety outcomes include (1) proportion of participants with any potentially relevant side effect, including provider diagnosis of angioedema, acute kidney injury (defined as relative increase in serum creatinine by 50% or an absolute increase by 0.3 mg/dl [>0.26 μmol/L]), electrolyte abnormalities (defined as serum potassium < 3.5 or >5.5 mEq/L or serum sodium <125 or > 145 mEq/L), syncope, or dizziness, (2) rate of relevant side effects at the participant level (i.e., count per participant), and (3) proportion of participants with any serious adverse event according to Good Clinical Practice definition.
Implementation
Implementation outcomes focus on domains of the RE-AIM framework including at the program, center, and individual level (Table 3).
Sample size
To estimate our sample size calculated for the primary effectiveness outcome, we used data from published literature to estimate the baseline prevalence of hypertension treatment (20%) and control (10%) rates in the Federal Capital Territory, Nigeria [25]. We based the anticipated effect on similar programs, including the KPNC Hypertension Control Program [2]. The power and sample size were estimated under a variety of conditions through Monte-Carlo simulation in SAS (v9.4, SAS Inc, Cary, North Carolina). Accounting for 10 sites dropping out, we estimated that 1200 patient visits per month across 50 sites over 9 months of baseline and 39 months of intervention would provide >80% power to detect a difference in treatment slope of 0.57% per month compared to underlying trend of 0.10% per month, resulting in hypertension treatment rate of 42.5% at the end of 48 months. Similarly, with the same sample size, we would have >80% power to detect a difference in control slope of 0.44% per month compared to underlying trend of 0.05% per month, resulting in hypertension control rate of 27.0% at the end of 48 months.
Data quality assurance and control
To ensure data quality, we built in field validation(s) and branching logic within the REDCap forms. Before obtaining data entry rights, all study team members and healthcare workers with data access privileges completed training and pilot tested data entry using hypothetical data. Centralized statistical monitoring are performed through weekly data and status quality reports. The data and status quality reports use the REDCap application programming interface functionality to export the program data, and then are restructured and summarized using statistical software packages, including R (v4.0.3, R Core Team, Vienna, Austria) and SAS (v9.4, SAS Inc., Cary, NC). The reports are reviewed and discussed bi-weekly. Because the systems are in place and code has been generated, the reports can be updated in real time.
Statistical analyses
All participants who are enrolled in the patient registry will be accounted for, in accordance with the consolidated standards for reporting trials (CONSORT) statement for transparent reporting of trials (Fig. 3; Additional file 6). As of May 2022, more than 16,000 individuals participated in the Program. Baseline demographic variables, such as age, sex, and relevant clinical variables will be summarized when results are reported. Summaries of continuous baseline variables will be presented as means and standard deviations or medians with minimum and maximum values as appropriate and based on distribution. Categorical variables will be described as frequencies and percentages.
The statistical analysis will be performed using the intention to treat principle (i.e., all patient visits recorded in the database will be included and considered exposed to the intervention or unexposed according to the overall program timeline, regardless of when the intervention was actually implemented). The statistical design of the program intervention includes evaluation of effectiveness and implementation co-primary outcomes, which is consistent with the type 2 hybrid design. For implementation outcomes, we will use quantitative and qualitative analysis using the RE-AIM framework to triangulate routinely collected data to evaluate the reach, secondary effectiveness, adoption, implementation, maintenance, acceptability, and cost of the implementation package [12]. We will seek to explore and explain variability across key subgroups, including age, sex, and geography.
For effectiveness outcomes, we will use an interrupted time series evaluation with aggregate measures of system-level monthly treatment and control rates to evaluate temporal trends. We will evaluate change in temporal trends for primary and secondary outcomes through segmented linear regression by aggregating data across primary healthcare centers by month. To evaluate effects of each implementation package component and time variance in implementation on our primary outcomes, we will use patient-level mixed-effects models including random effects to account for clustering at the primary healthcare center level. We will perform sensitivity analyses by both excluding and restricting repeated measures of the same patients over the program period.
For the home blood pressure and health coaching sub-study, we will use analysis of covariance, controlling for baseline clinic-measured systolic blood pressure and medication to estimate the effect of a community health extension worker-led health coaching and home blood pressure monitoring on a 12-week change in systolic blood pressure compared with propensity-matched patients in the participating sites as controls. For implementation outcomes, we will adapt the RE-AIM framework and use quantitative data to evaluate the reach, effectiveness, adoption, implementation, maintenance, and cost of the interventions.
Subgroup analyses
Subgroup analyses will examine primary outcomes within and across sites based on staffing levels, staff training, geography, and drug availability. Exploratory subgroup analyses will similarly examine primary outcomes based on age, sex, duration of registration, and treatment regimen.
Interim analyses
No interim quantitative analyses for effectiveness are planned for this study. Interim qualitative analyses were performed in November 2021 to determine what adaptations should be made to the varied implementation strategies. Mid-term qualitative interviews were conducted in November 2021 to evaluate the implementation outcomes of the HTN Program using the RE-AIM framework. Seven focus group discussions were conducted with CHEWS or community health officers (n=3), facility managers (n=2), and primary care physicians (n=1). Rapid synthesis indicated that even though the HTN Program has been able to reach many patients, the Program reach is not equitable, with men and younger patients being less likely to participate than women and older patients [26, 27]. Additional community mobilization was suggested as a strategy to increase reach.
Regarding effectiveness, there was a general perception that the HTN Program strategies have been effective, especially with patient registration and empanelment, team-based care, and emphasis of fixed-dose combination therapy. Implementation of patient registration and empanelment, team-based care, and fixed-dose combination therapy has been feasible. However, achieving a high rate of monthly follow-up visits have been more challenging not only because of inherent challenges in retention and seasonal transportation issues, but also CHEW’s workload and security threats in some communities.
Regarding maintenance, there were serious concerns about the transition from freely available drugs to a Drug Revolving Fund-based system that emphasizes access through reduced out-of-pocket costs. Some participants mentioned that this may affect enrollment and retention.
Missing data
Missing data are identified through bi-weekly data quality reports and resolved with the responsible site where feasible. Complete case analyses will be performed in the primary analysis, and multiple imputation will be used for sensitivity analyses to explore the robustness of results.
Monitoring
We have performed central statistical monitoring to evaluate sites’ performance and to direct risk-based, in-person, site monitoring and supportive supervision visits. In-person site monitoring visits have been conducted at minimum every 6 months with a target for quarterly visits for most sites. In-person visits are performed by at least two members of the HTN Program study team. Supportive supervision visits follow a checklist (Additional file 7) including quantification of site staff, inventory of equipment, observation of blood pressure measurement, and audit of patient paper case report forms. All site monitoring data are entered within a central REDCap database and summarized on a monthly basis to identify data quality problems, urgent staff or equipment needs, or other major data status or quality trends.
Context tracker
A context tracker was developed for simultaneous capture of individual-, site-, regional-, national-, and global-level events that may affect implementation and effectiveness of the trial. The context tracker is maintained by two members of the study team and discussed monthly during operations team meetings. Contextual factors will be considered in sensitivity analyses to triangulate routinely collected data to evaluate the reach, secondary effectiveness, adoption, implementation, maintenance, acceptability, and cost of the implementation package.
Discussion
The HTN Program aims to evaluate the effectiveness and implementation of hypertension services within primary healthcare centers in the Federal Capital Territory, Nigeria. The type 2 hybrid, interrupted time series design was employed because of several important features. First, simultaneous evaluation of implementation and effectiveness will generate information to inform adaptation and scaling of the intervention. Second, the interrupted time series design provides evidence for causal inference in evaluating the potential effects of an intervention compared with a pre-/post-intervention study design. Third, the design allows for evaluation of temporal trends, adaptation of implementation strategies, and assessment of the effects of contextual factors. Fourth, the interrupted time series design allows all primary healthcare centers to receive the intervention, which is important when the intervention is deemed likely to be beneficial. This design helps to avoid potential ethical concerns about sites being randomized to the control arm of a trial throughout the entire study period while having logistical advantages over a stepped wedge trial design given the large number of sites involved and perceived risk of site dropout, which was not realized.
However, the type 2 hybrid interrupted time series design also includes important limitations. We assumed a linear slope change effect between the control and intervention phases; however, this assumption may not be met in practice, limiting our ability to draw conclusions about the effectiveness of the intervention. The primary analysis of the HTN Program will employ segmented linear regression of aggregated measures of treatment and control at the system level, which does not account for imprecision at an individual patient or site level.
If successful in achieving its co-primary effectiveness outcomes, then this trial will provide robust evidence for implementation and effectiveness of this multi-level implementation package more broadly throughout the Federal Capital Territory, which may inform hypertension systems of care throughout Nigeria and potentially in other low- and middle-income countries. Data from our implementation outcomes will be important to understand what system-, site-, and personnel-level factors are necessary for successful implementation of this intervention.
Availability of data and materials
The datasets generated and/or analyzed during the current study will be available in the NHLBI BioLINCC repository, https://biolincc.nhlbi.nih.gov/home/ after completion of the study.
Abbreviations
- CHEW:
-
Community health extension worker
- CONSORT:
-
Consolidated Standards for Reporting Trials
- CVD:
-
Cardiovascular disease
- DSQR:
-
Data Status Quality Report
- HTN:
-
Hypertension Treatment in Nigeria
- NHCI:
-
National Hypertension Control Initiative
- KPNC:
-
Kaiser Permanent Northern California
- REDCap:
-
Research Electronic Data Capture
- SPIRIT:
-
Standardized Reporting Items: Recommendations for Intervention Trials
- StaRI:
-
Standards for Reporting Implementation Studies
- WHO:
-
World Health Organization
References
HEARTS technical package. Geneva: World Health Organization. https://www.who.int/publications/i/item/hearts-technical-package. Accessed 23 Feb 2022.
Jaffe MG, Lee GA, Young JD, Sidney S, Go AS. Improved blood pressure control associated with a large-scale hypertension program. JAMA. 2013;310(7):699–705.
Cazabon D, Farrell M, Gupta R, Joseph L, Pathni AK, Sahoo S, et al. A simple six-step guide to National-Scale Hypertension Control Program implementation. J Hum Hypertens. 2022;36:591–603. https://doi.org/10.1038/s41371-021-00612-6.
Fontil V, Gupta R, Moise N, Chen E, Guzman D, McCulloch CE, et al. Adapting and evaluating a health system intervention from Kaiser Permanente to improve hypertension management and control in a large network of safety-net clinics. Circ Cardiovasc Qual Outcomes. 2018;11(7):e004386.
Kaur P, Kunwar A, Sharma M, Mitra J, Das C, Swasticharan L, et al. India Hypertension Control Initiative-Hypertension treatment and blood pressure control in a cohort in 24 sentinel site clinics. J Clin Hypertens (Greenwich). 2021;23(4):720–9.
Valdes Gonzalez Y, Campbell NRC, Pons Barrera E, Calderon Martinez M, Perez Carrera A, Morales Rigau JM, et al. Implementation of a community-based hypertension control program in Matanzas, Cuba. J Clin Hypertens (Greenwich). 2020;22(2):142–9.
Giraldo GP, Joseph KT, Angell SY, Campbell NRC, Connell K, DiPette DJ, et al. Mapping stages, barriers and facilitators to the implementation of HEARTS in the Americas initiative in 12 countries: a qualitative study. J Clin Hypertens (Greenwich). 2021;23(4):755–65.
Adeloye D, Owolabi EO, Ojji DB, Auta A, Dewan MT, Olanrewaju TO, et al. Prevalence, awareness, treatment, and control of hypertension in Nigeria in 1995 and 2020: a systematic analysis of current evidence. J Clin Hypertens (Greenwich). 2021;23(5):963–77.
Nelson IO. Management of hypertension in Nigeria: the barriers and challenges. J Cardiol Cardiovasc Med. 2021;6(1):023–5.
Nigeria collaborates with WHO to curb hypertension, introduces control initiative. Geneva: World Health Organization. Published: 9 December 2020. Nigeria collaborates with WHO to curb hypertension, introduces control initiative | WHO | Regional Office for Africa.
New program announced to lower high blood pressure in unreached communities across. Nigeria New York, USA. Resolve to Save Lives. Published: 30 November 2020. https://resolvetosavelives.org/about/press/new-program-announcedto-lower-high-blood-pressure-in-unreached-communities-across-nigeria. Accessed 23 Feb 2022.
Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89(9):1322–7.
Chan AW, Tetzlaff JM, Gotzsche PC, Altman DG, Mann H, Berlin JA, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586.
Pinnock H, Barwick M, Carpenter CR, Eldridge S, Grandes G, Griffiths CJ, et al. Standards for reporting implementation studies (StaRI) statement. BMJ. 2017;356:i6795.
Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2017;46(1):348–55.
Mills KT, Obst KM, Shen W, Molina S, Zhang HJ, He H, et al. Comparative effectiveness of implementation strategies for blood pressure control in hypertensive patients: a systematic review and meta-analysis. Ann Intern Med. 2018;168(2):110–20.
Okoli RCB, Shedul G, Hirschhorn LR, Orji IA, Ojo TM, Egenti N, et al. Stakeholder perspectives to inform adaptation of a hypertension treatment program in primary healthcare centers in the Federal Capital Territory, Nigeria: a qualitative study. Implement Sci Commun. 2021;2(1):97.
Ojji DB, Baldridge AS, Orji IA, Shedul GL, Ojo TM, Ye J, et al. Characteristics, treatment, and control of hypertension in public primary healthcare centers in Nigeria: baseline results from the Hypertension Treatment in Nigeria Program. J Hypertens. 2022;40(5):888–96.
Orji IA, Baldridge AS, Omitiran K, Guo M, Ajisegiri WS, Ojo TM, et al. Capacity and site readiness for hypertension control program implementation in the Federal Capital Territory of Nigeria: a cross-sectional study. BMC Health Serv Res. 2021;21(1):322.
Part 46 - Protection of Human Subjects Code of Federal Regulations. 2022. https://www.ecfr.gov/current/title-45/subtitle-A/subchapter-A/part-46. Accessed 10 Mar 2022.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81.
Guideline for the pharmacological treatment of hypertension in adults. Geneva: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGOI accessed this online at: https://apps.who.int/iris/bitstream/handle/10665/344424/9789240033986-eng.pdf. Accessed 23 Feb 2022.
Improving hypertension control in 3 million people: country experiences of programme development and implementation. Geneva: World Health Organization; 2020. Licence: CC BY-NC-SA 3.0 IGO.I accessed this online at: https://apps.who.int/iris/handle/10665/336019.
Hypertension management training curriculum. Atlanta: Centers for Disease Control and Prevention. Available from: https://www.cdc.gov/globalhealth/healthprotection/ncd/training/hypertension-management-training.html. Accessed 10 Mar 2022.
Ogah OS, Okpechi I, Chukwuonye II, Akinyemi JO, Onwubere BJ, Falase AO, et al. Blood pressure, prevalence of hypertension and hypertension related complications in Nigerian Africans: a review. World J Cardiol. 2012;4(12):327–40.
Beebe J. Rapid qualitative inquiry : a field guide to team-based assessment. 2nd ed. Lanham: Rowman & Littlefield; 2014.
Gale RC, Wu J, Erhardt T, Bounthavong M, Reardon CM, Damschroder LJ, et al. Comparison of rapid vs in-depth qualitative analytic methods from a process evaluation of academic detailing in the Veterans Health Administration. Implement Sci. 2019;14(1):11.
Acknowledgements
We acknowledge the patients and teams at each of the 60 participating public primary health centers. We would like to thank members of the advisory board who contributed to the planning and execution of this study including Dr. Mangai Toma, Mrs. Chiamaka Omoyele, Dr. Nina Ezeigwe, Dr. Ndaeyo Iwot, Dr. Josephine Okechukwu, Dr. Mary Dewan, Mr. Michael Uwazie, Dr. Sunday Goji, PharmD Innocent Uche Nnubia, Dr. Ibrahim Katibi, and Dr. Emmanuel Agogo. We would like to thank members of the Data and Safety Monitoring Board who have overseen the conduct of this study, including Dr. Brian Rayner (chair), Dr. Amam Mbakwem, Dr. Justine Davies, Dr. James Sheppard, Dr. Patricia Ojiah, Dr. Amanda Thrift, and Dr. Adeloye Davies.
Funding
The study is supported by the National Heart, Lung, and Blood Institute (R01HL144708), Northwestern Robert J. Havey MD Institute for Global Health, and Resolve to Save Lives. The funders were not involved in the development of the study design; collection, management, analysis, and interpretation of data; writing of the report; and the decision to submit the report for publication. The program officer representing the National Heart, Lung, and Blood Institute participated in Data and Safety Monitoring Board activities. The Northwestern Robert J. Havey MD Institute for Global Health and Resolve to Save Lives contributed support for projects embedded within the overall Program.
Author information
Authors and Affiliations
Contributions
MDH and DBO conceptualized and obtained funding for the study. MDH, DBO, LRH, PT, NRK, and ASB oversaw project administration. KO, IAO, GLS, TMO, HE, GS, ENU, NBE, RCBO, AN, and DBO conducted the investigation, supervised lay health workers, and provided resources. ASB, AC, SO, JY, and BMA oversaw data curation and conducted formal analyses. OAS conducted and analyzed qualitative analyses. ASB wrote the original draft. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Informed consent was waived per the Common Rule. Ethical oversight was provided by the University of Abuja Teaching Hospital Health Research Ethics Committee. The study was also reviewed by the Federal Capital Territory Ethics Committee and Northwestern University Institutional Review Board. The trial was prospectively registered at www.clinicaltrials.gov under NCT04158154 on November 8, 2019; https://clinicaltrials.gov/ct2/show/NCT04158154.
Consent for publication
Not applicable.
Competing interests
MDH has pending patents for heart failure polypills. George Health Enterprises Pty Ltd (GH) and its subsidiary, George Medicines Pty Ltd, have received investment funds to develop fixed-dose combination products, including combinations of blood pressure-lowering drugs. GH is the social enterprise arm of The George Institute for Global Health.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
SPIRIT Checklist. Completed SPIRIT checklist for the HTN Program protocol manuscript.
Additional file 2.
StaRI Checklist. Completed StaRI checklist for the HTN Program protocol manuscript.
Additional file 3.
HTN Program Registry Log. Template of the site-based HTN Program registry logbook.
Additional file 4.
HTN Program Case Report Form. Template of the site-based paper case report form for the HTN Program which is included in each patients’ medical records.
Additional file 5.
HTN Program Training. Detailed listing of training provided to HTN Program community health extension workers, community health officers, record officers, pharmacists, and other healthcare workers.
Additional file 6.
HTN Program Statistical Analysis Plan. Statistical analysis plan for the HTN Program.
Additional file 7.
HTN Program Supervision Checklist. Checklist used by members of the HTN Program implementation team during usual supervision visits to participating sites.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Baldridge, A.S., Aluka-Omitiran, K., Orji, I.A. et al. Hypertension Treatment in Nigeria (HTN) Program: rationale and design for a type 2 hybrid, effectiveness, and implementation interrupted time series trial. Implement Sci Commun 3, 84 (2022). https://doi.org/10.1186/s43058-022-00328-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43058-022-00328-9