Abstract
Background
The debate about the optimal approach for aortic valve replacement continues. We compared the hospital and long-term outcomes (survival, aortic valve reintervention, heart failure readmissions, and stroke) between transcatheter vs. surgical (TAVR vs. SAVR) aortic valve replacement. The study included 789 patients; 293 had isolated SAVR, and 496 had isolated TAVR. Patients with concomitant procedures were excluded. Propensity score matching identified 53 matched pairs.
Results
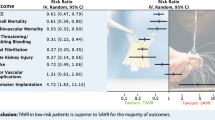
Patients who had TAVR were significantly older (P ˂ 0.001) and had significantly higher EuroSCORE II (P ˂ 0.001), NYHA class (P ˂ 0.001), and more prevalence of diabetes mellitus (P ˂ 0.001), hypertension (P ˂ 0.001), chronic lung disease (P = 0.001), recent myocardial infarction (P = 0.002), and heart failure (P ˂ 0.001), stroke (P = 0.02), atrial fibrillation (P = 0.004), and previous percutaneous coronary interventions (P ˂ 0.001) than SAVR patients. In the matched cohort, atrial fibrillation occurred more frequently after SAVR (P = 0.01), and hospital stay was significantly longer in SAVR patients (P ˂ 0.001). There were no differences in hospital mortality between groups (P ˃ 0.99). Survival at 1, 3, and 5 years was 97%, 95%, and 94% for SAVR and 91%, 79%, and 58% for TAVR patients. Survival was lower in TAVR patients before matching (P ˂ 0.001) and after matching (P = 0.045). Freedom from the composite endpoint of stroke, aortic valve reintervention, and heart failure readmission at 1, 3, and 5 years was 98.9%, 96%, and 94% for SAVR and 94%, 86%, and 75% for TAVR. The composite endpoint was significantly higher in the TAVR group than in SVR before matching (P ˂ 0.001), while there was no difference after matching (P = 0.07). There was no significant difference in the change in ejection fraction between groups (β: −0.88 (95% CI: −2.20–0.43), P = 0.19), and the reduction of the aortic valve peak gradient was significantly higher with TAVR (β: −7.80 (95% CI: −10.70 to −4.91); P ˂ 0.001).
Conclusions
TAVR could reduce postoperative atrial fibrillation and hospital stay. SAVR could have long-term survival benefits over TAVR with comparable long-term stroke, heart failure readmission, and aortic valve reinterventions between SAVR and TAVR.
Similar content being viewed by others
Background
The debate about the optimal approach for aortic valve replacement continues [1]. Transcatheter aortic valve replacement (TAVR) has emerged as an alternative to surgical aortic valve replacement (SAVR) in high-risk patients with severe aortic stenosis (AS) [2]. PARTNER 1 trial investigated the role of TAVR in high-risk patients and reported comparable outcomes to SAVR over a 5-year follow-up [3]. Furthermore, TAVR demonstrated non-inferiority outcomes compared to SAVR in intermediate-risk patients in PARTNER 2 [4] and SURTAVI trials [5]. Recently, TAVR showed lower stroke, rehospitalization, and death rates than SAVR in low-risk patients [6, 7].
Despite the expanding indications of TAVR, several unresolved issues remain. SAVR remains the standard approach in low-risk young patients, and the role of TAVR in this subset of patients is still debatable [8]. Additionally, there is no consensus if low ejection fraction could be an indication for TAVR [9], and the durability of TAVR prostheses could not be equivalent to SAVR bioprosthetic valves [10]. Most studies included patients with aortic stenosis, and the evidence about the role of TAVR in patients with severe aortic regurgitation is limited [11].
These knowledge gaps in managing patients with aortic valve disease with either SAVR or TAVR came from the strict inclusion criteria of the clinical trials with limited generalizability of the results, in addition to the inconsistency between the results of clinical trials and observational studies [12]. Therefore, we compared the hospital outcomes and long-term survival, aortic valve reintervention, heart failure readmissions, and stroke between patients who had TAVR vs. SAVR in our institution.
Methods
Design and patients
A retrospective cohort study was conducted on 789 patients who had SAVR or TAVR in a single center. SAVR was performed from 2002 to 2019 (n = 293) and TAVR from 2009 to 2019 (n = 496). We included patients who had isolated SAVR or TAVR only. Patients with a concomitant valve or coronary procedure (coronary artery bypass grafting or percutaneous coronary interventions) were excluded. Additionally, we excluded patients who had aborted TAVR before valve implantation. Patients who had SAVR were compared to TAVR patients. The patients were allocated to each intervention after 2009 based on the heart team discussion. The ethical committee approved the data collection for this study (reference no: R19021), and the need for the patient’s consent to participate in the research was waived because of the retrospective design.
Data and outcome
We collected preoperative demographics [age, gender, body mass index (BMI)], comorbidities (diabetes mellitus, hypertension, myocardial infarction (MI), recent heart failure (HF) within 2 weeks, chronic lung disease, stroke, atrial fibrillation), and laboratory results (creatinine clearance and hemoglobin). The patient’s functional status was assessed using New York Heart Association (NYHA) dyspnea class, and surgical risk stratification was stratified using EuroSCORE II. The latest echocardiography before intervention was reviewed, and the following data were recorded, ejection fraction (EF), left ventricular end-diastolic diameter (LVEDD), left ventricular end-systolic diameter (LVESD), peak aortic valve pressure gradient, indexed left ventricular mass, and the degree of aortic regurgitation (AR) or stenosis (AS). Preoperative data were collected according to the EuroSCORE II definition [13].
Postprocedural outcomes were the need for a permanent pacemaker, new-onset atrial fibrillation, periprocedural MI, ICU and hospital stay duration, and hospital mortality. The primary outcomes were long-term survival and the composite endpoint of stroke, aortic valve reintervention, and heart failure rehospitalization.
The types of valves used for TAVR were Sapien (Edwards Lifesciences, Irvine, CA, USA) and CoreValve (Medtronic, Inc, Minneapolis, MN, USA). Mechanical valves used in our study were St. Jude mechanical valves (SJM; St. Jude Medical Inc.; Minneapolis, MN, USA), ATS (Medtronic; Minneapolis, MN, USA), Carbomedics Sorin (LivaNova PLC, Sorin, Sluggia), and On-X (CryoLife, Kennesaw, GA, USA). Tissue valves were Magna valves (Edwards Lifesciences, Irvine, CA, USA), Trifecta (Abbott, Plymouth, MN, USA), perceval sutureless valves (LivaNova Group, Milan, Italy), and Hancock (Medtronic Inc; Minneapolis, MN, USA). All patients had TAVR through the transfemoral approach.
Echocardiographic follow-up
There were 2792 echocardiographic studies available for follow-up. Ejection fraction and the peak aortic valve pressure gradient were collected over time and compared between SAVR and TAVR patients. The vital status of the patients was confirmed in June 2021.
Statistical analysis
Data were presented as mean (standard deviation) or median (25th–75th percentiles) for continuous variables and frequencies and percentages for categorical variables. A comparison was performed using the t-test for normally distributed continuous data or the Mann-Whitney test for non-normal data. The chi-squared test or Fisher exact test was used with categorical data whenever appropriate.
Propensity score matching was performed with a 1:1 match using the nearest neighbor match with a caliber of 0.4 and the logit model (Fig. 1 Supplement). An absolute bias of 20% was considered satisfactory, and the standardized mean difference was used to compare the matched variables (Fig. 2 Supplement). The propensity score identified 53 matched pairs. Post-match comparison of continuous variables was performed using the paired t-test or Wilcoxon signed-ranked test for matched pairs. McNemar was used to compare matched categorical variables.
Time-to-event data were plotted using the Kaplan-Meier curve and compared with the log-rank test before the match and Cox regression with clustered robust standard error for matched data. Repeated measure analysis was performed using random effect regression before matching and random effect regression after matching with robust clustered standard error. Competing risk regression was performed using the Fine and Gray method for the composite endpoint in the presence of death as a competing factor. All analyses were performed using Stata 17 (Stata Corp, College Station, TX, USA).
Results
Preprocedural data
Patients who had TAVR were significantly older, with more prevalence of female gender, and had a higher BMI. TAVR patients had significantly higher EuroSCORE II, NYHA class, and more prevalence of diabetes mellitus, hypertension, chronic lung disease, recent MI and HF, stroke, atrial fibrillation, and previous percutaneous coronary interventions (PCI) than SAVR patients. Hemoglobin levels and creatinine clearance were significantly lower in TAVR than in SAVR patients. EF was significantly lower in TAVR patients; meanwhile, the left ventricular dimensions and mass were higher in SAVR patients. Aortic stenosis was more prevalent in TAVR patients, while aortic regurgitation was more in SAVR patients. All patients in the TAVR group had aortic stenosis with or without regurgitation. After matching, there was no difference in preoperative patients’ characteristics, comorbidities, laboratory, and echocardiographic data. No matching was performed on the degree of aortic stenosis or regurgitation, as these factors were mixed pathologies (Table 1).
Procedural data
The valve size was significantly bigger in TAVR than in SAVR patients. Cardiopulmonary bypass time was 96 (79–120) min, and ischemic time was 76 (61–97) min. In the SAVR group, tissue valves were used in 153 patients (52.22%) and mechanical valves in 140 patients (47.78%). Eight patients had emergency TAVR for critical aortic stenosis with hemodynamic compromise.
Hospital outcomes
In the matched cohort, permanent pacemaker insertion (PPM) was required more in TAVR patients but did not reach a significant level. Atrial fibrillation occurred more frequently after SAVR, and hospital stay was significantly longer in SAVR than in TAVR patients. There were no differences between the groups regarding postoperative creatinine levels, postprocedural MI, ICU stay, and hospital mortality. Bleeding requiring exploration occurred in 10 (3.58%) in the SAVR group, and bleeding at the access site occurred in 84 (16.94%) patients in the TAVR group (Table 2).
Long-term follow-up
The median follow-up was 24 months (25th–75th percentiles: 6–56 months). Survival at 1, 3, and 5 years was 97%, 95%, and 94% for SAVR and 91%, 79%, and 58% for TAVR patients. Survival was lower in TAVR patients before matching (P ˂ 0.001) and after matching (HR: 6.37 (95% CI: 1.04–39.07); P = 0.045) (Fig. 1 A and B).
Stroke occurred in 11 patients in the SAVR group and 42 patients in the TAVR group and heart failure readmission in 5 vs. 33 patients in the SAVR vs. TAVR group, respectively. Aortic valve reintervention occurred in 20 (16 redo SAVR and 4 valve in valve) vs. 4 (2 SAVR vs. 2 valve in valve) patients in SAVR vs. TAVR groups, respectively. Freedom from the composite endpoint of stroke, aortic valve reintervention, and heart failure readmission at 1, 3, and 5 years was 98.9%, 96%, and 94% for SAVR and 94%, 86%, and 75% for TAVR. The composite endpoint was significantly higher in the TAVR group than SVR before matching (P ˂ 0.001), while there was no difference after matching (HR: 5.04 (95% CI: 0.90–28.41); P = 0.07) (Fig. 2 A and B). Competing risk analysis showed no differences between groups in the composite endpoint in the presence of death as a competing factor (subdistribution HR: 1.43 (95% CI: 0.91–2.25); P = 0.119).
The 5-year incidence of grade 2 or higher paravalvular leak was higher with TAVR (6 (2.32%) vs. 21 (11.23%); P ˂ 0.001). There was no significant difference in the change in EF between groups (β: −0.88 (95% CI: −2.20–0.43), P = 0.19), and the EF increased significantly over time (β: 0.03 (0.02–0.05); P ˂ 0.001). The improvement in EF started immediately post-procedure in TAVR patients and after 6 months after SAVR (Fig. 3). There was a significant reduction of peak AV gradient post procedure (β: −0.53 (95% CI: −0.61–0.46); P ˂ 0.001), and the reduction was significantly higher with TAVR (β: −7.80 (95% CI: −10.70 to −4.91); P ˂ 0.001) (Fig. 4).
Discussion
Preprocedural characteristics
The optimal approach for managing aortic valve disease is still a hot topic, with continuous updates on recommendations and guidelines [14, 15]. In this study, we presented our experience in TAVR and compared hospital and long-term outcomes between SAVR and TAVR. The baseline characteristics of TAVR patients varied widely from those who had SAVR. TAVR patients were significantly older than SAVR patients. This finding is expected since the value of TAVR in young patients has not been established yet [8]. The age of our TAVR patients is comparable to those in PARTNER trials [2, 4, 6]. This fundamental difference in age is attributed to SAVR being the standard of care in young patients. Those patients will benefit from having mechanical valves to decrease the odds of aortic valve reoperations [16]. Consequently, TAVR patients had higher EuroSCORE II and more prevalence of comorbidities. Patients were recruited until 2019, and the evidence to use TAVR in low-risk patients was introduced recently [7], and high-risk surgical patients are assigned to TAVR.
Gender distribution significantly differed between TAVR and SAVR, and female patients were significantly higher in the TAVR group. Calcific aortic stenosis has no sex predilection; however, rheumatic and degenerative aortic valve diseases are more common in females [17]. The high prevalence of females who underwent TAVR in our series could be attributed to the surgical risk in females. Surgery in women is more demanding, and the incidence of stroke and operative mortality after SAVR was higher in women compared to men [18,19,20]. Additionally, the PARTNER trial showed that women randomized to TAVR had better survival compared to those randomized to SAVR [2].
Patients with aortic regurgitation were more in the SAVR group. This finding is because most TAVR studies were performed on aortic stenosis, and the aortic dilatation in patients with regurgitation may not be suitable for TAVR. Alharbi and associates evaluated the results of TAVR in patients with pure aortic regurgitation, and there was no difference in hospital mortality between TAVR and SAVR [11].
Procedural outcomes
In our series, postoperative pacemaker insertion was higher in TAVR patients. This difference was significant in the unmatched cohort and non-significant after matching. Heart block after TAVR is one of the most encountered complications [21]. Several mechanisms could contribute to this, including the proximity to the conduction system and the nature of the expanding valves [22]. There was no difference in the postoperative PPM between groups after matching, which could be related to using sutureless valves in the SAVR group. On the other hand, new-onset atrial fibrillation was significantly higher in the SAVR group, which is similar to other series [23]. Therefore, TAVR could be recommended in high-risk patients for atrial fibrillation. The paravalvular leak was extensively studied after TAVR, and its incidence ranged from 7 to 40% due to various definitions and grading used in different studies [24]. Paravalvular leak reported in our series was 11% in a 5-year follow-up.
Hospital mortality did not differ significantly between TAVR and SAVR; however, long-term survival was better in SAVR patients. In a meta-analysis by Swift and colleagues, the risk of all-cause mortality was increased after TAVR but did not reach a significant level [1]. Meanwhile, Siontis and colleagues reported a reduced risk of death with TAVR, and the survival benefit was evident in patients who had transfemoral TAVR [25]. However, this difference was not evident over longer follow-up periods.
We reported no significant difference between groups in the composite endpoint of stroke, aortic valve reintervention, and heart failure rehospitalization. Swift and colleagues reported a decreased risk of stroke with TAVR but did not reach a significant level [1]. PARTNER 3 trial reported a reduced cardiac rehospitalization rate after TAVR [26]. Few studies evaluated aortic valve reintervention after TAVR; however, the durability of TAVR valves was comparable to SAVR valves after 5 and 6 years [27, 28].
Additionally, we evaluated the long-term echocardiographic follow-up in our series, specifically the change in EF and peak aortic valve pressure gradient. TAVR and SAVR were associated with improvement in ejection fraction, while the pattern was different. TAVR was associated with an immediate improvement in the EF, while the effect of SAVR on ejection fraction was evident after six months. The peak gradient was significantly lower in TAVR, which could be attributed to the larger valves used in TAVR. The delayed change in EF in the SAVR group could be attributed to cardiopulmonary bypass and myocardial protection.
The debate about the optimal approach for aortic valve surgery will continue. Several gaps need to be addressed, and the evolving technology makes the comparison uneven. The comparison did not consider some technical issues, including the miniaturized cardiopulmonary bypass [29], aortic valve repair [29, 30], the minimally invasive approach, and robotic SAVR [31,32,33]. In a meta-analysis, minimally invasive SAVR was associated with lower mid-term mortality compared to TAVR [34]. The future inclusion of these factors in the comparison could be a game changer.
Study limitations
The study has several major limitations. First, the design is retrospective with its inherent selection bias. The propensity score matched the measured data; however, several patients’ characteristics and comorbidities could affect the treatment outcomes but were not included in the analysis. Second, the study is a single-center experience, and the generalization of the results may not be applicable. Third, there was great variability in the baseline characteristics of the included patients, which affected the number of matched patients and the results. Fourth, the timeline for TAVR and SAVR is different, which may have introduced selection bias. Fifthly, the number of patients with SAVR is low because we included isolated aortic valve procedures only. In the era of TAVR, patients with single valves and a high risk of surgery were assigned to the TAVR group. Additionally, rheumatic fever is the most common cause of valve lesions in our region, and it rarely affects the aortic valve without mitral affection. Lastly, the number of events is low, and this could be a reason for not detecting the statistical significance of some outcomes.
Conclusions
TAVR had a lower postprocedural incidence of atrial fibrillation; however, SAVR could bear a survival benefit over TAVR, with no difference in stroke, aortic valve reintervention, and heart failure rehospitalization between SAVR and TAVR. Both approaches were associated with improved ejection fraction; TAVR had an immediate effect on ejection fraction, while the effect of SAVR became evident after 6 months. The peak aortic valve pressure was lower with TAVR, while it had a higher paravalvular leak. Both approaches for managing aortic valve disease should be tailored according to the patient’s needs and characteristics.
Availability of data and materials
The authors declare that the data supporting the findings of this study are not available per hospital-related regulations.
Abbreviations
- AR:
-
Aortic regurgitation
- AS:
-
Aortic stenosis
- BMI:
-
Body mass index
- EF:
-
Ejection fraction
- HF:
-
Heart failure
- LVEDD:
-
Left ventricular end-diastolic diameter
- LVESD:
-
Left ventricular end-systolic diameter
- MI:
-
Myocardial infarction
- NYHA:
-
New York Heart Association
- PCI:
-
Percutaneous coronary intervention
- SAVR:
-
Surgical aortic valve replacement
- TAVR:
-
Transcatheter aortic valve replacement
References
Swift SL, Puehler T, Misso K, Lang SH, Forbes C, Kleijnen J et al (2021) Transcatheter aortic valve implantation versus surgical aortic valve replacement in patients with severe aortic stenosis: a systematic review and meta-analysis. BMJ Open 11(12). Available from: https://bmjopen.bmj.com/content/11/12/e054222
Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG et al (2011) Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 364(23):2187–2198
Mack MJ, Leon MB, Smith CR, Miller DC, Moses JW, Tuzcu EM et al (2015 Jun) 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet (London, England) 385(9986):2477–2484
Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK et al (2016) Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 374(17):1609–1620
Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Søndergaard L, Mumtaz M et al (2017) Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med 376(14):1321–1331
Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M et al (2019) Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med 380(18):1695–1705
Waksman R, Craig PE, Torguson R, Asch FM, Weissman G, Ruiz D et al (2020) Transcatheter aortic valve replacement in low-risk patients with symptomatic severe bicuspid aortic valve stenosis. JACC Cardiovasc Interv 13(9):1019–1027
Bocchino PP, Angelini F, Alushi B, Conrotto F, Cioffi GM, Tersalvi G et al (2020) Transcatheter aortic valve replacement in young low-risk patients with severe aortic stenosis: a review. Front Cardiovasc Med 7:608158
Arafat AA, Alawami MH, Hassan E, Alshammari A, AlFayez LA, Albabtain MA et al (2022) Surgical vs transcatheter aortic valve replacement in patients with a low ejection fraction. Angiology 13:33197221121012
Pibarot P, Ternacle J, Jaber WA, Salaun E, Dahou A, Asch FM et al (2020) Structural deterioration of transcatheter versus surgical aortic valve bioprostheses in the PARTNER-2 trial. J Am Coll Cardiol 76(16):1830–1843. https://doi.org/10.1016/j.jacc.2020.08.049
Alharbi AA, Khan MZ, Osman M, Khan MU, Munir MB, Syed M et al (2020) Transcatheter aortic valve replacement vs surgical replacement in patients with pure aortic insufficiency. Mayo Clin Proc 95(12):2655–2664
Wang D, Huang L, Zhang Y, Cheng Z, Zhang X, Ren P et al (2020) Transcatheter aortic valve implantation versus surgical aortic valve replacement for treatment of severe aortic stenosis: comparison of results from randomized controlled trials and real-world data. Brazilian J Cardiovasc Surg 35(3):346–367
Nashef SAM, Roques F, Sharples LD, Nilsson J, Smith C, Goldstone AR et al (2012) EuroSCORE II†. Eur J Cardio-Thoracic Surg 41(4):734–745. https://doi.org/10.1093/ejcts/ezs043
Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F et al (2021) 2020 ACC/AHA Guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 143(5):e72–e227. https://doi.org/10.1161/CIR.0000000000000923
Otto CM, Kumbhani DJ, Alexander KP, Calhoon JH, Desai MY, Kaul S et al (2017) 2017 ACC Expert Consensus decision pathway for transcatheter aortic valve replacement in the management of adults with aortic stenosis: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol 69(10):1313–1346
Arafat AA, AlQattan H, Zahra A, Alghamdi R, Alghosoon H, AlGhamdi F et al (2022) Using tissue mitral valves in younger patients: a word of caution. J Card Surg 37:4227–4233
DesJardin JT, Chikwe J, Hahn RT, Hung JW, Delling FN (2022) Sex differences and similarities in valvular heart disease. Circ Res 130(4):455–473. https://doi.org/10.1161/CIRCRESAHA.121.319914
Kulik A, Lam B-K, Rubens FD, Hendry PJ, Masters RG, Goldstein W et al (2009) Gender differences in the long-term outcomes after valve replacement surgery. Heart 95(4):318–326
Youssef G (2021) Valvular heart diseases in women. Egypt Hear J Off Bull Egypt Soc Cardiol 73(1):58
Chaker Z, Badhwar V, Alqahtani F, Aljohani S, Zack CJ, Holmes DR et al (2017) Sex differences in the utilization and outcomes of surgical aortic valve replacement for severe aortic stenosis. J Am Heart Assoc 6(9):e006370
Baan JJ, Yong ZY, Koch KT, Henriques JPS, Bouma BJ, Vis MM et al (2010) Factors associated with cardiac conduction disorders and permanent pacemaker implantation after percutaneous aortic valve implantation with the CoreValve prosthesis. Am Heart J 159(3):497–503
Young Lee M, Chilakamarri Yeshwant S, Chava S, Lawrence LD (2015) Mechanisms of heart block after transcatheter aortic valve replacement - cardiac anatomy, clinical predictors and mechanical factors that contribute to permanent pacemaker implantation. Arrhythmia Electrophysiol Rev 4(2):81–85
Jeong HK, Yoon N, Kim JH, Lee N, Hyun DY, Kim MC et al (2021) Postoperative atrial fibrillation impacts on outcomes in transcatheter and surgical aortic valve replacement. Front Cardiovasc Med 8:789548
Bhushan S, Huang X, Li Y, He S, Mao L, Hong W et al (2022) Paravalvular leak after transcatheter aortic valve implantation its incidence, diagnosis, clinical implications, prevention, management, and future perspectives: a review article. Curr Probl Cardiol 47(10):100957. Available from: https://www.sciencedirect.com/science/article/pii/S0146280621001729
Siontis GCM, Overtchouk P, Cahill TJ, Modine T, Prendergast B, Praz F et al (2019) Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of symptomatic severe aortic stenosis: an updated meta-analysis. Eur Heart J 40(38):3143–3153. https://doi.org/10.1093/eurheartj/ehz275
Leon MB, Mack MJ, Hahn RT, Thourani VH, Makkar R, Kodali SK et al (2021) Outcomes 2 years after transcatheter aortic valve replacement in patients at low surgical risk. J Am Coll Cardiol 77(9):1149–1161
Didier R, Eltchaninoff H, Donzeau-Gouge P, Chevreul K, Fajadet J, Leprince P et al (2018) Five-year clinical outcome and valve durability after transcatheter aortic valve replacement in high-risk patients. Circulation 138(23):2597–2607
Blackman DJ, Saraf S, MacCarthy PA, Myat A, Anderson SG, Malkin CJ et al (2019) Long-term durability of transcatheter aortic valve prostheses. J Am Coll Cardiol 73(5):537–545
Guo MH, Boodhwani M (2019) Aortic valve repair: from concept to future targets. Semin Thorac Cardiovasc Surg 31(4):650–655
Amr MA, Fayad E (2022) Early outcomes of aortic valve repair versus replacement for aortic regurgitation: a single-center experience. Cardiothorac Surg 30(1):2. https://doi.org/10.1186/s43057-021-00063-2
Torky MA, Arafat AA, Fawzy HF, Taha AM, Wahby EA, Herijgers P (2021) J-ministernotomy for aortic valve replacement: a retrospective cohort study. Cardiothorac Surg 29(1):16. https://doi.org/10.1186/s43057-021-00050-7
Hancock HC, Maier RH, Kasim A, Mason J, Murphy G, Goodwin A et al (2021) Mini-sternotomy versus conventional sternotomy for aortic valve replacement: a randomised controlled trial. BMJ Open 11(1):e041398. Available from: https://bmjopen.bmj.com/content/11/1/e041398
Wei LM, Cook CC, Hayanga JWA, Rankin JS, Mascio CE, Badhwar V (2022) Robotic aortic valve replacement: first 50 cases. Ann Thorac Surg 114(3):720–726
Sayed A, Almotawally S, Wilson K, Munir M, Bendary A, Ramzy A et al (2021) Minimally invasive surgery versus transcatheter aortic valve replacement: a systematic review and meta-analysis. Open Hear 8(1):e001535. Available from: https://openheart.bmj.com/content/8/1/e001535
Acknowledgements
Not applicable
Funding
No funding was received for this project. This research did not receive any grants from funding agencies in the public, commercial, or nonprofit sectors.
Author information
Authors and Affiliations
Contributions
AM, study design, data collection, and drafting a manuscript; MA, study design, superivision, and revising the manuscript; FA, data collection and critical appraisal; LA, data collection and critical appraisal; AAM, study design, revising, and supervision; MA, study design, supervision, critical appraisal; AAK, study design and critical appraisal; HI, data interpretation and revising; CP, study design and revision; AIA, study design, revision, and supervision; and AAA, study design, analysis, interpretation, and revising. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
IRB approval number R19017, Prince Sultan Cardiac Center, Riyadh, Saudi Arabia, 2019. Consent to participate was waived by the ethical committee.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
The distribution of propensity score (SAVR: surgical aortic valve replacement; TAVR: transcatheter aortic valve replacement).
Additional file 2: Figure S2.
Standardized bias percentage across the covariates before and after matching.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Almedimigh, A.A., Albabtain, M.A., Alfayez, L.A. et al. Isolated surgical vs. transcatheter aortic valve replacement: a propensity score analysis. Cardiothorac Surg 31, 2 (2023). https://doi.org/10.1186/s43057-022-00094-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43057-022-00094-3