Abstract
Background
Giant coronary artery aneurysms (GCAA) are a rare disease entity with an estimated incidence of 0.02%. Atherosclerosis is the most common underlying factor in adulthood. Management guidelines lack the support of large-scale studies.
Case presentation
We present a case of a 58-year-old Caucasian male with complaints of stable dyspnea who was found to have a GCAA of the right coronary artery (RCA). Further evaluation revealed an aneurysm of 5.0 cm in diameter with a tortuous course, fistulation to the distal vena cava superior, and mass effect on the left atrium. Surgical deroofing and ligation of the aneurysm with venous bypassing of the right coronary artery were performed. There were no postoperative complications. Cardiac function had improved at 1-month follow-up and remained improved at 1-year follow-up.
Conclusions
Diagnosis and treatment strategy concerning GCAA remain challenging. Surgical treatment is advised in cases of giant aneurysms, multivessel disease, left main coronary artery (LMCA) involvement, mechanical complications (fistula, compression, or rupture), and concomitant valve surgery. Coronary angiography remains the gold standard for evaluation. However, coronary computed tomography angiography (CCTA) and cardiac magnetic resonance imaging (CMR) can add an important value for the clinician to assess myocardial viability and planning of surgical intervention.
Similar content being viewed by others
Background
Giant coronary artery aneurysms (GCAA) exceeding a diameter of 5.0 cm are an uncommon subgroup of coronary artery aneurysms (CAA) with a proposed incidence of 0.02%. The right coronary is most commonly affected [1]. Coronary artery aneurysms are found in about 0.3–4.9% of patients undergoing coronary angiography and are reportedly present in 1.4% of postmortem examinations [1].
In terms of pathogenesis, studies report atherosclerosis to be the underlying factor in >90% of CAAs in adulthood [1].
In this case report, we present a patient with a giant coronary artery aneurysm who presented with a history of atypical angina pectoris. This paper will elaborate on the surgical treatment and the added value of preoperative coronary computed tomography angiography (CCTA) and cardiac magnetic resonance imaging (CMR).
Case presentation
We present a 58-year-old Caucasian male with a past medical history of clinical depression, gout, arterial hypertension, and morbid obesity with a current BMI of 44.69. Except for the previously mentioned, no other relevant cardiac risk factors were present.
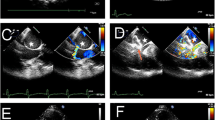
In August 2020, he was diagnosed with a new onset of heart failure with mid-range ejection fraction and atrial fibrillation. He complained of dyspnea and palpitations. The ejection fraction was estimated at 50% in the presence of a left bundle branch block (LBBB). He required hospitalization for electric cardioversion twice in the following months. A transthoracic echocardiography (TTE) during a routine follow-up after the second hospitalization showed a large structure anterior to the aortic root. At that moment he had stable dyspnea, New York Heart Association (NYHA) class 1, no complaints of angor pectoris, palpitations, syncope, or any other symptoms. Upon auscultation, a systolic continuous systolic murmur was heard at the left sternal edge. Further imaging (Fig. 1A–D) by means of coronary computed tomography angiography (CCTA) and phase-contrast cardiac magnetic resonance (CMR) showed a giant right coronary aneurysm (max diameter = 5.0 cm) with a tortuous course, fistulation to the distal vena cava superior and mass effect on the left atrium. Pulmonary blood flow (Qp) measured at the level of the pulmonary trunk was 160 ml. Pulmonary blood flow and systemic blood flow (Qp/Qs) ratio was 1.36, indicating a significant left-to-right shunt. Coronary angiography showed a large right-sided coronary aneurysm with fistulation to the distal vena cava superior and subsequent contrast opacification of the right atrium. The left coronary vasculature was normal, with collateral branches originating from the circumflex artery to the distal right coronary artery (Fig. 2A–D). After multidisciplinary counseling, the decision was made to perform an elective surgical ligation of the aneurysm given its size and mass effect.
Coronary computed tomography angiography (CCTA) images (A, B) and phase-contrast cardiac magnetic resonance (CMR) images (C, D). A Normal ostium of the right coronary artery (RCA) with short proximal normal segment (6 mm length, arrow). B Giant aneurysm of the RCA with dilatation up to 5.0 cm (arrow) and coronary artery fistula with a tortuous course, ultimately draining into the distal superior vena cava (SVC) (arrowhead). C Systemic blood flow (Qs) measured at the level of the proximal ascending aorta was 118 ml. D Pulmonary blood flow (Qp) measured at the level of the pulmonary trunk was 160 ml. Qp/Qs ratio was 1.36, indicating a significant left-to-right shunt
A–C Coronary angiography showing the aneurysm with contrast filling of the right atrium in the absence of opacification of the distal right coronary artery (RAC). Catheterization showed slightly increased pressure of the pulmonary artery (42/20 mmHg), pulmonary capillary wedge pressure (PCW) was normal (19 mmHg). D Collateral branches originating from the ramus circumflex (RCx) to the distal RAC were visualized
During surgery, a small groin incision was made to expose the common femoral vessels in case of a need for emergency cardiopulmonary bypass (CPB). Then, a classic midline sternotomy was performed with immediate visualization of the giant aneurysm (Fig. 3). We did a classic aortic and superior vena cava cannulation; the inferior vena cava was cannulated at the level of the groin. Antegrade cardioplegia was not successful because of shunting by the fistulated aneurysm. By means of retrograde cardioplegia, cardiopulmonary bypass was successfully initiated. Dissection of the aneurysm was started near the coronary ostium and was completed at the level of the superior vena cava fistula. A complete deroofing of the aneurysm was performed and diffuse atherosclerosis was seen in absence of thrombosis (Fig. 4). Both the fistula and the coronary ostium were subsequently ligated, as well as small side branches to the aneurysm. A larger branch with a 1.5-mm diameter, which we suspected to be the true distal right coronary artery, was bypassed by a saphenous vein graft. Histopathology showed atherosclerosis, no other possible cause was identified. There were no postoperative complications, and the patient was discharged home on day seven.
A TTE 1 month postoperatively showed an improved left ventricular function. There were no subjective symptoms. Cyclo-ergometry was electrographically and clinically negative for cardiac ischemia. At approximately one year postoperatively, the patient showed no signs of cardiac disease.
Discussion
Giant coronary artery aneurysms (GCAA) present an uncommon subgroup of coronary artery aneurysm (CAA) with a proposed incidence of 0.02%. Whereas coronary artery aneurysms are defined as dilatation of the coronary artery exceeding 50% of the reference vessel diameter, aneurysms are deemed “giant” [2] if their diameter transcends the reference vessel diameter by greater than four times or if they are >8 mm in diameter [2, 3]. Coronary aneurysms differ from ectasia in their more focal presentation, rather than diffuse dilatation of the coronary artery [2].
Atherosclerosis is the most common etiology of CAA. This causes mechanical stress on the vessel wall [4] and initiates an inflammatory response with subsequent induction of proteolysis of the extracellular matrix proteins [5]. There is an imbalance of increased levels of matrix metalloproteinases (MMPs) and a decrease of tissue inhibitors inside the aneurysm. The elevated levels of MMPs degrade the arterial media and lead to thinning of the arterial wall and increased wall stress leading to dilatation of the artery [5]. In a study of Lamblin M et al. (2002), the 5A allele of MMP3 was found to be significantly more prevalent in patients with CAA. This modification seems to play a moderating role in the development of coronary artery aneurysms in patients with atherosclerosis [6].
Another more recently reported type of CAA is the CAAs caused by coronary interventions. The anti-inflammatory and antiproliferative drugs in the newest generation stents halt intima proliferation. The polymer of these stents induces hypersensitivity reactions and vasculitis, resulting in the weakening of the vessel wall [5]. Iatrogenic aneurysms caused by damage to the vessel wall are also reported after balloon angioplasty [7].
Other known causes of coronary artery aneurysms in adults are Takayasu disease, systemic vasculitis, connective tissue disorders (e.g. Marfan, Ehlers-Danlos), infections causing mycoid aneurysms, drugs (e.g. cocaine, amphetamines), and protein disorders. Kawasaki disorder is the most common cause of aneurysms in children [7].
Most aneurysms remain asymptomatic or cause atypical symptoms (atypical angina, dyspnea, hiccups, signs of compression). The majority are incidental findings. Our patient suffered from HfmrEF with an EF of 50% in combination with asynchronous cardiac contractility due to an LBBB. This explained his complaints of dyspnea and led towards the reported finding. There is limited data on complications such as embolization and rupture of these aneurysms, which gives rise to the discussion on optimal timing for invasive treatment [8].
Coronary angiography remains the golden standard for evaluation. The additional value of coronary computed tomography angiography (CCTA) in patients with giant CAA mostly relates to its excellent three-dimensional visualization of the CAA which avoids possible underestimation of aneurysm size in cases with partial thrombosis and provides the surgeon with a roadmap of side and distal branches of the involved coronary and an overview of the surrounding structures [7, 9]. Cardiac magnetic resonance imaging (CMR) allows for the assessment of myocardial viability and quantification of left-to-right shunt. Additionally, in cases with an atypical partially thrombosed CAA, CMR may help to differentiate the CAA from a cardiac tumor [8, 10].
The management of these giant coronary artery aneurysms consists of a surgical intervention in cases of giant aneurysms, multivessel disease, left main coronary artery (LMCA) involvement, mechanical complications (fistula, compression, or rupture), concomitant valve surgery, and multiple CAAs [7]. However, given the rarity of this pathology, the data on which reviews are based remains limited. We believe that publishing these cases and sharing our experience will result in better insight for clinicians and better treatment planning.
Conclusions
Giant coronary artery aneurysms (GCAA) are clinically challenging. Decision-making and treatment strategy are often based on expert opinions and small clinical studies. Surgical treatment is advised in cases of giant aneurysms, multivessel disease, left main coronary artery (LMCA) involvement, mechanical complications (fistula, compression, or rupture), and concomitant valve surgery. Coronary angiography remains the golden standard for evaluation. Multimodality evaluation including CCTA and CMR can add significant value. We advise that they should be obtained to allow for the most accurate preoperative diagnosis and procedural planning.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- CAA:
-
Coronary artery aneurysm
- GCAA:
-
Giant coronary artery aneurysm
- RCA:
-
Right coronary artery
- LMCA:
-
Left main coronary artery
- CCTA:
-
Coronary computed tomography angiography
- CMR:
-
Cardiac magnetic resonance imaging
- TTE:
-
Transthoracic echocardiography
- NYHA:
-
New York Heart Association functional classification
- Qp:
-
Pulmonary blood flow rate
- Qs:
-
Systemic blood flow rate
- CPB:
-
Cardiopulmonary bypass
- MMP:
-
Matrix metalloproteinase
- LBBB:
-
Left bundle branch block
References
Swaye PS, Fisher LD, Litwin P, Vignola PA, Judkins MP, Kemp HG et al (1983) Aneurysmal coronary artery disease. Circulation 67(1):134–138. [cited 2021 Nov 17] Available from. https://doi.org/10.1161/01.CIR.67.1.134
Sheikh AS, Hailan A, Kinnaird T, Choudhury A, Smith D (2019) Coronary artery aneurysm: evaluation, prognosis, and proposed treatment strategies. Heart Views 20(3):101 [cited 2021 Nov 17]. Available from: /pmc/articles/PMC6791093/
Kato H, Sugimura T, Akagi T, Sato N, Hashino K, Maeno Y et al (1996) Long-term Consequences of Kawasaki Disease. Circulation 94(6):1379–1385. 15 [cited 2021 Nov 17] Available from. https://doi.org/10.1161/01.CIR.94.6.1379
Díaz-Zamudio M, Bacilio-Pérez U, Herrera-Zarza MC, Meave-González A, Alexanderson-Rosas E, Zambrana-Balta GF et al (2009) Coronary artery aneurysms and ectasia: Role of coronary CT angiography. Radiographics 29(7):1939–1954. 1 [cited 2021 Nov 17] Available from. https://doi.org/10.1148/rg.297095048
Kawsara A, Núñez Gil IJ, Alqahtani F, Moreland J, Rihal CS, Alkhouli M (2018) Management of Coronary Artery Aneurysms. JACC Cardiovasc Interv 11(13):1211–1223. [cited 2021 Nov 17] Available from. https://doi.org/10.1016/j.jcin.2018.02.041
Lamblin N, Bauters C, Hermant X, Lablanche JM, Helbecque N, Amouyel P (2002) Polymorphisms in the promoter regions of MMP-2, MMP-3, MMP-9 and MMP-12 genes as determinants of aneurysmal coronary artery disease. J Am Coll Cardiol. 40(1):43–48
Pham V, de Hemptinne Q, Grinda J-M, Duboc D, Varenne O, Picard F (2020) Giant coronary aneurysms, from diagnosis to treatment: A literature review. Arch Cardiovasc Dis 113(1):59–69 Available from: https://www.sciencedirect.com/science/article/pii/S1875213619302232
Pahlavan PS, Niroomand F (2006) Coronary artery aneurysm: A review. Clin Cardiol 29(10):439–443. Available from. https://doi.org/10.1002/clc.4960291005
Ramirez FD, Hibbert B, Simard T, Pourdjabbar A, Wilson KR, Hibbert R et al (2012) Natural History and Management of Aortocoronary Saphenous Vein Graft Aneurysms. Circulation 126(18):2248–2256. Available from. https://doi.org/10.1161/CIRCULATIONAHA.112.101592
Hayashida S, Yagi T, Suzuki Y, Tachibana E (2019) Usefulness of multimodality cardiac imaging in a patient with ST elevation myocardial infarction caused by two giant coronary artery aneurysms. BMJ Case Rep. 12(8):10–12
Acknowledgements
Not applicable.
Funding
The authors have not received any funding for this research from either public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors fulfill the ICMJE Criteria for Authorship and all authors were equally involved in critical revision and approving the work for publication. VVG and AB were involved in conception of the work and drafting the work. HDP was involved in data acquisition and case description. HDP, AB, and VVG performed the surgery. RS was responsible for drafting the interpretation of the radiological aspects of the work. HR was the referring cardiologist who was also responsible for case description and review. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was given by the Ethics Committee of KU Leuven. A copy of this approval can be made available upon request.
Consent for publication
A written consent for publication from the patient was obtained. A copy of this approval can be made available upon request.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van Grinsven, V., Binst, A., Rombouts, H. et al. Giant right coronary artery aneurysm with vena cava superior fistula: a case report and radiological findings. Cardiothorac Surg 30, 19 (2022). https://doi.org/10.1186/s43057-022-00081-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43057-022-00081-8