Abstract
Background
Cor triatriatum is a rare congenital heart disease representing the 0.4% of all congenital cardiac anomalies. To date, no specific genetic alteration has been associated to cor triatriatum. The left-sided presentation (cor triatriatum sinister (CTS)) generally consists in a fibromuscular membrane that divides the left atrium into two chambers, therefore generating a varying grade of flow obstruction depending on the shape, location, and membrane fenestration size. Cor triatriatum sinister can be isolated or associated to other congenital heart defects such as ostium secundum atrial septal defect, patent foramen ovale or abnormal pulmonary veins drainage.
Case presentation
Our case is a 63-year-old woman who was diagnosed with a non-restrictive membrane during a hospitalization for acute heart failure. In the following 6 months, she started to become symptomatic. However, the onset of symptoms was more likely related to mitral valve regurgitation worsening and previously unknown coronary artery disease, rather than to CTS. She underwent bi-atrial surgical ablation (Cox Maze IV procedure) for atrial fibrillation (AF), surgical resection of interatrial membrane with mitral annuloplasty, and myocardial revascularization.
Conclusion
The onset and severity of symptoms in patients with CTS mostly depend on membrane fenestration size, grade of stenosis generated and pulmonary veins drainage site. However, some cases may remain asymptomatic until adulthood; the degree of pulmonary hypertension and congestive heart failure is determined by the presence of additional cardiac anomalies and the fibromuscular membrane fenestration. In some cases, CTS may remain asymptomatic, thus the diagnosis can be incidental.
Similar content being viewed by others
Background
Cor triatriatum is a rare congenital heart disease representing the 0.4% of all congenital cardiac anomalies [1].
To date, no specific genetic alteration has been associated to cor triatriatum. It can occur in left atrium (cor triatriatum sinister (CTS)) and seldom in the right atrium (cor triatriatum dexter).
The left-sided presentation (CTS) generally consists in a fibromuscular membrane that divides the left atrium into two chambers, generating a varying grade of flow obstruction depending on the shape, location, and membrane fenestration size. The two chambers are named “proximal”, the one receiving pulmonary venous drainage, located in postero-superior position, and “distal”, the one containing left atrial appendage and atrioventricular valve, located in antero-inferior position. CTS can be isolated or associated with other congenital heart defects, such as ostium secundum atrial septal defect, patent foramen ovale, or abnormal pulmonary veins drainage [2].
Different classifications exist for CTS. Lam classification, which is based on site of pulmonary veins (PV) drainage (proximal chamber, coronary sinus, or elsewhere), is the most used. The diaphragmatic type is the typical presentation of CTS: the membrane appears horizontally with normal atrial geometry and PV drainage in the proximal chamber, without atrial septal defect (67%) [3].
Clinical presentation of CTS is variable, and it is very similar to that of mitral valve stenosis. The membrane creates a supra-valvular obstruction leading to an increase in left atrial pressure that results in pulmonary hypertension, which, over time, may determine tricuspid regurgitation and right ventricular failure.
The onset and severity of symptoms in patients with CTS mostly depend on membrane fenestration size, grade of stenosis generated and pulmonary veins drainage site. However, some cases may remain asymptomatic until adulthood; the degree of pulmonary hypertension and congestive heart failure is determined by the presence of additional cardiac anomalies and the fenestration of fibromuscular membrane. In some cases, CTS may remain asymptomatic, thus the diagnosis is incidental.
The diagnosis can be easily established with echocardiography including measurement of the trans-membrane pressure gradient to evaluate the functional significance. Cardiac magnetic resonance (CMR) may also give a clearer anatomic view.
Case presentation
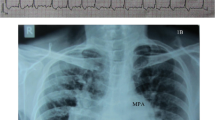
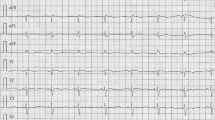
A 63-year-old woman was admitted in intensive care coronary unit with acute heart failure in February 2019, symptomatic for orthopnea, dyspnea, dizziness, and palpitations. The patient was previously asymptomatic with personal anamnesis of hypertension, dyslipidaemia, and obesity (BMI 36). The electrocardiogram showed a new onset of atrial fibrillation (AF) with 110–120 bpm heart rate. Chest x-ray showed severe pulmonary edema. High doses of intravenous diuretic drugs were used to restore circle compensation, and an electrical cardioversion was performed to restore sinus rhythm.
Echocardiography showed biventricular normal function and moderate mitral regurgitation due to annular dilation (antero-posterior diameter 39 mm, inter-commissural diameter 42 mm) without evidence of leaflet abnormalities. However, during the exam it was also detected a widely dilated left atrium with an intracavitary membrane, defined as non-restrictive cor triatriatum (mean gradient 2 mmHg).
To better characterize the membrane and left atrium anatomy, a cardiac magnetic resonance (CMR) with contrast was performed, allowing its classification as Lam type A cor triatriatum sinister (Fig. 1). No atrial septal defects were detected at CMR, as well as no PV drainage abnormalities; the four PVs drained in the proximal chamber. Before discharge, right heart catheterization was also performed, showing moderate post-capillary pulmonary hypertension (PAPs 40 mmHg, PCWP 25 mmHg).
After medical therapy optimization, the patient was discharged at home. In the following six months, the woman experienced progressive worsening of symptoms, with strong limitation to physical activity. Therefore, the patient was eventually admitted in cardiac surgery unit for mitral valve repair and concomitant surgical excision of the membrane. At the time of admission, the patient was in atrial flutter. Preoperative transesophageal echocardiography showed circular membrane with a wide communication between the two chambers, no transmembrane gradients, no mitral valve opening abnormalities, severe mitral regurgitation with three central and paracentral jets due annular dilation, normal dimensions and function of the left ventricle, no kinetic anomalies and severe pulmonary hypertension (PAPs 65 mmHg, systemic arterial pressure 140/90 mmHg). Preoperatively coronary angiography was also performed, revealing unexpected coronary disease with involvement of Left Main and three-vessel disease (Fig. 2).
The patient underwent bi-atrial surgical ablation using bipolar radiofrequency and cryo-ablation for AF (Cox-Maze IV), surgical resection of interatrial membrane, mitral annuloplasty with a Profile 3D n°28, and myocardial revascularization with bypass using bilateral mammary artery (LIMA on LAD, RIMA on OM) and saphenous vein graft on right coronary artery.
She was discharged after 7 days in sinus rhythm, and excellent result of mitral annuloplasty without any signs of stenosis. At 3-month follow-up, the patient was asymptomatic, in sinus rhythm, with good tolerance to physical effort. Postoperative echocardiography showed a residual trivial mitral valve regurgitation (Fig. 3).
Discussion
To date, given the rarity of CTS diagnosis, data on its clinical history are limited.
The main conclusions of this clinical case report are that CTS is often an incidental finding and its diagnosis may require multimodality imaging, since echocardiography alone may not be sufficient to describe the anatomical presentation of the defect.
Diagnosis is usually based on transesophageal and transthoracic echocardiography, specifically on the measurement of the trans-membrane pressure gradient. However, in most cases patients may require integrative diagnosis with CMR. Sakamoto and colleagues described two cases of CTS in adults where the use of CMR provided additional anatomic information that was not evident on trans-thoracic echocardiography, particularly regarding the location of atrial communication and anatomy of the pulmonary veins [4].
Moreover, clinical presentation could be extremely variable depending on the grade of stenosis generated from the membrane. In this case, the patient was asymptomatic until the sudden onset of atrial tachyarrhythmia due to the worsening of mitral valve regurgitation, which eventually lead to acute heart failure condition and pulmonary edema. CTS was incidentally detected during diagnostics. However, at the time of the diagnosis, in condition of decompensation, the membrane did not generate trans-membrane gradients and this hemodynamic condition remained stable during the 6-month follow-up.
This leads to the consideration that patients with CTS could remain lifelong asymptomatic if the membrane fenestration does not generate gradients between the two chambers from the beginning and particularly if not associated to other congenital defects or significant acquired valve diseases.
Furthermore, as already reported in literature, patients affected by Lam A1 CTS often receive an early diagnosis because of the lack of atrial septal defects, generally associated with other CTS phenotypes. In this case, restrictive membrane determined early onset of symptoms due to a rapid increase in pulmonary pressure [3].
Echocardiographic follow-up of patients with CTS did not show an increase in obstruction severity over time, meaning that this congenital defect is not progressive [4]. As reported from Fuchs and colleagues in their 26-year experience on this pathology, asymptomatic patients, and in particular patients without restrictive CTS, can be managed conservatively without surgical intervention and do not require close clinical monitoring [5].
Indeed, the 6-month clinical follow-up of the patient allowed us to discriminate that the onset of symptoms was more likely related to mitral valve regurgitation worsening and previously unknown coronary artery disease, rather than to CTS.
Conclusions
As already reported in other cases in literature, this patient underwent CTS excision because of other cardiac conditions that influenced the decision at the time of surgery. Surgery required for CTS is a simple treatment and most importantly an effective solution; indeed, surgical excision of the membrane provides rapid relief from symptoms, with effective long-term results. In our experience, it is vital that all patients with cor triatriatum are continuously monitored in order to define the optimal timing for surgery.
Availability of data and materials
Yes, we record data about patient’s characteristics and our surgery strategy.
Abbreviations
- CTS:
-
Cor triatriatum sinister
- PV:
-
Pulmonary veins
- AF:
-
Atrial fibrillation
- CMR:
-
Cardiac magnetic resonance
References
Jegier W, Gibbons JE, Wiglesworth FW (1963) Cortriatriatum: clinical, hemodynamic and pathological studies surgical correction in early life. Pediatrics 31:255–267
Saxena P, Burkhart HM, Schaff HV, Daly R, Joyce LD, Dearani JA (2014) Surgical repair of cor triatriatum sinister: the Mayo Clinic 50-year experience. Ann Thorac Surg 97:1659–1663
Lam CR, Green E, Drake E (1962) Diagnosis and surgical correction of 2 types of triatrial heart. Surgery 51:127–137
Sakamoto I, Matsunaga N, Hayashi K, Ogawa Y, Fukui J (1994) Cine-magnetic resonance imaging of cor triatriatum. Chest 106(5):1586–1589. https://doi.org/10.1378/chest.106.5.1586
Fuchs M, Connolly H, Said S, Egbe A (2018) Outcomes in patients with cor triatriatum sinister. Congenit Heart Dis 13(4):628–632. https://doi.org/10.1111/chd.12624 Epub 2018 Jul 22
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
Di Bacco: writing discussion. D’Alonzo: imaging and research. Repossini, Muneretto, and Benussi: surgeons on operating table. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The patient gave the consent to participate (recording data and imaging).
Consent for publication
The patient gave us the consent for publication the case report.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Di Bacco, L., D’Alonzo, M., Repossini, A. et al. Treatment of non-restrictive cor triatriatum sinister during concomitant cardiac surgery. Cardiothorac Surg 30, 15 (2022). https://doi.org/10.1186/s43057-022-00076-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43057-022-00076-5