Abstract
Background
Degenerative aortic arch aneurysms are known to develop through a pathological process of arterial atherosclerosis, which could be accompanied by peripheral artery diseases and resultant development of intrapelvic collateral arteries to the ischemic lower limbs. The aim of this study was to investigate the relationship between peripheral collateral circulation and postoperative paraplegia after total arch repair with a frozen elephant trunk in patients with degenerative aortic arch aneurysms and peripheral artery diseases.
Methods
Between October 2014 and March 2020, 27 patients (20 men; 69.8 ± 7.7 years old) underwent total arch repair with a frozen elephant trunk. Two of the 27 patients developed paraplegia postoperatively. The patients were divided into two groups, spinal cord ischemia (SCI) group (2 patients) and no-SCI group (25 patients). The aortic shagginess score, arterial calcification (subclavian artery; hypogastric artery) score, and the number of hypogastric artery branches, assessed using preoperative contrast-enhanced computed tomography images, were compared between the two groups.
Results
The ankle brachial artery pressure index (i.e., lower side value each patient) was lower in the SCI group than that in the no-SCI group (0.64, 0.71, and 1.09±0.07, respectively). There was no difference between the two groups in the arterial calcification scores or the aortic shagginess score. The number of hypogastric artery branches was greater in the SCI group than in the no-SCI group (66, 66, and 30.7±7.5, respectively).
Conclusions
Enhanced collateral circulation to the ischemic lower limbs in patients with combination of degenerative aortic arch aneurysms and peripheral artery diseases may be involved in paraplegia the upper thoracic spinal cord injury after total arch repair with a frozen elephant trunk.
Similar content being viewed by others
Background
Degenerative aortic arch aneurysms are known to develop through a pathological process of arterial atherosclerosis, which could be accompanied by peripheral artery diseases (PAD) and resultant development of intrapelvic collateral arteries (i.e., branches originated from the hypogastric artery) to the ischemic lower limbs. Spinal cord low perfusion caused by segmental (e.g., Adamkiewicz) artery occlusion has been regarded as a crucial factor relating to the onset of spinal cord ischemia (SCI). Thoracic aortic replacements without costal artery reconstructions or thoracic endovascular aortic repairs for thoracic aortic aneurysms have a risk of SCI caused by collateral artery occlusion during surgery. Additionally, loss of a collateral supply to the segmental arteries from the subclavian artery or hypogastric (internal iliac) artery is another determinant of SCI, which is considered to be likely based on the “Collateral network concept” reported by Griepp et al. [1,2,3]. A frozen elephant trunk (FET) technique, developed by Kato et al. [4] has recently been used worldwide, and total arch replacement with an FET has grown in number for surgical treatments of degenerative aortic arch aneurysms. The FET technique is thought to have a risk of SCI during surgery, because it occludes some of thoracic segmental arteries. Little is known about the involvement of the collateral circulation altered by PADs in the development of postoperative paraplegia in patients undergoing total arch repair with a concomitant FET technique for degenerative aortic arch aneurysms. We investigated relationship between peripheral collateral circulation and postoperative paraplegia in patients who underwent total arch repair with an FET for degenerative aortic arch aneurysms.
Methods
The patient characteristics and preoperative data are summarized in Table 1. Twenty-seven patients (20 men [74%]; aged 69.8 ± 7.7 years [44–82 years]) with degenerative aortic arch aneurysms underwent total arch repair with an FET in Akita University Hospital from October 2014 through March 2020. The patients were divided into two groups, SCI group (2 patients with paraplegia) and no-SCI group (25 patients without paraplegia). The two groups were compared in terms of the following data. Preoperative data (age, sex, body weight, body height, hemoglobin concentration, ankle brachial artery pressure index (ABPI), past medical history, comorbidities), operative data (emergent or elective, concomitant procedures, operative time, cardiopulmonary bypass (CPB) time, selective cerebral perfusion (SCP) time, aortic cross clamp (AXC) time, circulatory arrest (CA) time, device information), and postoperative data (mortalities, morbidities, minimum values of hemoglobin, mixed venous oxygen saturation, cardiac index, partial pressure of arterial oxygen, and mean blood pressure within 72 h after surgery) were retrospectively compared.
Evaluations of aortic shagginess, arterial calcification, and arterial collaterals
Preoperative thin-slice contrast-enhanced computed tomography (eCT) images were analyzed using an image analysis workstation (Synapse Vincent®, version 6. 1. 0003; Fujifilm Medical Co, Tokyo, Japan) to assess three-dimensional positional relationship between the FET distal end and vertebral body levels. Hosaka et al. proposed “the shagginess score” as a method of quantifying aortic shagginess [5]. Aortic shagginess in our patients was assessed using the method of “the shagginess score” preoperatively in the range between the left subclavian artery and celiac artery levels on the axial images of the thin-slice (1.0–2.5 mm) eCT. Arterial calcification (bilateral subclavian artery, hypogastric artery) was analyzed and scored preoperatively on the axial images of the thin-slice eCT (no calcification = 0, less than half a circumference = 1, more than half a circumference to less than a full circumference = 2, full circumference = 3, occlusion = 4). The number of hypogastric artery branches and segmental arteries were counted visually on the three-dimensional images, which was converted from the axial images on the thin-slice eCT using the image analysis workstation. The gradation processing was performed to enable clear identification of the hypogastric artery branches and segmental arteries. The hypogastric artery branches were counted on the post-processing image separated into right and left at the iliac artery level (Fig. 1).
Three-dimensional computed tomographic images of the pelvic level from the 2 patients with postoperative paraplegia (SCI patient 1 and SCI patient 2 in a and b, respectively) and a patient without postoperative paraplegia (no-SCI patient, c). SCI patient 1: ABI was 0.71/1.23 (right superficial femoral artery occlusion). No. of hypogastric arteries was 45 and 21 at the right side and left side, respectively. SCI patient 2: ABI was 1.05/0.64 (left common iliac artery occlusion). No. of hypogastric arteries was 28 and 38 at the right side and left side, respectively. No-SCI patient: 70-year-old woman. ABI was 1.22/1.12. No. of hypogastric arteries was 15 and 13 at the right side and left side, respectively
Surgical procedure for arch aneurysms
The basic surgical technique has been previously described in detail elsewhere [6]. Basically, we did not perform preoperative cerebrospinal fluid drainage. Intraoperative monitoring of arterial pressure (both upper extremities, one lower extremity and left superficial temporal artery), central venous pressure, pulmonary arterial pressure, cardiac index, and mixed venous oxygen saturation was performed. No motor-evoked potential was used. In short, we perform surgery for aortic arch aneurysms using an FET graft based on the “zone 0 arch repair” strategy, which consists of (a) graft anastomosis to the left axillary artery for a systemic perfusion route, (b) FET deployment from the ascending aorta (zone 0) into the descending aorta, (c) ascending aortic replacement, and (d) arch vessel reconstructions. This technique simplifies the procedure by bringing the distal anastomosis closer to the front. A commercially available FET graft (J Graft Frozenix, Japan Lifeline Co, Ltd., Tokyo, Japan) is routinely used for surgery. The concomitant procedures were performed in 8 patients (coronary artery bypass in 2; David operation in 1; Bentall operation in 1; tricuspid annuloplasty in 1; procedure for atrial fibrillation in 3).
Statistical analysis
We used Easy R “EZR” software for statistical analysis [7]. Continuous data were presented as mean and standard deviation and categorical variables as numbers and percentages. We presented the actual data for the two patients with spinal cord ischemia.
The ethics committee of Akita University Hospital granted approval for publication of the present study (No. 2641), and the need for individual informed consent was waived in this retrospective study.
Results
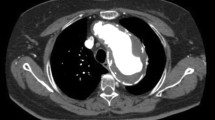
The postoperative mortality was 7.4% (2/27). Postoperative neurological complications were observed in 4 patients (paraplegia in 2 [7.4%]; cerebral infarction in 2 [7.4%]). The postoperative magnetic resonance images (MRI) in the 2 paraplegia patients revealed spinal cord infarction at the levels of C3–Th2 in one patient and Th1–Th5 in the other (Fig. 2a, b, respectively).
Implanted FET data (Table 2)
The FET diameter ranged from 25 to 39 mm, and the most-used FET diameter was 35 mm (7 patients [25.9%]). The FET stent length ranged from 60 to 150 cm, and the most-used FET stent length was 120 mm (16 patients [59.2%]). The level of the FET distal end ranged 4 to 8th thoracic vertebrates (Th4–Th8), and the FET distal end was most frequently positioned at the level of Th7 (13 patients [48.1%]). There was no difference between the SCI and no-SCI groups in terms of the FET diameter, FET stent length, or level of the FET distal end. In the 2 paraplegia patients, the FET distal end was positioned at the level of Th8 in one patient and Th7 in the other.
Perioperative data
The average age of patients was older in the SCI group than in the no-SCI group (Table 1). There was no difference between the two groups in the lowest rectal temperature, CPB time, AXC time, SCP time, or CA time (Table 1). No difference was found between the two groups in the postoperative minimum values of hemoglobin concentration, mixed venous oxygen saturation, cardiac index, partial pressure of arterial oxygen, or mean blood pressure within 72 h after surgery (Table 3).
Evaluations of aortic shagginess, arterial calcification, and arterial collaterals (Table 4)
The ABPI was able to be measured in 2 and 12 patients in the SCI and no-SCI groups, respectively, and it was lower in the SCI group than in the no-SCI group (0.64, 0.71, and 1.09±0.07, respectively). There was no difference between the two groups in the calcification scores of the subclavian artery or those of hypogastric artery. There was no difference between the two groups in “the shagginess score” of the aorta (13.90, 14.38, and 12.22±2.40 in the SCI group and no-SCI group, respectively). The number of hypogastric artery branches, counted on the three-dimensional thin-slice eCT image, was greater in the SCI group than in the no-SCI group in each (right or left) side. There was no difference between the two groups in the number of the intercostal arteries (Th8–Th12), whereas the number of the lumbar arteries (L1–L5) was greater in the SCI group than in the no-SCI group.
Discussion
The present study demonstrated that compared to the no-SCI group, the SCI group had lower ABI but exhibited a greater number of collateral arteries originated from the hypogastric arteries, suggesting that an increase in peripheral collateral arteries resulting from PADs may be involved in development of SCI after arch repair with FETs for degenerative arch aneurysms.
PADs have been reported to be a factor of developing the collateral perfusion to peripheral arteries from the hypogastric (internal iliac) arteries. Gao et al. observed in a study using swine that the collateral source for peripheral perfusion after ligation of the external iliac artery originated from the internal iliac artery [8]. In our PAD (low ABPI) patients, the number of the collateral arterial branches from the internal iliac artery was greater in the SCI group than the no-SCI group (Table 4), which suggests that PAD-induced enhancement of collateral circulation might be related to the development of SCI. Griepp et al. proposed the “Collateral Network” concept that spinal cord perfusion is supplied not only from horizontal blood flow connections (among segmental arteries) but also from vertical blood flow connections (between the subclavian and internal iliac arteries), forming a complex collateral network [1,2,3, 9]. This implies that vertical blood flow connections for collateral perfusion to the ischemic lower limbs are likely to play a role in the development of spinal cord low perfusion.
The vertical blood flow connections serve as a potent protector against SCI although abrupt occlusion of the segmental arteries at the level of Th8–L2 is known to have a risk of SCI. Etz et al. have reported that even if the segmental arteries are ligated, spinal cord perfusion is maintained through vertical collateral circulation from the subclavian and hypogastric arteries [10]. On the other hand, patent segmental arteries at the level of Th8–L2 can be protective against SCI due to vertical blood flow connections even if both upper intercostal and lumber arteries are occluded. In our experience, 3 out of the 25 patients without PAD, who had a past history of abdominal aortic aneurysm repair (resulting in lumbar artery occlusion at the levels distal to L2), had no paraplegia after arch aneurysm repair with an FET (resulting in abrupt intercostal artery occlusion at the levels proximal to Th8). However, 2 patients with PAD, who had no past history of abdominal aortic aneurysm repair, developed postoperative paraplegia after arch aneurysm repair with an FET. The postoperative MRI images in the 2 paraplegia patients revealed spinal cord infarction at the cervical or upper thoracic level (Fig. 2a, b). These findings in our experience suggest that enhancement of collateral circulation to the lower limbs by increased branches of the hypogastric arteries may play a role of stealing blood from the spinal cord vascular beds through vertical blood flow connections, possibly resulting in upper spinal cord ischemia after an FET graft is deployed.
Age, smoking, diabetes, chronic kidney disease, hypertension, PAD, and Crawford type II have been shown to be preoperative risk factors of paraplegia after thoracic aortic repairs [9, 11,12,13,14,15,16,17,18]. As shown in the present study, advanced age and PAD may be important factors of SCI because enhanced peripheral collateral circulation caused by systemic atherosclerosis has a possibility of alterations in spinal cord perfusion after FET deployment. A shaggy aorta or arterial calcification may have a risk of postoperative paraplegia. However, there was no difference between the SCI and no-SCI groups in terms of the shagginess score of the aorta or the calcification score of the subclavian and hypogastric arteries, suggesting that spinal cord perfusion may not be influenced by aortic shagginess or arterial calcification.
Although neither intraoperative factors (lowest rectal temperature and circulatory arrest time) nor postoperative factors (minimum values of hemoglobin, mixed venous oxygen saturation, cardiac index, partial pressure of arterial oxygen, and mean blood pressure within 72 h after surgery) showed a distinct relationship to postoperative paraplegia, maintaining higher arterial blood pressure (mean > 80 mmHg) and hemoglobin concentration (> 10 g/dl) with sufficient systemic oxygenation may be important to enhance spinal cord perfusion during the early postoperative period in PAD patients who underwent arch repair with an FET for degenerative arch aneurysms.
The present study has some limitations. This is a single-center and retrospective study. The number of patients is small, and only 2 patients developed postoperative SCI. Further investigation will be needed for elucidating the effects of PAD on the FET-induced paraplegia.
Conclusions
Enhanced collateral circulation to the ischemic lower limbs in patients with combination of degenerative aortic arch aneurysms and PADs may be involved in the development of upper thoracic SCI through vertical blood flow connections, possibly resulting in FET-induced paraplegia after surgery.
Availability of data and materials
Not applicable.
Abbreviations
- SCI:
-
Spinal cord ischemia
- PAD:
-
Peripheral artery disease
- FET:
-
Frozen elephant trunk
- ABPI:
-
Ankle brachial artery pressure index
- CPB:
-
Cardiopulmonary bypass
- SCP:
-
Selective cerebral perfusion
- AXC:
-
Aortic cross clamp
- CA:
-
Circulatory arrest
- eCT:
-
Contrast-enhanced computed tomography
- MRI:
-
Magnetic resonance image
References
Griepp RB, Griepp EB (2007) Spinal cord perfusion and protection during descending thoracic and thoracoabdominal aortic surgery: The Collateral Network Concept. Ann Thorac Surg 83:S865–S869
Griepp RB, Rrgin MA, Galla JD, Lansman S, Khan N, Quintana C, McCollough J et al (1996) Looking for the artery of Adamkiewicz: a quest to minimize palaplegia after operations for aneurysms of the descending thoracic and thoracoabdominal aorta. J Thorac Cardiovasc Surg 112:1202–1215
Griepp EB, Griepp RB (2010) The Collateral Network Concept. Minimizing paraplegia secondary to thoracoabdominal aortic aneurysm resection. Tex Heart Inst J 37:672–674
Kato M, Onishi K, Kaneko M, Ueda T, Kishi D, Mizushima T et al (1996) New graft-implanting method for thoracic aortic aneurysm or dissection with a stented graft. Circulation 94(Suppl II):II188-93
Hosaka A, Motoki M, Kato M, Sugai H, Okubo N (2019) Quantification of aortic shagginess as a predictive factor of perioperative stroke and long-term prognosis after endovascular treatment of aortic arch disease. J Vasc Surg 69:15–23
Yamamoto H, Kadohama T, Yamaura G, Tanaka F, Takagi D, Kiryu K et al (2020) Total arch repair with frozen elephant trunk using the “zone o arch repair” strategy for type A acute aortic dissection. J Thorac Cardiovasc Surg 159:36–45
Kanda Y (2013) Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant 48:452–458
Gao Y, Aravind S, Patel NS, Fuglestad MA, Ungar JS, Mietus CJ et al (2020) Collateral development and arteriogenesis in hindlimbs of swine after ligation of arterial inflow. J Surg Res 249:168–179
Miranda V, Sousa J, Mansilha A (2018) Spinal cord injury in endovascular thoracoabdominal aortic aneurysm repair: prevalence, risk factors and preventive strategies. Int Angiol 37:112–126
Etz CD, Kari FA, Mueller CS, Silovitz D, Brenner RM, Lin H et al (2011) The collateral network concept: a reassessment of the anatomy of spinal cord perfusion. J Thorac Cardiovasc Surg 141:1020–1028
Hanna JM, Anderson ND, Aziz H, Shah AA, McCann RI, Hughes GC (2013) Results with selective preoperative lumber drain placement for thoracic endovascular aortic repair. Ann Thorac Surg 95:1968–1975
Katsargyris A, Okinomou K, Kouvelos G, Renner H, Ritter W, Verhoeven ELG (2015) Spinal cord ischemia after endovascular repair of thoracoabdominal aortic aneurysm with fenestrated and debranched stent grafts. J Vasc Surg 62:1450–1456
Sobel JD, Vartanian SM, Gasper WJ, Hitamoto JS, TAM C, Relly LM (2015) Lower extremity weakness after endovascular aneurysm repair with multibranched thoracoabdominal stent grafts. J Vasc Surg 61:623–629
Feezor RJ, Martin TD, Hess PJ, Daniels MJ, Beaver TM, Klodell CT et al (2008) Extent of aortic coverage and incidence of spinal cord ischemia after thoracic endovascular aneurysm repair. Ann Thorac Surg 86:1809–1814
Drinkwater SL, Goebells A, Haydar A, Bourke P, Brown L, Hamady M, the Regional Vascular Unit, St Mary’s Hospital, Imperial College NHS Trust et al (2010) The incidence of spinal cord ischaemia following thoracic and thoracoabdominal aortic endovascular intervention. Eur J Vasc Enovasc Surg 40:729–735
Bisdas T, Panuccio G, Sugimoto M, Torsello G, Austermann M (2015) Risk factors for spinal cord ischemia after endovascular repair of thoracoabdominal aortic aneurysms. J Vasc Surg 61:1408–1416
Dias NV, Soneson B, Kristmundsson T, Holm H, Resch T (2015) Short-term outcome of spinal cord ischemia after endovascular repair of thoracobdominal aortic aneurysms. Eur J Vasc Endovasc Surg 61:623–629
Scali ST, Wang SK, Feezor RJ, Huber TS, Martin TD, Klodell CT et al (2014) Preoperative prediction of spinal cord ischemia after thoracic endovascular aortic repair. J Vasc Surg 60:1481–1490
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
KK did the writing. HY, TK, and DT reviewed the manuscript. YI and II did the illustration. TW, TA, YY, and WI took part in the patient data collection. The authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
IRB approval: The ethics committee of Akita University Hospital granted approval for publication of the present study (No. 2641), and the need for individual informed consent was waived in this retrospective study.
Consent for publication
Consent of the patients was obtained through the written consent form of our institute.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kiryu, K., Yamamoto, H., Kadohama, T. et al. Risk factors for spinal cord ischemia in frozen elephant trunk–induced upper spinal cord ischemia in patients with combination of degenerative arch aneurysms and peripheral artery diseases: a possible mechanism. Cardiothorac Surg 29, 22 (2021). https://doi.org/10.1186/s43057-021-00060-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43057-021-00060-5