Abstract
Background
Phyllodes tumours (PT) of the breast are rare primary breast neoplasms, mostly seen in fourth to fifth decades of life. These tumours are exceptionally rare in children and young females. The clinico-radiological features often overlap with fibroadenoma. Though imaging plays a pivotal role in providing an initial diagnostic approach, the final confirmation is dispensed on histopathology, which further classifies PT into either benign, low-grade malignant (borderline), or high-grade malignant. On reviewing the literature, only 20 cases have been reported so far in young females, with majority of them being benign and very few numbers of malignant PT.
Case presentation
Herein, we report the clinical and imaging features of PT in four young females, of benign, borderline and high-grade malignant categories as confirmed on histopathology, thereby adding few more numbers to the already existing cases. Additionally, our fourth case depicts rare presentation of high-grade malignant recurrent PT in adolescent female with nodal and unilateral ovarian metastases.
Conclusions
In conclusion, a possibility of PT should be considered in differential diagnosis in a large rapidly enlarging breast mass even in young females with characteristic imaging features, as it alters the plan of management.
Similar content being viewed by others
Background
Phyllodes tumour (PT) is an unusual primary breast tumour constituting less than 1% of total breast tumours and 2–3% of all fibroepithelial breast tumours, with reported incidence of 1 in 100,000 [1,2,3,4,5,6]. Muller in 1838 firstly described it and coined the term "cystosarcoma phyllodes" due to leaf like tumoural extensions within cystic areas of the lesion [1,2,3,4,5,6]. The median age of presentation is fifth decade, with rare occurrence in children, adolescents and young females. Various breast masses encountered in adolescent females are either due to physiologic/developmental abnormalities, inflammatory lesions, fibrocystic changes or tumours [4]. The most common neoplasm in adolescent female breast is fibroadenoma, with breast carcinomas being an unusual sight. Likewise, a thorough literature search revealed only 20 cases of PT in adolescents [1]. Though PT are usually large sized at presentation and possess different biological behaviour, their clinico-radiological picture often overlaps with giant fibroadenomas, posing a diagnostic dilemma.
The relevance behind adequate distinction between PT and fibroadenoma lies in the fact that PT requires surgical management, while fibroadenomas can be safely followed up without further investigations or managed by simple enucleation [6]. Further, it is essential to perform wide excision with adequate margins in all pathological types of PT in an attempt to avoid local recurrence and need for subsequent surgery [6]. Radiologists can play an important role in raising a suspicion and dispensing an alternative preoperative diagnosis of PT over fibroadenoma in young females, based on characteristic imaging pointers on ultrasound(US) and MRI as enumerated in Table 1, thereby guiding the surgeons for adequate management [6,7,8]. Additionally, MRI also plays an essential role in assessment of underlying chest wall invasion if any. Furthermore, CECT chest and abdomen may be done in recurrent/ malignant cases for detecting nodal/distant metastases [6,7,8].
US guided core needle biopsy with histopathology examination not only confirms the aetiology and differentiates PT from giant fibroadenomas, but also aids in the categorization of PT into benign, low-grade malignant (borderline), or high-grade malignant [9,10,11]. Major chunk of adolescent PTs are benign, with a handful of malignant cases [1,2,3,4,5].
Local recurrences can occur in all categories of PT, usually developing within 2 to 3 years. The local recurrence rates in benign, borderline and malignant tumours are nearly 10% to 17%, 14% to 25%, and 23% to 30%, respectively [12,13,14]. Metastasis, however, is almost exclusively seen in malignant PT, disseminating primarily to lungs, bones and brain, and rarely to liver and heart, heralding poor prognosis [12,13,14]. Nodal and ovarian metastases are an exceptionally rare sight in PTs. To the best of our knowledge, only 2 case reports of PTs with ovarian metastasis have been published so far [15,16,17].
The management goal of PT in adolescents is henceforth to maximize breast conservation, with an aim to prevent local recurrence, achieved mostly by wide local excision (WLE) of the tumour with adequate margins.
We herein report clinico-radiological features of PT in four young females ranging from benign to malignant categories as confirmed on histopathology. Moreover, the novelty of our report is that our fourth case contributes in filling the extreme void in existing literature on malignant PTs with nodal and ovarian metastases.
Case presentation
Case 1
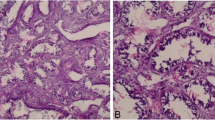
A 17-year-old female presented with complaints of progressively increasing left breast lump for the last 6 months, with new onset of pain for last 15 days; without any nipple discharge. On clinical examination, a large lobulated left breast mass was palpated, occupying all the quadrants of left breast with limited mobility, firm to hard in consistency, causing severe thinning of overlying skin without any skin inflammatory changes or ulcerations. Right breast was normal. Ultrasound (US) showed a well circumscribed predominantly solid heterogeneous mass lesion, involving whole of the left breast. The mass depicted lobulated margins and hypoechoic cleft like anechoic spaces with focal cystic areas showing low level internal echoes within (Fig. 1a). Posteriorly, it was abutting the pectoralis major muscle without any obvious signs of invasion. No significant axillary lymphadenopathy was seen. Colour Doppler revealed internal vascularity within the solid components. On MRI, a large encapsulated, irregular, lobulated heterogeneous intensity soft tissue mass lesion with circumscribed margins was seen involving all the quadrants of left breast. The mass lesion appeared isointense on T1WI, hyperintense on T2WI, with multiple T1W/ T2W/ T2W fat saturated hyperintense linear clefts within (Fig. 1b). Multiple flow voids were seen extending into the lesion from its periphery, communicating with right axillary and internal mammary vessels (Fig. 1b). No skin ulcerations or pectoralis muscle invasion was seen. Dynamic contrast enhanced (DCE) MRI demonstrated intense, heterogeneous, gradually progressive enhancement (Type I kinetic curve) with internal non-enhancing septations and clefts (Fig. 1c, d). The lesion showed focal area of diffusion restriction on diffusion weighted images (DWI: ADC value 0.6 × 10−3) (Fig. 1e, f). Based on these imaging features, a preoperative diagnosis of PT was suggested. Patient underwent surgical excision biopsy, which confirmed the radiological diagnosis of borderline (low-grade malignant) PT (Fig. 1g, h).
(case 1) a Left breast ultrasound image shows a heterogeneously hyperechoic lobulated mass lesion with anechoic linear clefts and cystic spaces within (yellow long arrow). b Sagittal T2W MR image of the left breast shows a large well circumscribed heterogeneous signal intensity mass lesion occupying the entire breast with multiple linear T2 hyperintense fluid signal clefts and cystic spaces (yellow short arrow). Multiple hypointense peripheral flow voids are also seen (red arrows). c Axial DCE MR image of both breasts shows heterogeneous intense enhancement of the left breast mass lesion with non-enhancing clefts and cystic spaces. Normal dense glandular enhancing right breast parenchyma is seen. d DCE type I kinetic curve is seen. e DWI image shows areas of focal hyperintensity (green arrow). f Corresponding ADC map shows focal moderate hypointensity (restricted diffusion). g–i H & E × 10, shows stromal overgrowth and hypercellularity with mild pleomorphism (g). IHC shows cytoplasmic BCL2 positivity in stromal cells (h). IHC shows CD34 positivity in stromal cells (i)
Case 2
A 19-year-old female presented with a rapidly enlarging painless left breast lump. On clinical examination, the lesion was firm to hard in consistency with limited mobility. On US, a well circumscribed, lobulated, predominantly hypoechoic, lesion with parallel orientation, internal anechoic cystic spaces and mild posterior acoustic enhancement was seen (Fig. 2a). No obvious microcalcifications, surrounding architectural distortion, nipple retraction, skin thickening or significant axillary lymphadenopathy seen. The lesion depicted internal vascularity on Colour Doppler (Fig. 2b). On MRI, the mass lesion was seen involving all the quadrants of left breast, with circumscribed margins without any underlying pectoralis muscle invasion (Fig. 2c). It was isointense on T1WI and hyperintense on T2WI with cystic spaces within. On DCE MRI, the mass showed intense heterogeneous enhancement with non-enhancing cystic components and a type I kinetic curve (Fig. 2d). On DWI images, patchy diffusion restriction was observed in the solid components, with mean ADC value of truly restricted areas being 1.5 × 10−3 (Fig. 2e, f). Based on these features, a preoperative diagnosis of PT was made. Finally, this lesion was proven to be a benign PT on excision biopsy.
(case 2 and 3) a Left breast ultrasound image shows a well circumscribed, lobulated, predominantly hypoechoic, wider than taller lesion with internal anechoic cystic spaces and mild posterior acoustic enhancement. b Colour Doppler image shows internal vascularity within the lesion. c Axial T1W MR image of the left breast shows a large, well circumscribed heterogeneous signal intensity mass lesion occupying the entire breast with multiple hyperintense clefts and cystic spaces. No pectoralis muscle invasion seen (red arrow). d Axial DCE MR image of left breast shows heterogeneous intense enhancement of the mass lesion with non-enhancing clefts and cystic spaces. e DWI image shows patchy areas of focal hyperintensity in solid components. f Corresponding ADC map shows focal mild hypointensity (area of true restricted diffusion). g, h US image of right breast shows a well circumscribed hypo to hyperechoic lobulated mass lesion with focal microlobulations (red arrow), areas of posterior acoustic shadowing (red asterisk), internal echogenic septae (yellow arrows) and few small hypoechoic cystic spaces (white arrow)
Case 3
A 22-year-old female presented to breast clinic with complain of pain and lump in right breast for 3 months. Clinically, a firm to hard mobile mass was felt in the outer quadrants of breast. Ultrasound depicted a well circumscribed hypo to hyperechoic lobulated mass lesion with focal microlobulations, areas of posterior acoustic shadowing, internal echogenic septae and few small hypoechoic cystic spaces (Fig. 2g, h). Based on these imaging features, a diagnosis of BIRADS 4a lesion was suggested which was confirmed to a malignant PT on surgical excision biopsy.
Case 4
A 20 year old female patient post WLE status for malignant PT, presented with a rapidly enlarging swelling in the post-operative bed and abdominal fullness for three months, two years after the initial surgery. Clinical examination revealed a large friable reddish brown ulcerated mass at the post-operative bed with a vaguely palpable abdominopelvic mass in the right iliac fossa. Patient underwent CECT chest and abdomen which revealed an irregular heterogeneously enhancing infiltrating mass lesion in the right anterolateral chest wall with rib involvement, few enlarged right axillary, internal mammary and pericardiophrenic lymph nodes (Fig. 3a, c, d). Additionally, a similar morphology right ovarian abdominopelvic mass lesion with moderate ascites was seen (Fig. 3b). A diagnosis of recurrent malignant PT with nodal and right ovarian metastases was made which was proven on HPE. The patient was referred to multidisciplinary and oncology team for further management.
(case 4) a Coronal CECT chest image shows a large irregular heterogeneously infiltrating mass lesion (yellow asterisk) with infiltration of the chest wall and pleura with rib involvement and periostitis (red arrow). b Coronal CECT Abdomen and pelvis shows a large heterogeneously enhancing solid cystic right adnexal mass lesion with non-visualized right ovary (white asterisk) and moderate ascites. Normal left ovary (orange arrow) and uterus (yellow dotted arrow) seen. c, d Axial CECT images show enlarged right axillary node (red dotted arrow), pericardiophrenic node (yellow circle) and internal mammary lymph node (red circle) with bilateral mild pleural effusion and passive atelectasis
Conclusions
Reviewing the literature, most common age group for phyllodes tumour is premenopausal and they are extremely rare in adolescents. However, if a large rapidly growing breast mass with characteristic imaging features is encountered, as seen in our cases, a possibility of PT should be kept, even in this young age group, as it alters the plan of management, owing to its potential ability for recurrences and metastasis based on the final histopathological category.
Availability of data and materials
The content of the manuscript has not been published or submitted for publication elsewhere.
Abbreviations
- PT:
-
Phyllodes tumour
- US:
-
Ultrasound
- MRI:
-
Magnetic Resonance Imaging
- CECT:
-
Contrast Enhanced Computed Tomography
- WLE:
-
Wide Local Excision
- PTs:
-
Phyllodes tumours
- T1WI:
-
T1 Weighted Image
- T2WI:
-
T2 Weighted Image
- DCE MRI:
-
Dynamic Contrast enhanced Magnetic Resonance Imaging
- DWI:
-
Diffusion Weighted Imaging
- ADC:
-
Apparent Diffusion Coefficient
- BIRADS:
-
Breast Imaging Reporting And Data System
References
Erginel B, Celet OB, Yesil OS, Yuksel S, Gun SF, Celik A et al (2015) Management of a benign phyllodes tumor in a 13-year-old girl with trans-position of the nipple areola complex and breast reconstruction. Acta Chir Belg 115:256–259
Galazios G, Dafopoulos K, Gardikis S, Sigalas J, Tamiolakis D, Liberis V et al (2003) Cystosarcoma phyllodes in a 13-year-old Muslim girl treated with conservative surgery: a case report. Eur J Gynaecol Oncol 24:89–90
Pietruszka M, Barnes L (1978) Cystosarcoma phyllodes: a clinical analysis of 42 cases. Cancer 41:1974–1983
Greydanus DE, Parks DS, Farrell EG (1989) Breast disorders in children and adolescent. Pediatr Clin North Am 36:601–638
Rajan PB, Cranor ML, Rosen PP (1998) Cystosarcoma phylloides in adolescant girls and young women: a study of 45 patients. Am J Surg Pathol 22:64–69
Duman L, Gezer NS, Balcı P, Altay C, Başara I, Durak MG et al (2016) Differentiation between phyllodes tumors and fibroadenomas based on mammographic sonographic and MRI features. Breast Care 11:123–127
Wiratkapun C, Piyapan P, Lertsithichai P, Larbcharoensub N (2014) Fibroadenoma versus phyllodes tumor: distinguishing factors in patients diagnosed with fibroepithelial lesions after a core needle biopsy. Diagn Interv Radiol 20:27–33
Jacklin RK, Ridgway PF, Ziprin P, Healy V, Hadjiminas D, Darzi A (2006) Optimising preoperative diagnosis in phyllodes tumour of the breast. J Clin Pathol 59:454–459
Dillon MF, Quinn CM, McDermott EW, O’Doherty A, O’Higgins N, Hill AD (2006) Needle core biopsy in the diagnosis of phyllodes neoplasm. Surgery 140:779–784
Lee AH, Hodi Z, Ellis IO, Elston CW (2007) Histological features useful in the distinction of phyllodes tumour and fibroadenoma on needle core biopsy of the breast. Histopathology 51:336–344
West KW, Rescorla FJ, Scherer LR, Grosfeld JL (1995) Diagnosis and treatment of symptomatic breast masses in the pediatric population. J Pediatr Surg 30:182–186
Limaiem F, Kashyap S (2023) Phyllodes Tumor of the Breast. [Updated 2023 Jan 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing
Joshi SC, Sharma DN, Bahadur AK, Maurya R, Kumar S, Khurana N (2003) Cystosarcoma phylloides: our institutional experience. Autralas Radiol 47:434–437
Tan PH, Thike AA, Tan WJ, Thu MM, Busmanis I, Li H et al (2012) Phyllodes Tumour Network Singapore. Predicting clinical behaviour of breast phyllodes tumours: a nomogram based on histological criteria and surgical margins. J Clin Pathol 65:69–76
Tada Y, Yasunaga M, Tomonobe H, Yamada Y, Hori E, Okugawa K et al (2022) A case of malignant phyllodes tumor of the breast metastasizing to the ovary. Int J Surg Pathol 30:427–431
Durga G, Gandhi JS, Mehta A (2018) Malignant phyllodes tumor metastatic to bilateral ovaries: a Krukenberg-like presentation. J Cancer Res Ther 14:1138–1141
Testori A, Meroni S, Errico V, Travaglini R, Voulaz E, Alloisio M (2015) Huge malignant phyllodes breast tumor: a real entity in a new era of early breast cancer. World J Surg Oncol 13:81
Acknowledgements
Not applicable.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
Dr. AB, Dr. JG and Dr. RM prepared the initial draft, edited and formatted the draft. Dr. PG and Dr. NB contributed to relevant data collection of cases and final formatting. ‘All authors have read and approved the final manuscript’.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Patient consent was taken. Ethical approval not applicable.
Consent for publication
Obtained from the patients and parents wherever applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gupta, J., Bhayana, A., Gupta, P. et al. Case series on phyllodes tumour of breast in young females: Unusual clinico-radiological presentations in unusual age group—Thinking beyond fibroadenomas!. Egypt J Radiol Nucl Med 55, 33 (2024). https://doi.org/10.1186/s43055-024-01207-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-024-01207-0