Abstract
Background
Preeclampsia, a condition very frequently linked to maternal and fetal deaths worldwide, remains a pressing concern due to delays in recognition and response. Effective screening tests for early detection of high-risk cases and appropriate preventive measures are currently lacking. Well planned prenatal care, timely detection, monitoring, and appropriate management are vital to prevent preeclampsia-related fatalities.
Methods
This prospective study evaluated the use of shear wave elastography (SWE) in identifying placental structural issues caused by preeclampsia in high-risk pregnancies. A total of 143 high-risk pregnant women with singleton pregnancies and an anterior placental position were included in the study.
Results
Women with preeclampsia exhibited significantly elevated SWE values in both center (27.98 ± 16.12 vs. 4.57 ± 6.57 kPa) and peripheral areas of the placenta (29.14 ± 16.12 vs. 4.80 ± 7.70 kPa) when compared to non-preeclampsia women (p = 0.000). Cutoff values of 8.70 kPa and 8.15 kPa at the Center and edge of the placenta respectively, accurately predicted preeclampsia in second-trimester pregnancies, with 84.62% sensitivity and 94% specificity. However no significant difference was observed between elastography values obtained from the center and edge of the placenta.
Conclusions
In conclusion, shear wave elastography can help diagnose preeclampsia early by assessing placental stiffness.
Similar content being viewed by others
Background
Preeclampsia is defined as the presence of hypertension and proteinuria occurring after 20 weeks of gestation in a previously normotensive patient [1]. It is a major cause of maternal mortality and morbidity, with preterm birth occurring in approximately 5–8% of cases and perinatal death occurring in 1–3% of cases worldwide [2]. In India, the prevalence of preeclampsia is 2% (WHO [3]. Risk factors associated with the development of preeclampsia include chronic hypertension, antiphospholipid antibody syndrome (APLA), previous history of preeclampsia, advanced maternal age, chronic kidney disease history [4], genetic susceptibility [5], etc. It is more prevalent in women experiencing their first pregnancy (primigravida) and the risk rises with longer intervals between subsequent pregnancies." [5]. Since there are so many commonly prevalent risk factors associated with the development of preeclampsia, it becomes necessary to identify the pregnancies which are at higher risk, for initiating timely preventive measures to improve maternal and fetal outcomes.
Various diagnostic techniques, invasive (maternal serum biomarkers) and non-invasive (such as uterine artery Doppler), have also been used to diagnose preeclampsia. Soluble Fms-like tyrosine kinase-1 (sFlt-1), Soluble Endoglin (sEng), and Placental Growth Factor (PIGF) are the mounting biomarkers for the diagnosis of preeclampsia [6]. The sFlt-1: PlGF ratio is elevated in pregnant women with preeclampsia and could be used as a diagnostic aid for preeclampsia when considered alongside with other clinical findings. Some studies also suggest the use of Uterine Artery Doppler as an early predictor of preeclampsia and Intrauterine growth retardation (IUGR) [7]. However, the reliability of these techniques for predicting preeclampsia is uncertain since positive predictive values for both techniques are unclear [7,8,9]. Additionally, the conventional method of B-mode ultrasonography (USG) primarily provides structural information about the placenta, lacking the ability to assess its biomechanical properties and the relationship between structural disorganization and clinical symptoms associated with preeclampsia [10]. Recently, Ultrasound elastography has gained attention as a non-invasive imaging technique for evaluating tissue stiffness [11]. Shear wave elastography (SWE) is considered the most suitable type of ultrasound elastography as it not only obtains tissue morphological information but also quantifies soft tissue's elasticity values and helps in obtaining information about the mechanical properties related to degeneration, injury, and healing quantitatively [10]. SWE utilizes the principle of inducing transverse shear waves through acoustic radiation force, offering real-time quantitative data on tissue stiffness with high reproducibility and without artifacts or compression effects [12].
Previous studies have examined placental elastography using different techniques, such as acoustic radiation force impulse [13] in conditions like gestational diabetes mellitus [14], preeclampsia and fetal anomalies. These studies consistently demonstrated an increase in placental stiffness compared to normal pregnancies [15]. However, the use of (SWE), which provides deeper tissue response, remains limited [16]. Moreover, most of the existing studies focused on patients already diagnosed with preeclampsia, and there is a lack of focused research on pregnancies at increased risk [14]. Few reports are available on shear wave elastography of the placenta, indicating a scarcity of studies assessing its utility. Therefore, the objective of this study is to evaluate the use of SWE in identifying structural disorganization of the placenta due to preeclampsia in high-risk pregnancies, aiming to facilitate timely detection and monitoring of the disease.
Methods
Study population
This is a prospective cohort study conducted in the radio-diagnosis department of District Civil Hospital, Panchkula, Haryana, India, from Nov 2019 to April 2021. A total of 145 high-risk pregnant women, at a gestational age of 18–26 weeks, who were referred from the department of obstetrics and gynecology and other health facilities, were recruited for the study. High-risk pregnant women with singleton pregnancies and an anterior placental position, who were identified as being at risk of developing preeclampsia based on pre-eclampsia community guideline (PRECOG) [17], were included in the study. The decision to restrict the imaging plane to anterior placenta serves the purpose of preventing deeper beam penetration over the fetus. This approach aims to avoid potential inaccuracies in elasticity calculations, particularly in cases with a posterior placental location in the uterus. Pregnant women with fetal congenital malformation, who had preeclampsia at the time of examination and who were not giving consent for study were excluded. Of the initially recruited 145 patients, 2 were excluded due to congenital malformations. The study included a robust sample of 143 patients who met the inclusion criteria. The progress of all pregnancies was tracked until delivery.
A written informed consent was obtained from all participants before the shear wave measurements were performed. All methods and experiments conducted in the study were in accordance with relevant guidelines and regulations and were approved by the Institutional Ethics Committee.
Measurement
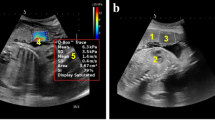
Placental shear wave elastography (SWE) was used as the primary measurement method to assess the stiffness of the placenta. The data collection method involved performing ultrasonography using an Esaote SPA MyLab Eight eXP ultrasound machine with a convex array probe of frequency 2–5 MHz (Fig. 1).
Data collection
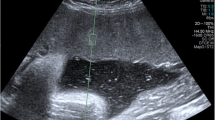
The predefined settings on the Ultrasonography machine were modified to achieve the minimum depth required for clear and accurate imaging. To ensure clear and artifact-free images, an ample amount of transmission gel was applied. During the examination, patients were positioned in supine posture, and were instructed to hold their breath for 5 s. The ultrasound assessment included observing sonographic features such as the normal obstetric scan and shear wave elastography values both at the center and edge of placenta. The measurements were taken from areas that were free from blood vessels and were located away from cord (Fig. 2). A total of 10 elastography values were measured, with 5 obtained from the center (Fig. 3) and 5 from the edge of placenta (Fig. 4). The mean values of these measurement were calculated. All measurements and quality checks were executed by a radiology resident, under the attentive supervision of two radiologists with 23 to 26 years of experience, and with the assistance of a gynecologist with 23 years of expertise. Participants were followed up until the delivery to detect the development of preeclampsia at any stage. During patient admission demographic data, detailed medical and obstetric history of patient was recorded.
Outcome
The primary objective of the study was to investigate the predictive value of shear wave elastography (SWE) for prediction of preeclampsia (PE) among the high-risk pregnant women. The Secondary outcome was to assess whether there were any differences in elastography values between the center and edge of the placenta. Perinatal outcomes, including period of gestation at delivery and newborn birthweight were also recorded.
Statistical analysis
Data was inputted in Microsoft Excel 2010 and then analyzed using SPSS (Statistical Package for the Social Science; SPSS) version 21. The descriptive data was presented by reporting the mean (standard deviation, SD) for continuous variables, while categorical variables were expressed as frequencies and percentages. The Fischer exact test was used to compare categorical variables between groups, and the independent t-test was employed to assess differences in continuous data. The accuracy of predicting the presence of PE was assessed using the receiver operating characteristic (ROC) curve. The result with p-value < 0.05 was considered as statistically significant.
The sample size of 145 patients was determined based on 2% prevalence of the outcome among the unexposed group, a 15% prevalence among the exposed group, an 80% power, and a 5% error level.
Results
In the study, a cohort of 143 patients was included. Out of these patients, 12 were lost to follow-up and 2 experienced miscarriages. As a result, the final analysis encompassed a total of 129 pregnancies with 10% loss to follow up rate (Fig. 5).
The patient characteristics analyzed in the study are summarized in Table 1. Approximately half of the patients included in the study fell within the age range of 19–24 years. The mean gestation age of the patients included was 21.79 ± 2.62 weeks. A total of eight risk factors associated with preeclampsia were documented [17, 18]. Notably, most patients (78%) were identified as nulliparous, meaning they had no previous pregnancies. The average SWE value of the women included in the study during the initial screening was (10.06 ± 15.06) at the center of placenta and (10.49 ± 15.62) at the placental edge (Table 2).
The study demonstrates statistically significant differences between patients with preeclampsia and those with normal pregnancies regarding various factors including age, gestational period at delivery, SWE value (both at center and edge of placenta), and birth weight (Table 3). Out of the total pregnancies 20% women developed preeclampsia. Women diagnosed with preeclampsia displayed a notably higher mean age (p = 0.002). The average elasticity values in both the central (27.98 ± 16.12 vs. 4.57 ± 6.57 kPa) and peripheral areas of placenta (29.14 ± 16.12 vs. 4.80 ± 7.70 kPa) were significantly elevated as compared to normal pregnancies (p = 0.000). Women with preeclampsia had shorter mean gestational age at delivery (36.15 ± 2.12 weeks) in comparison to those with uncomplicated pregnancies [(38.46 ± 2.12 weeks); (p < 0.05)]. Moreover, babies born to women with preeclampsia exhibited lower birth weights [2433 ± 457.9 g vs. 3072 ± 235.6 g in normal pregnancies (p < 0.05)].
The distribution of risk factors between the two groups did not indicate any statistically significant difference (as shown in Table 4).
In terms of the mean values of SWE, the ROC analysis revealed that the optimal cutoff values were 8.70 kPa at the center and 8.15 kPa at the edge of placenta to predict preeclampsia in second trimester pregnancies, achieving 84.62% sensitivity and 94% specificity (as illustrated in Table 5 and Fig. 6). This study shows no significant difference in elastography values between the center and edge of placenta (p = 0.212) (Table 2). The most frequently observed complication among patients who experienced preeclampsia was Intrauterine growth restriction (IUGR). Among the patients with preeclampsia, 53.85% had low birth weight accompanied by IUGR, whereas none of the patients who did not develop preeclampsia faced this issue.
Discussion
To the best of our knowledge, this is the first prospective study demonstrating the role of shear wave elastography in detecting structural disorganization of the placenta seen in patients predisposed to developing preeclampsia in high-risk pregnancies in Indian context. In low- and middle-income countries (LMICs), the failure to identify preeclampsia and delayed response to its signs and symptoms contribute to a significant number of maternal and fetal deaths [18]. This study's strength lies in its extensive sample size, conducted in a District Civil Hospital serving a lower-middle-income population.
Approximately 20% of the pregnant women included in this study developed preeclampsia, while the remaining 80% did not. These findings align with a previous study conducted by Cimsit et al., in 2014, which also reported a 20% incidence of preeclampsia among their patients [19]. The participants in our study were between the ages of 19 and 45, with an average age of 25 years. Similarly, Altunkeser et al. conducted a study in 2018, focusing on the placenta of healthy pregnant individuals aged 18 to 46, with an average age of 26 years [20]. All the subjects recruited for our study were in their second trimester of pregnancy, specifically between 18 and 26 weeks of gestational age. On the other hand, a study by Kilic et al. in 2015 enrolled subjects in both the second and third trimesters of their pregnancies [21].
In this study, we examined how the presence of various risk factors in a subject relates to the occurrence of preeclampsia. However, no statistically significant difference was found between the risk factors of women who developed preeclampsia and those who did not (Table 4). It is worth noting that most subjects in both groups were nulliparous. Specifically, 65.3% of the patients who developed preeclampsia were nulliparous. A previous study by Opitasari et al. [22] reported that nulliparous women had a 78% higher risk of pre-eclampsia compared to primiparous women [adjusted relative risk (RRa) = 1.78; P = 0.000].
The gestational age at the time of birth was compared between the two groups. Preeclamptic subjects had a lower mean gestational age at delivery, specifically 36.16 ± 2.14 weeks, with over 60% of them experiencing preterm birth. In contrast, subjects without preeclampsia had a normal gestational age at birth of 38.09 ± 3.78 weeks, indicating completion of pregnancy to near term. A study by Akbas et al. in 2019 reported similar results, where women with preeclampsia had a mean gestational age of 36.73 ± 2.26 weeks at delivery, while the control group had a mean gestational age of 38.2 ± 1.94 weeks at delivery.
In the present study, birth weight between the two groups was also compared. Subjects who did not develop preeclampsia had a mean birth weight of 3074.71 g and subjects with preeclampsia had a mean birth weight of 2803.33 g. Study conducted by Xiong et al., in 2002 also shows statistically significantly lower birth weight babies among mothers with preeclampsia. In this study, it was found that approximately 54% of all subjects diagnosed with preeclampsia had babies with Intrauterine growth restriction (IUGR), which is a fetal complication observed in preeclampsia cases at an occurrence rate of 10–25% [23].
In women with preeclampsia, there was a significant increase in shear wave elasticity values at both the center (27.98 ± 16.12 kPa) and edge (29.14 ± 16.12 kPa) of placenta, compared to the values at the center (4.57 ± 6.57 kPa) and edge (4.80 ± 7.70 kPa) of placenta in normal pregnancies. A study conducted by Fujita et al. in 2018 also reported a significant difference (p < 0.001) in placental elasticity between healthy subjects and those with preeclampsia [11]. In both groups, when comparing the elasticity values between the center and edge of placenta in the same patient, no significant differences were observed. A similar study conducted by Li et al. [24] also utilized real-time quantitative shear wave elasticity and yielded similar results, showing no significant distinction between the values at the center and edge of placenta.
The diagnostic application of shear wave elasticity in predicting preeclampsia in high-risk pregnancies was analyzed by Receiver Operator Characteristic (ROC) curve. We observed cut-off values at the center and edge of placenta to be 8.70 kPa and 8.15 kPa respectively. The accuracy of the test at the center and edge of placenta was found to be 92.24% and 90.6% respectively. A study conducted by Sirinoglu et al., in 2021 observed cut off value of 7.43 kPa to predict PE in the placentas of first-trimester pregnancies, with 88% sensitivity and 78% specificity [25]. This study correlates well with our findings and we speculate that shear wave elasticity can be used as non-invasive marker for predicting preeclampsia in high-risk pregnancies.
This study possessed several strengths. Preeclampsia (PE) placentas exhibited significant variability in stiffness, which was thoroughly characterized. To ensure the elimination of sampling bias, we adopted a comprehensive approach by conducting SWE measurements over a large acquisition area, encompassing both the center and peripheral regions of placenta. The fact that limits our study is that the shear wave elasticity values were taken only at the time of screening i.e., during second trimester of pregnancy and not during third trimester. Secondly, histopathological studies to correlate structural changes in placenta with the shear wave elasticity values in placenta were not done. Interobserver variation was avoided to minimize repeated scanning of the same fetus. However, the measurements of tissue stiffness with SWE are not operator dependent and we obtained multiple measurements from different areas of same placenta (viz. central and peripheral regions)..
Conclusions
In conclusion, shear wave elastography is a valuable tool for screening and detecting preeclampsia during the second trimester of pregnancy. It helps identify changes in placental elasticity, allowing for early detection. Prompt diagnosis of preeclampsia in community settings is crucial for the health and safety of both the mother and the fetus. By detecting it early, we can prevent delays in providing proper care and timely referral of patients to specialists and ultimately reduce preventable mortality rates for both mothers and fetuses, especially in low- and middle-income countries.
Availability of data and materials
The datasets used and analyzed during the study are available from the corresponding author on reasonable request.
Abbreviations
- SWE:
-
Shear wave elastography
- WHO:
-
World health organization
- APLA:
-
Antiphospholipid antibody syndrome
- USG:
-
Ultrasonography
- PRECOG:
-
Pre-eclampsia community guideline
- PE:
-
Preeclampsia
- SPSS:
-
Statistical Package for the Social Science
- SD:
-
Standard deviation
- ROC:
-
Receiver operating characteristic curve
- POGA:
-
Period of gestational age
- IUGR:
-
Intrauterine growth restriction
- KPa:
-
Kilopascal
- PPV:
-
Positive predictive value
- NPV:
-
Negative predictive value
- AUC:
-
Area under the curve
- LMICs:
-
Low- and middle-income countries
- RRa:
-
Adjusted relative risk
References
Banala C, Moreno S, Cruz Y, Boelig RC, Saccone G, Berghella V et al (2020) Impact of the ACOG guideline regarding low-dose aspirin for prevention of superimposed preeclampsia in women with chronic hypertension. Am J Obstet Gynecol 223(3):419-e1. https://doi.org/10.1016/j.ajog.2020.03.004
Xiong X, Buekens P, Pridjian G et al (2007) Pregnancy-induced hypertension and perinatal mortality. J Reprod Med 52(5):402–406
Abalos E, Cuesta C, Carroli G, Qureshi Z, Widmer M, Vogel JP et al (2014) WHO multicountry survey on maternal and newborn health research network. pre-eclampsia, eclampsia and adverse maternal and perinatal outcomes: a secondary analysis of the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG Int J Obstet Gynaecol 121:14–24. https://doi.org/10.1111/1471-0528.12629
Armaly Z, Jadaon JE, Jabbour A et al (2018) Preeclampsia: novel mechanisms and potential therapeutic approaches. Front Physiol 9:973. https://doi.org/10.3389/fphys.2018.00973
Kim YJ, Williamson RA, Murray JC, Andrews J, Pietscher JJ, Peraud PJ et al (2001) Genetic susceptibility to preeclampsia: roles of cytosine-to-thymine substitution at nucleotide 677 of the gene for methylenetetrahydrofolate reductase, 68–base pair insertion at nucleotide 844 of the gene for cystathionine β-synthase, and factor V Leiden mutation. Am J Obstet Gynecol 184(6):1211–7. https://doi.org/10.1067/mob.2001.110411
Phipps E, Prasanna D, Brima W et al (2016) Preeclampsia: updates in pathogenesis, definitions, and guidelines. Clin J Am Soc Nephrol 11(6):1102–13. https://doi.org/10.2215/CJN.12081115
Papageorghiou AT, Christina K, Nicolaides KH (2004) The role of uterine artery Doppler in predicting adverse pregnancy outcome. Best Pract Res Clin Obstet Gynaecol 18(3):383–96. https://doi.org/10.1016/j.bpobgyn.2004.02.003
Espinoza J, Kusanovic JP, Bahado-Singh R, Gervasi MT, Romero R, Lee W et al (2010) Should bilateral uterine artery notching be used in the risk assessment for preeclampsia, small-for-gestational-age, and gestational hypertension? J Ultrasound Med 29(7):1103–15. https://doi.org/10.7863/jum.2010.29.7.1103
Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC, Sennström M et al (2016) Predictive value of the sFlt-1: PlGF ratio in women with suspected preeclampsia. N Engl J Med 374(1):13–22. https://doi.org/10.1056/NEJMoa1414838
Docking SI, Ooi CC, Connell D (2015) Tendinopathy: is imaging telling us the entire story?. J Orthopaedic Sports Phys Ther 45(11):842–52. https://doi.org/10.2519/jospt.2015.5880
Fujita Y, Nakanishi TO, Sugitani M et al (2019) Placental elasticity as a new non-invasive predictive marker of pre-eclampsia. Ultrasound Med Biol 45(1):93–7. https://doi.org/10.1016/j.ultrasmedbio.2018.09.007
Taljanovic MS, Gimber LH, Becker GW, Latt LD, Klauser AS, Melville DM et al (2017) Shear-wave elastography: basic physics and musculoskeletal applications. Radiographics 37(3):855–70. https://doi.org/10.1148/rg.2017160116
Karaman E, Arslan H, Çetin O, Şahin HG, Bora A, Yavuz A et al (2016) Comparison of placental elasticity in normal and pre-eclamptic pregnant women by acoustic radiation force impulse elastosonography. J Obstet and Gynaecol Res 42(11):1464–70. https://doi.org/10.1111/jog.13078
Yuksel MA, Kilic F, Kayadibi Y, Alici Davutoglu E, Imamoglu M, Bakan S et al (2016) Shear wave elastography of the placenta in patients with gestational diabetes mellitus. J Obstet Gynaecol 36(5):585–8. https://doi.org/10.3109/01443615.2015.1110120
Alan B, Tunç S, Agacayak E et al (2016) Diagnosis of pre-eclampsia and assessment of severity through examination of the placenta with acoustic radiation force impulse elastography. Int J Gynecol Obstet 135(1):43–6. https://doi.org/10.1016/j.ijgo.2016.03.037
Simon EG, Callé S, Perrotin F et al (2018) Measurement of shear wave speed dispersion in the placenta by transient elastography: a preliminary ex vivo study. PLoS ONE 13(4):e0194309. https://doi.org/10.1371/journal.pone.0194309
Milne F, Redman C, Walker J, Baker P, Bradley J, Cooper C et al (2005) The pre-eclampsia community guideline (PRECOG): how to screen for and detect onset of pre-eclampsia in the community. BMJ 330(7491):576–80. https://doi.org/10.1136/bmj.330.7491.576
Salam RA, Das JK, Ali A et al (2015) Diagnosis and management of preeclampsia in community settings in low and middle-income countries. J Fam Med Primary Care 4(4):501. https://doi.org/10.4103/2249-4863.174265
Cimsit C, Yoldemir T, Akpinar IN (2015) Strain elastography in placental dysfunction: placental elasticity differences in normal and preeclamptic pregnancies in the second trimester. Arch Gynecol Obstet 291:811–7
Akbas M, Koyuncu FM, Artunç-Ulkumen B (2019) Placental elasticity assessment by point shear wave elastography in pregnancies with intrauterine growth restriction. J Perinat Med 47(8):841–6. https://doi.org/10.1515/jpm-2019-0238
Kılıç F, Kayadibi Y, Yüksel MA, Adaletli İ, Ustabaşıoğlu FE, Öncül M et al (2015) Shear wave elastography of placenta: in vivo quantitation of placental elasticity in preeclampsia. Diagn Interv Radiol 21(3):202. https://doi.org/10.5152/dir.2014.14338
Opitasari C, Andayasari L (2014) Parity, education level and risk for (pre-) eclampsia in selected hospitals in Jakarta. Health Sci J Indonesia 5(1):35–9. https://doi.org/10.22435/hsji.v5i1Jun.3529.35-39
Gupta LM, Gaston L, Chauhan SP (2008) Detection of fetal growth restriction with preterm severe preeclampsia: experience at two tertiary centers. Am J Perinatol 25(04):247–9. https://doi.org/10.1055/s-2008-1075034
Li WJ, Wei ZT, Yan RL et al (2012) Detection of placenta elasticity modulus by quantitative real-time shear wave imaging. Clin Exp Obstet Gynecol 39(4):470–3
Sirinoglu HA, Uysal G, Nazik H et al (2021) Efficacy of shear wave elastography in predicting preeclampsia in the first trimester. Rev Assoc Med Bras 67:1558–63. https://doi.org/10.1590/1806-9282.20210491
Acknowledgements
The authors would like to acknowledge the study participants for their time and contributions to the study.
Funding
This study had no funding from any source.
Author information
Authors and Affiliations
Contributions
VS conceptualized the study and reviewed the literature. VS and MM collected the data. RK and SS contributed to the data collection. VS and LJ analyzed the data. VS, MM and LJ organized the results, and wrote, revised and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study involving human participants was approved by Biomedical research ethics committee, Pt. B.D. sharma post graduate institute of medical sciences, UHS Rohtak, Haryana (EC/NEW/INST/2022/HR/0189). A written informed consent was obtained from all the patients to participate in the study.
Consent for publication
All patients included in this research gave written informed consent to publish the data obtained in the study.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Singh, V., Kapoor, R., Modi, M. et al. Can placental shear wave elastography predict preeclampsia in high-risk pregnant women during second trimester? Insights from a prospective cohort study. Egypt J Radiol Nucl Med 55, 45 (2024). https://doi.org/10.1186/s43055-024-01205-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-024-01205-2