Abstract
Background
Patients with chronic kidney disease (CKD) are at risk of a many of central nervous system complications such as stroke and cognitive impairment. Studies based on diffusion tensor imaging (DTI) confirmed that the integrity of white matter in CKD patients has changed and these alterations can be observed before tissue morphological changes occurring on conventional MRI. Early use of DTI in CKD patients can distinguish early individuals with normal cognitive system function and will develop cognitive impairment in later stages.
Results
As compared with controls, patients with CKD showed increased MD and decreased FA values in different brain regions with frontal, parietal regions and genu of corpus callosum more affected more than the occipital regions. For combined FA and MD in differentiating cases with CKD, there was statistically significant difference in both thalami, internal capsule, CC, cingulum, ILF and CST as p value < 0.05.
Conclusions
DTI with fiber tractography can present the mainstay of imaging in evaluating the white matter integrity in CKD patient has normal conventional MRI with better diagnostic accuracy in differentiation between healthy and altered white matters.
Similar content being viewed by others
Background
Chronic kidney disease (CKD) affects 0.1% of the general population [1]. It occurs when the abnormality in the kidney function or structure lasts more than 3 months with GFR less than 60 mL/min [2].
Patients with CKD are at risk of a many of central nervous system complications such as stroke, cognitive impairment and encephalopathy [3, 4].
Cognitive impairment affects the quality of life of patients with CKD [5]. It can be reflected by neuroimaging abnormalities caused by different degrees of neurological injury [6]. Because CKD may cause axonal degeneration, demyelination or loss of axon [8], most of the neuroimaging studies focused on the abnormal structure of white matter [7].
In recent years, studies based on diffusion tensor imaging confirmed that the integrity of white matter in CKD patients has changed [9] and these alterations can be observed before tissue morphological changes occurring on conventional MRI [10].
DTI-derived indices (FA and MD) as well as fiber tractography can reflect the integrity of the fiber bundle [11] and white matter tracts in addition to localization of white matter damage to specific brain regions [12]. So, early use of DTI in CKD patients can distinguish early individuals with normal cognitive system function and will develop cognitive impairment in later stages [10].
These study aims to evaluate the ability of DTI to detected brain changes in CKD patients before development of neurological manifestations and abnormalities on conventional MRI.
Material and methods
Patients
The current study was approved by the local ethics committee, and each participant provided informed consent. A total of 170 participants were enrolled in this study from August 2021 to May 2023: 150 patients who had CKD and 20 control healthy participants. Patients (n = 50) with the following criteria were included: (1) no apparent neurological or psychological manifestations in patients with chronic kidney disease, either non-end-stage (NES-CKD) or end-stage renal disease (ESRD) (Table 1), and (2) no evident white matter abnormalities on conventional MRI. They were 34–74 years old (median age = 57.5 years). Patients (n = 100) with apparent conventional MRI abnormalities were excluded.
MRI technique
A 3 T MR scanner (Ingenia; Philips Healthcare Medical Systems, veenpluis, Netherlands) was used to collect the imaging data. To rule out anatomical brain lesions, the standard MR sequences (T1WI, T2WI and FLAIR) were employed for imaging in each participant. Axial, coronal and sagittal planes were the various imaging planes used.
A single-shot EPI sequence with a repetition time of 2950 ms and an echo duration of 70 ms, an image matrix of 128 × 128, a field of view of 235 and a b value of 1000 mm2/s was used to acquire the DTI.
Images post-processing
The DICOM standard was used to store every image. Initially, DTI data registration was done automatically. Later, fiber tracking with sophisticated tools was initiated. In addition to 3D fiber tractography maps, the eigenvalues of the CC, CST, cingulum, internal and exterior capsules were calculated and bilaterally assessed using the relevant region of interest (Figs. 1, 2, 3 and 4).
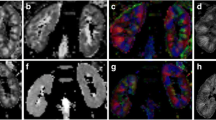
In a 45-year-old healthy female, laboratory investigations revealed normal serum creatinine 0.8 mg/dl [4]. A FLAIR image shows no abnormal SI. B, C axial FLAIR colored image with DTI: (B) of right and left frontal lobes shows normal FA and MD values at both sides (FA = 0.40, MD = 0.82) and (FA = 0.42, MD = 0.86), respectively. C of right and left parietal lobes with normal FA and MD values at both sides with (FA = 0.42, MD = 0.71) and (FA = 0.42, MD = 0.70), respectively. D to I Sagittal FLAIR images with DTT: D normal body of CC shows normal FA and MD values and number of fibers with (FA = 0.41, MD = 0.86, no. of fibers = 9200), E normal genu and splenium of CC show normal FA and MD values and number of fibers with (FA = 0.45, MD = 0.81, no. of fibers = 2756) and (FA = 0.42, MD = 0.87, no. of fibers 2500), F right and left CST shows normal FA and MD values and number of fibers with (FA = 0.52, MD = 0.80, no. of fibers = 21,832) and (FA = 0.51, MD = 0.87, no. of fibers = 22,950), respectively, G normal inferior longitudinal fasciculus shows normal FA and MD values and numbers of fibers (FA = 0.42, MD = 0.79. no. of fibers = 20,850), H cingulum with normal FA and MD values (FA = 0.40, MD = 0.73, no. of fibers = 5965) and I uncinate fasciculus with normal FA and normal MD values (FA = 0.44, MD = 0.73, no. of fibers = 20,550)
A 54-year-old male patient suffered chronic kidney diseases, and laboratory investigations revealed high serum creatinine 4.9 mg/dl. A FLAIR image shows no abnormal SI. B–D Axial FLAIR colored images with DTI: B right and left parietal lobes show decreased FA values and normal MD values with (FA = 0.35, MD = 0.85) and (FA = 0.37, MD = 0.86), respectively, C right and left temporal lobes show decreased FA value and at the right side, normal FA value at left side and normal MD values at both sides (FA = 0.34, MD = 0.80) and (FA = 0.47, MD = 0.86), respectively, and D right and left frontal lobes show decreased FA and normal MD values and no. of fibers at both sides (FA = 0.37, MD = 0.73) and (FA = 0.34, MD = 0.70), respectively. E–I Sagittal FLAIR colored images with DTT: E genu and splenium of CC show increased MD value at splenium, normal MD value at genu and normal FA values at both of them (FA = 0.47, MD = 0.88, no. of fibers = 2560) and (FA = 0.45, MD = 1.02, no. of fibers 1590, respectively). F Body of CC with normal FA values and increased MD values, with (FA = 0.45, MD = 0.99, no. of fibers = 3270), G normal right and left CST shows normal FA and MD values with (FA = 0.40, MD = 0.79, no. of fibers = 2200) and (FA = 0.42, MD = 0.80, no. of fibers = 2580), respectively. H normal inferior longitudinal fasciculus shows normal FA and MD values and no. of fibers (FA = 0.52, MD = 0.71, no. of fibers 2650), I normal cingulum with normal FA and MD values (FA = 0.48, MD = 0.62, no. of fibers 1725)
A 59-year-old female patient suffered chronic kidney diseases, with laboratory investigations revealed high serum creatinine 4.6 mg/dl. A FLAIR image shows no abnormal SI. B–D Axial FLAIR colored image with DTI. B right and left frontal lobes shows decreased FA values at both sides, increased MD values at both sides (FA = 0.38, MD = 0.95) and (FA = 0.36, MD = 0.94), respectively. C Right and left parietal lobes with decreased FA value at both sides, increased MD value at the right side and normal MD values are noted at left side with (FA = 0.29, MD = 0.90) and (FA = 0.35, MD = 0.84), respectively. D Right and left temporal lobes showed decreased FA value at both sides, increased MD value at the right side, normal MD value at left side with (FA = 0.36, MD = 0.90) and (FA = 0.29, MD = 0.83), respectively
Sagittal T2 colored images with DTT in same patient. A Body of corpus callosum with (normal FA = 0.451, increased MD = 1.03 and normal number of fibers 9570). B Genu and splenium of corpus callosum show thinning of tracts of the genu in comparison with the tracts of the splenium with (FA = 0.366, MD = 0.903, no. of fibers = 497) and (FA = 0.463, MD = 0.964, no. of fibers = 3021, respectively). C Right and left CST show mild thinning of left CST in comparison with the right one with (FA = 0.458, MD = 0.959, no. of fibers = 2250) and (FA = 0.428, MD = 0.120, no. of fibers = 1520), respectively. D Inferior longitudinal fasciculus shows (FA = 0.468, MD = 0.848, no. of fibers = 950). E Sagittal DTT image of the cingulum showed normal tract with (FA = 0.431, MD = 0.792 and number of fibers = 850)
Statistical analysis
Data were entered and analyzed using IBM-SPSS software. The Chi-square test was used to assess the association between two nominal variables. The independent samples t-test was used to compare normally distributed quantitative data between two groups. The Mann–Whitney U-test was used, and results were considered as statistically significant if p value ≤ 0.050. ROC curve was used to find the cutoff value that best discriminates diseased from non-diseased. An area under the ROC curve was reported (AUC). Appropriate charts were used to graphically present the results whenever needed.
Results
This study was conducted on 170 people, including 150 patients suffering from chronic kidney disease without cognitive impairment and 20 healthy people. In total, 100 patients were excluded from completing the study because they had abnormal white matter on FLAIR and T2WI and the study was completed only on 20 healthy people and 50 patients who had normal white matter on conventional MRI. The patient group included 34 males and 16 female patients with CKD (their median age = 57.5 years). Their median serum creatinine was 2.65 mg/dl, ranging from 1.1 to 8.8 mg/dl, and their median eGFR was 25.5 ml/min/1.73 m2, ranging from 6 to 74 ml/min/1.73 m2. The control group consisted of 20 healthy individuals. Their median serum creatinine was 0.85 mg/dl, ranging from 0.6 to 1.0 mg/dl, and their median eGFR was 107 ml/min/1.73 m2, ranging from 92 to 134 ml/min/1.73 m2 as illustrated in Table 1 which showed that serum creatinine was statistically significantly higher, and both eGFR and hemoglobin were statistically significantly lower in CKD cases vs. control subjects.
There is a significant difference between CKD patients and controls in the measurement of means of FA and MD (10−3 mm2/s) in different brain lobes (Figs. 5, 6, 7 and 8), thalami, internal capsules and corpus callosum (Figs. 9 and 10), as p value < 0.05 and demonstrated in Tables 2 and 3. The median of FA values in the four brain lobes was significantly lower than controls while that of MD values was higher compared to the controls tables (Tables 2 and 3).
ROC curves was performed to study the significant cutoff values of FA discriminating between CKD and healthy control cases showed that the best significance being at right occipital (A) and left frontal regions (B) (cutoff value ≤ 0.43 and 0.41). Significant predictive power of FA showing AUC 0.973 and 0.983, p value < 0.001, 100% and 93.3% sensitivity, and 95% and 90% specificity), respectively
Roc curves was performed to study the significant cutoff values of MD discriminating between CKD and healthy control cases showed that the best significance being at right occipital region cutoff value > 0.79. Significant predictive power of MD within right occipital region showing AUC 0.993, p value < 0.001, 100% sensitivity, 95% specificity
ROC curve demonstrates that A genu of CC cutoff value ≤ 0.43 and showed also significant predictive power of FA with (AUC 0.977, p value < 0.001), 93.3 sensitivity, 95% specificity, B, C also body and splenium of corpus callous MD (cutoff value > 0.89 and 0.90) showed significant predictive power (AUC 0.993 and 0.975, p value < 0.001, 96.7% and 100% sensitivity, and 95% and 90% specificity, respectively)
The median of MD values in the right frontal lobe was 0.895 (range 0.73–1.14), left frontal was 0.900 (range 0.76–1.02), right parietal was 0.805 (range 0.73–0.91), left parietal was 0.840 (range 0.75–1.03), right temporal was 0.830 (range 0.73–1.1), left temporal was 0.870 (range 0.77–1.1) right occipital region was 0.990 (range 0.83–1.50) and left occipital lobe was 0.935 (range 0.12–1.30) (Table 2).
The median of FA value in right frontal lobe was 0.355 (range 0.23–0.42), left frontal was 0.350 (range 0.24–0.42), right parietal was 0.350 (range 0.28–0.41), left parietal was 0.360 (range 0.25/0.40), right temporal was 0.365 (range 0.29–0.43), left temporal was 0.365 (range 0.29–0.43), right occipital was 0.350 (range 0.28–0.43) and left occipital lobe was 0.360 (range 0.24–0.42) (Table 3).
Discussion
It is frequently seen that neurological impairment occurs in people with chronic kidney disease (CKD). Because cognitive dysfunction is so common, this neurodegeneration is strongly associated with microstructural damage to the brain's white matter. Nonetheless, it is still unknown how precisely cognitive system developmental abnormalities are caused [11].
This study was conducted on healthy peoples and CKD patients who did not show symptoms of cognitive impairment and have normal conventional MRI, and imaging data were acquired on a 3 T MR scanner. The results were compared with previous studies that were based on CKD patients who suffer from cognitive impairment. Their studies used 1.5 T scanner, and the results are taken as a reference for us.
The microstructural arrangement of the white matter in the brain can be examined by DTI [13]. The characteristic of our work is the variations in DTI parameters in several brain areas in both CKD patients and control individuals. The degree of anisotropy in tissues that reflects the integrity of the fiber bundle can be evaluated using fractional anisotropy, or FA. There is more diffusion anisotropy when the FA value is larger [14]. Mean diffusivity (MD), independent of dispersion direction, can represent the resistance of the entire molecule. More free water molecules are present in the tissue when there is a larger MD brought on by modifications in the white matter architecture [15].
Previous DTI-based studies have found that CKD patients who suffer from cognitive impairment have abnormal WM integrity in multiple WM tracts in the corpus callosum (CC), anterior thalamic radiation (ATR) and inferior longitudinal fasciculus (ILF) due to the toxic effect of CKD itself [16].
The metabolic disorders and toxic effect of CRD caused by uremia or dialysis may be the major factor for the alteration of the DTI indices in the corpus callosum and other white matter tissues which result in cognitive dysfunction. The decrease in FA may suggest the axonal degeneration or demyelination caused by CRD, and the increase in the diffusivity indices may reflect the loss of axon and myelin [17].
This idea was made clear in this study, which showed that fronto-parietal regions and corpus callosum were the most affected WM tract followed by thalami and ILF. The median FA demonstrated a statistically significant reduction in the cases with CKD in comparison with the control subjects in the following regions: fronto-parietal regions, genu of CC, anterior limb of internal capsules as well as caudate nuclei. The median MD values were increased compared to controls in the following regions: corpus callosum including genu, body and splenium mainly, occipital and frontal regions with fronto-parietal region predominance. This aligns with study by Drew et al. [14] who demonstrated that patients with CKD showed reduced FA and increased MD values particularly in the fronto-temporal regions among CKD patients in comparison with control subjects indicating more isotropic diffusion owing to WM abnormalities.
According to Jiang et al. [16], the anterior limb of the internal capsule, specifically, was found to have abnormal white matter microstructure in CKD patients, as evidenced by decreased white matter fractional anisotropy and increased mean diffusivity. Also, we noted that the anterior limb of internal capsule is affected in about 60% of patients.
Steinbach et al. [18] reported that the corpus callosum plays a basic role in cognitive functions and reduced FA within the body of the corpus callosum was noted in CKD patients. Guo et al. [19] mentioned that FA values have decreased in CKD patients and more evident in the frontal regions including the fornix and genu of corpus callosum that develops gradually with the course of CKD. These studies are in line with our study stating that FA was reduced in body of CC in 67% of patients. In the region of CC, FA value in the genu of corpus callosum was 0.380 (0.33–0.46), body of CC was 0.40 (0.37–0.48) and splenium of CC was 0.420 (0.36–0.49) that was significantly lower than the control group.
FA in cingulum and internal capsule was reduced according to Chen et al. [20]. FA decreased and MD increased in the internal capsule and thalami compared with that of the control group regarding Zhang et al. [21]. This aligns our study in which FA was reduced in cingulum in 50% of patients, posterior limb of internal capsule in 20% of patients, left thalami in 30% of patients and right in 3% of patients.
Some specific white matter tracts such as the CST and ILT showed decreased FA and increased MD [20]. These results match our study in which left CST affected in 50% of patients and ILF in 25% of patients.
We noticed in this study that using 3 T MRI in our work gave the same results as other research that used 1.5 T MRI.
We have faced some limitations in this study. First, the study was based only on CKD patients who do not have symptoms of cognitive impairment. If also included those with symptoms of cognitive impairment, it would be better in term of final results. Second, there is no follow up of the patients, so we do not know whether they showed symptoms on cognitive impairment or not in the future. Third, we did not compare the degree of changes in DTI parameters with the severity of CKD, and in the future, patients with different stages of CKD should be included to explore the correlation between disease progression and white matter changes.
Conclusions
DTI with fiber tractography present a promising advanced technique in evaluating white matter integrity among neurologically normally apparent CKD patients. Altered DTI parameter and DTT can predict the occurrence of cognitive impairment in the future with an acceptable rate. Using a 3 T MRI scanner gives the same results as using a 1.5 T MRI scanner.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CKD:
-
Chronic kidney disease
- DTI:
-
Diffusion tensor imaging
- DTT:
-
Diffusion tensor tractography
- FA:
-
Fractional anisotropy
- MD:
-
Mean diffusivity
- CC:
-
Corpus callosum
- ATR:
-
Anterior thalamic radiation
- ILF:
-
Inferior longitudinal fasciculus
- WM:
-
White matter
- CST:
-
Corticospinal tract
References
Romagnani P, Remuzzi G, Glassock R, Levin A, Jager J et al (2017) Chronic kidney disease. Nat Rev Dis Primers 3:17088. https://doi.org/10.1038/nrdp.2017.88
Drew DA, Weiner DE, Sarnak MJ (2019) Cognitive impairment in CKD: pathophysiology, management, and prevention. Am J Kidney Dis 74:78290. https://doi.org/10.1053/j.ajkd.2019.05.017
Arnold R, Issar T, Krishnan V, Pussell A (2016) Neurological complications in chronic kidney disease. JRSM Cardiovasc Dis 5:2048004016677687. https://doi.org/10.1177/2048004016677687
Matsuda-Abedini M, Fitzpatrick K, Harrell WR, Gipson DS, Hooper SR et al (2018) Brain abnormalities in children and adolescents with chronic kidney disease. Pediatr Res 84(3):387–392. https://doi.org/10.1038/s41390-018-0037-5
Penton AA, Lau H, Babikian VL, Shulman J, Cervantes-Arslanian A et al (2020) Chronic kidney disease as risk factor for enlarged perivascular spaces in patients with stroke and relation to Racial Group. Stroke 51:334851. https://doi.org/10.1161/STROKEAHA.119.028688
Sember V, Bogataj Š, Ribeiro C, Paravlić A, Pajek M et al (2021) Accelerometry correlates in body composition, physical fitness, and disease symptom burden: a pilot study in end-stage renal disease. Front Physiol 12:737069. https://doi.org/10.3389/fphys.2021.737069
Meurs M, Roest M, Groenewold A, Franssen F, Westerhuis R et al (2016) Gray matter volume and white matter lesions in chronic kidney disease: exploring the association with depressive symptoms. Gen Hosp Psychiatry 40:18–24. https://doi.org/10.1016/j.genhosppsych.2016.02.005
Murea M, Hsu C, Cox J, Hugenschmidt E, Xu J et al (2015) Freedman, structural and functional assessment of the brain in European Americans with mild-to-moderate kidney disease: diabetes heart study-MIND. Nephrol Dial Transplant 30:1322–1329. https://doi.org/10.1093/ndt/gfv030
Liu M, Wu Y, Wu X, Ma X, Yin Y, Fang H et al (2020) White matter microstructure changes and cognitive impairment in the progression of chronic kidney disease. Front Neurosci 14:559117. https://doi.org/10.3389/fnins.2020.559117
Zhang R, Liu K, Yang L, Zhou T, Qian S et al (2015) Reduced white matter integrity and cognitive deficits in maintenance hemodialysis ESRDpatients: a diffusion-tensor study. Eur Radiol 25:661–668. https://doi.org/10.1007/s00330-014-3466-5
Kim S, Park W, Bai S, Jeong Y, Hong H et al (2011) Diffusion tensor imaging findings in neurologically asymptomatic patients with end stage renal disease. Neuro Rehabil 29(1):111–116. https://doi.org/10.3233/NRE-2011-0684
Elsorogy G, Abdelrazek M, Awad A, Abdelwahab M (2022) Altered diffusion tensor imaging (DTI) parameters in brain parenchyma after traumatic brain injury. Egypt J Radiol Nucl Med 53(1):1–9
Chou C, Hsieh TJ, Lin L, Hsieh YT, Li WZ et al (2013) Widespread white matter alterations in patients with end-stage renal disease: a voxelwise diffusion tensor imaging study. Am J Neuroradiol 34(10):1945–1951. https://doi.org/10.3174/ajnr.A3511
Drew A, Koo B, Bhadelia R, Weiner E, Duncan S et al (2017) White matter damage in maintenance hemodialysis patients: a diffusion tensor imaging study. BMC Nephrol 18:213. https://doi.org/10.1186/s12882-017-0628-0
Chou C, Ko H, Hsieh TJ, Chang M, Chung S (2019) A preliminary report of longitudinal white matter alterations in patients with end-stage renal disease: a three-year diffusion tensor imaging study. PLoS ONE 14(4):e0215942. https://doi.org/10.1371/journal.pone.0215942
Jiang Y, Gao Q, Liu Y, Gao B, Che Y et al (2021) Reduced white matter integrity in patients with end-stage and non-end-stage chronic kidney disease: a tract-based spatial statistics study. Front Hum Neurosci. https://doi.org/10.3389/fnhum.2021.774236
Mu J, Ma L, Ding D, Ma X, Li P, Li R et al (2021) White matter characteristics between amygdala and prefrontal cortex underlie depressive tendency in end stage renal disease patients before the dialysis initiation. Brain Imaging Behav 15:1815–1827. https://doi.org/10.1007/s11682-020-00376-6
Steinbach EJ, Harshman LA (2022) Impact of chronic kidney disease on brain structure and function. Front Neurol. https://doi.org/10.3389/fneur.2022.797503
Guo H, Liu W, Li H, Yang J (2021) Structural and functional brain changes in hemodialysis patients with end-stage renal disease: DTI analysis results and ALFF analysis results. Int J Nephrol Renovasc Dis. https://doi.org/10.2147/IJNRD.S297402
Chen HJ, Zhang LJ, Lu GM (2015) Multimodality MRI findings in patients with end-stage renal disease. BioMed Res Int. https://doi.org/10.1155/2015/697402
Zhang C, Yu H, Cai Y, Wu N, Liang S et al (2022) Diffusion tensor imaging of the brain white matter microstructure in patients with chronic kidney disease and its correlation with cognition. Front Neurol. https://doi.org/10.3389/fneur
Acknowledgements
The authors acknowledge subjects for their participation and cooperation in this study.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
SA and DR collected the data, revised the sample collection, diagnosed the images, and wrote and prepared the manuscript in its full presentation. LE, ME and WM supervised the final work and revised the study outcomes. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee. The study was accepted by IRB, Faculty of Medicine, Mansoura University, under the code of MS.21.01.1352. An informed written consent was taken from each participant involved in this study prior to the conduct of any study-related activities. All data obtained from participants were confidential and were not used outside the study. The patients had the rights to withdraw from the study at any time without giving any reason.
Consent for publication
All patients included in this study gave a written informed consent to publish the data contained in this study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moustafa, S.A.F., Rizk, D.A.E., El Serougy, L.G. et al. Diffusion tensor imaging of brain changes in patients with chronic kidney disease before cognitive impairment with 3 T MRI device. Egypt J Radiol Nucl Med 55, 6 (2024). https://doi.org/10.1186/s43055-023-01173-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-023-01173-z