Abstract
Background
Diagnosis of tumour thrombosis and differentiating it from benign thrombosis are essential for managing patients, planning treatments, and minimising unneeded anticoagulation therapy. Bland thrombi occur in both cancer and non-cancer patients; tumour thrombi and bland can coexist. 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) is useful in detecting and diagnosing tumour thrombosis and distinguishing it from benign thrombosis.
Objective
This study’s aim was to assess the value of 18F-FDG PET/CT in distinguishing benign from malignant portal vein thrombosis (PVT) in liver cirrhosis patients.
Methods
A retrospective study was conducted on 38 patients who had PVT that was histopathologically confirmed and performed 18F-FDG PET/CT scans at our institute between January 2021 and April 2022. For all patients, sociodemographic data, visual analysis, semiqualitative analysis (SUVmax value), and associated hepatic pathology were collected.
Results
The SUVmax values were significantly higher in the tumour thrombosis group (6.26 ± 1.94), compared to the bland thrombosis group (1.79 ± 0.69), (P < 0.001). The ROC curve of semiqualitative analysis (SUVmax) revealed a sensitivity of 96.3% and a specificity of 36.4%, at area under curve of 0.827 with SUVmax > 3.5 as the pathological cut-off value to distinguish tumour from bland thrombi.
Conclusions
By using semiqualitative analysis, 18F-FDG PET/CT is a valuable new technique in differentiating between neoplastic and bland PV thrombi, with optimal cut-off SUVmax value > 3.5 as a criterion.
Similar content being viewed by others
Background
Tumour thrombosis is an unusual clinical condition. Diagnosis of tumour thrombosis and differentiating it from benign thrombosis is essential for managing patients, planning treatments, and minimising unneeded anticoagulation therapy. Bland thrombi occur in both cancer and non-cancer patients; tumour thrombi and bland can coexist [1].
A blood clot inducing narrowing or occlusion of the portal vein is known as portal vein thrombosis (PVT) [2]. The thrombus may involve any vein of the portal venous system [3]. PVT is frequently associated with liver cirrhosis. Cancer, abdominal sepsis, pancreatitis, systemic lupus erythematosus, or other conditions, especially those involving hypercoagulable states, can result in PVT [4].
HCC frequently invades the portal vein resulting in tumoural thrombosis [5]. This occurs in approximately 35% of HCC patients and is related to advanced tumours and a bad prognosis [6].
Recent studies have primarily used multislice CT (MSCT) and colour Doppler ultrasound to identify and characterise thrombi. MSCT can detect morphological changes, but 18F-FDG PET/CT is capable of detecting the metabolic abnormalities that occur before those changes. Numerous sporadic reports [7,8,9] and retrospective studies [10, 11] have demonstrated the valuable PET/CT role in the detection of tumour thrombosis. Prior research revealed different cut-off values of the maximum standardised uptake (SUVmax) for the different tumours, concluding that PET/CT can distinguish tumour from benign thrombi based on the SUV [12].
Any solid malignant tumour has the potential to develop malignant venous thrombosis, but it is most common in the liver, renal, colon, as well as pancreatic cancers. Serious prognostic implications result from venous thrombosis, particularly in the portal vein. As a result, distinguishing tumour thrombosis from benign PVT is critical. Pathological examination is the gold standard. In the clinical practice, however, imaging and laboratory results are frequently combined to establish the diagnosis [13].
Methods
An IRB-approved retrospective study (Registration number: 00341/2022) was conducted on 38 patients who had liver cirrhosis with PVT, either with or without malignant hepatic tumours, and had performed 18 F-FDG PET/CT during January 2021 to April 2022.
Inclusion criteria
-
Patients having liver cirrhosis with PVT diagnosed through a contrast-enhanced MSCT study underwent histopathological examination of hepatic focal lesions one month before PET/CT scan and clinical and imaging follow-up for those with no focal hepatic lesions.
-
Both sexes ranged in age from 40 to 60.
Exclusion criteria
-
Patients who do not have PVT.
18F-FDG PET/CT scanning
All patients performed 18F-FDG PET/CT scans (Siemens, Biograph mCT 128, Siemens Medical Solutions, Knoxville, USA). The patients were instructed to refrain from eating or drinking for a roughly 6 h prior to the injection, including cessation of tube feedings, dextrose-containing intravenous fluids, and parenteral hyperalimentation. Do minimal physical activity 24 h prior the scan. No caffeine and nicotine products are allowed (12 h before scan). A high-protein, low-carbohydrate diet is required (24 h before scan). If the patients have routine non-diabetic medications take them with plain water at the usual times. Wear warm clothes at home and on the way to the institute. Only plain water is permitted, 1 L of plain water as tolerated before arriving to the institute. Do serum creatinine (within 15 day), and remove metal objects, such as a belt, jewellery, dentures, and eyeglasses, which might interfere with image results. Euglycaemia (a blood glucose level must not exceed 150 mg/dL) was determined by measuring serum glucose levels prior to injection. Then, a dose of 370–550 MBq of 18F-FDG was administered via antecubital vein injection along with a saline infusion. PET/CT scan started approximately 45–60 min after tracer injection.
The patients were scanned in the supine position with the patients’ arms raised above their heads. A low-dose 128 slice MSCT scan was acquired with a field of view (FOV) of 780 mm, 150 kV, 50 mAs, and a 512 × 512 matrix size from the skull base to the midthigh.
Then, the PET scan was obtained with a 3 min acquisition time per bed position, and imaging was done using sequentially overlapping bed positions.
Following the PET scan, a contrast-enhanced 128 slice MSCT was acquired from skull base to midthigh. A total dose of 80 to 100 ml and at a rate of 1.5–3 ml/kg body weight, the contrast agent was injected intravenously automatically. The FOV was 78 cm, with 512 × 512 reconstruction matrix, 120 kV and 150 mAs. The coronal and the sagittal images were reconstructed in 1 mm increments with a slice thickness of 1.5 mm for multi-planar evaluation.
Imaging analysis
Two radiologists (M.H. and S.A., with 13 and 8 years of experience, respectively) reviewed the PET/CT scans independently. The tumour and PVT were known to these medical professionals, but they were not aware of the histological and clinical follow-up findings. Consensus was used to resolve disagreements.
The PET/CT scans were evaluated visually and semiqualitatively (SUVmax measurement).
Visual analysis
The multi-modality workstation was used to perform visual analysis of the PET/CT scans. Increased metabolic activity in the form of focal nodular or linear branching patterns conformed to the portal vein was used to show the presence of thrombus on the PET/CT scans.
The FDG uptake level of the thrombus, when compared to the uptake of the descending aorta at the same level, can be categorised into four categories: no uptake; uptake less than; uptake equal to; and uptake more than the descending aorta. The last category was regarded as specific for tumour thrombosis. The first three categories were regarded as particular to bland thrombosis.
On the contrast-enhanced 128 slice MSCT, the filling defect of the involved vein (main portal vein, its intrahepatic right and left branches), as well as direct invasion from the tumour, was also reported.
Semiqualitative analysis
The focal metabolic activity (SUVmax) of the involved vein was measured on the PET/CT scans. The ROI was identified using contrast-enhanced 128 slice MSCT at the PVT site. All lesions had SUVmax measured. These values were classified as tumour or bland thrombosis uptakes based on histology or radiological criteria and clinical follow-up.
Study outcome
The following criteria were used to define tumoural thrombosis; both the visual analysis: where the tumoural thrombus FDG uptake level is more than the uptake of the descending aorta, the other criterion is SUVmax value > 3.5. The PET/CT findings were histopathologically confirmed as well as clinical and imaging follow-up. Tumour thrombus had a significantly higher SUVmax than benign thrombus.
Statistical analysis
SPSS version 21 was used to compile and analyse all data (SPSS Inc., Chicago, IL). The continuous variables were represented by mean, mode, range, and standard deviation and were compared using the Student’s t test, whereas categorical variables were represented by percentages, and were compared using the chi-squared test (X2). The cut-off values of the receiver operating characteristic (ROC) curves were measured. P value ≤ 0.05 was considered statistically significant.
Results
Of the 51 patients who attended to our radiology department, 13 patients were prohibited retrospectively from the study (did not meet the inclusion criteria as no PVT), and 38 patients joined this study. Thus, 11 patients had bland (benign) thrombosis, and 27 patients had tumour (malignant) thrombosis. Each group was divided into male and female.

Flowchart of the studied patients.
This retrospective study included 38 (30 male and 8 female) patients, age mean was 56.47 ± 5.40 years, and patient’s ages ranged between 47 and 67 years (Table 1).
In our study, most of the studied cases had affected right portal vein branch (78.9%).
Liver cirrhosis with HCC was the most common among the cases studied (65.8%) (Fig. 1).
Visual analysis
PET/CT images visual analysis showed different FDG uptake levels in the thrombus. FDG uptake level in the thrombus higher than that of the descending aorta was found in 27 cases (71.05% of all the studied cases), all of which were histopathological proven to be tumour thrombi (Figs. 2, 3), while no uptake, uptake ≤ the uptake of the descending aorta was noticed in 3, 6, and 2 cases (7.89%, 15.79%, and 5.26%, respectively, of all the studied cases) and all were histopathological proven to be bland thrombi (Table 2) (Figs. 4, 5) with a significant difference (P = 0.001).
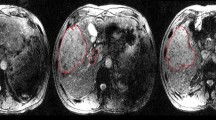
A 61-year-old female patient with liver cirrhosis and right hepatic, and caudate lobes HCC lesions associated with segmental right portal vein and IVC malignant thrombosis and mild ascites. a, b Axial contrast-enhanced MSCT images. c, d Axial fused PET/CT images. e, f Axial PET images. g Coronal PET MIP image. h Sagittal contrast-enhanced MSCT image revealed linear increased FDG uptake (SUVmax 8.6) corresponding to segmental right portal vein malignant thrombosis (red arrows), right hepatic lobe and caudate lobe HCC lesions (white arrows), an active satellite nodule (yellow arrows)
A 67-year-old male patient with liver cirrhosis and right hepatic lobe HCC lesion associated with right portal vein malignant thrombosis. a–c Axial contrast-enhanced MSCT, axial fused PET/CT, and axial PET images. d–f Coronal contrast-enhanced MSCT, coronal fused PET/CT, and coronal PET images revealed linear increased FDG uptake (SUVmax 4) corresponding to right portal vein malignant thrombosis (orange arrows) and increased FDG uptake corresponding to right hepatic lobe HCC lesion (white arrows) and porta hepatis metastatic lymph nodes (blue arrows). Also note increased FDG uptake in the regional spine corresponding to metastatic spinal deposits
A 47-year-old male patient with liver cirrhosis and subtotal benign thrombosis in the left portal vein and mild ascites. a–c Axial contrast-enhanced MSCT, axial fused PET/CT, and axial PET images. d–f Sagittal contrast-enhanced MSCT, sagittal fused PET/CT, and sagittal PET images revealed low-grade FDG uptake (SUVmax 1.8) corresponding to subtotal benign left portal vein thrombosis (orange arrows)
A 61-year-old male patient with liver cirrhosis, subtotal benign main portal vein thrombosis and marked ascites. a–c Axial contrast-enhanced MSCT, axial fused PET/CT, and axial PET images. d–f Coronal contrast-enhanced MSCT, coronal fused PET/CT, and coronal PET images revealed low-grade FDG uptake (SUVmax 1.4) corresponding to subtotal benign main portal vein thrombosis (orange arrows)
In the 24 studied cases, focal nodular FDG uptake pattern was frequent in tumour thrombosis (20 cases) (representing 52.6% of all cases) than in bland thrombosis (only 4 cases) (representing 10.5% of all cases), with a significant difference between both groups (P = 0.029). On the contrary, linear branching FDG uptake pattern was equal in both tumour and bland thrombosis (7 cases each), and there was no significant difference in focal nodular and linear branching FDG uptake pattern between the two groups (P = 0.435) (Table 2).
No significant differences were in the age or the gender between the bland thrombosis and tumour thrombosis study groups (P > 0.05).
Semiqualitative analysis
Furthermore, the SUVmax values (Fig. 6) were significantly higher in tumour thrombosis group (6.26 ± 1.94) than in bland thrombosis group (1.79 ± 0.69), (P < 0.001) (Table 3).
Tumour thrombus SUVmax (6.26 ± 1.94, range 4.2–10, median 6.5) was significantly higher (P < 0.001) than bland thrombus (1.79 ± 0.69, range 0.0–2.8, median 1.5).
The ROC curve of semiqualitative analysis (SUVmax) (Fig. 7) revealed a sensitivity of 96.3% and a specificity of 36.4%, at area under curve of 0.827 with SUVmax > 3.5 as the pathological cut-off value to distinguish tumour from bland thrombi (Table 4).
Discussion
The diagnosis of tumour thrombosis and differentiating it from benign thrombosis is essential for managing patients, planning treatments, and minimising unneeded anticoagulation therapy [1].
In this study, we assessed the value of FDG PET/CT in differentiating the tumoural from the bland PVT in patients with liver cirrhosis.
The visual analysis was done in our study with the FDG uptake of the descending aorta which is taken as a reference, and FDG uptake in the thrombus more than that of the descending aorta was noticed in most of (71.05%) the studied cases, all of which were histopathological proven to be tumour thrombi, while no uptake, uptake ≤ that of the descending aorta was observed in 7.89%, 15.79%, and 5.26%, respectively, of all the studied cases, and all were histopathological proven to be bland thrombi with a significant difference (P = 0.001).
In all cases, focal nodular FDG uptake pattern was frequent in tumour thrombosis (52.6% of all cases) than in bland thrombosis (10.5% of all cases), with a significant statistical difference between both groups (P = 0.029). On the contrary, linear branching FDG uptake was equal in both tumour and bland thrombosis, and there was no significant difference in focal nodular and linear branching FDG uptake between the two studied groups (P = 0.435).
Hu et al. [14] discovered that the visual analysis of the FDG uptake level was done and had a sensitivity (91.5%), specificity (64%), and an accuracy (81.9%). 18F-FDG uptake pattern in tumour thrombi appears as a linear or a small focal tumour infiltration. There may not be any significant tumour infiltration and high FDG uptake in a large thrombus [15].
In our study, only the malignant group had direct tumour extension into the vessel lumen on post contrast 128 slice MSCT images. The dilatation of the involved vessel is associated with venous thrombosis, but it does not distinguish between malignant and benign portal venous thrombosis.
In addition, both the tumoural and the benign venous thrombi showed increased FDG uptake, and tumoural thrombi displayed greater FDG uptake than benign thrombi, according to Sharma et al. [10] study of 24 cancer patients having hypermetabolic venous thrombosis. Despite the fact that the aforementioned study only included 5 cases of HCC. In all HCC patients, the FDG avid PVT was confirmed to be malignant.
In addition, Sun et al. [12] examined PET/CT scans of seven patients having HCC associated with PVT, the five cases who had tumoural PVT displayed increased FDG uptake, whereas the remaining two cases who had benign PVT did not display FDG avidity.
In the current study, the SUVmax was significantly higher in tumour thrombosis group (6.26 ± 1.94) than in bland thrombosis group (1.79 ± 0.69) (P < 0.001). This finding is matching with a study results performed by Hu et al. [14], who assessed the PET/CT scans in 72 cancer patients and discovered that the SUVmax in tumoural PVT group (6.37 ± 2.67) was significantly higher than SUVmax in the bland thrombi group (2.87 ± 1.47) (P < 0.01). Also, Nguyen et al. [16] discovered that PET/CT imaging findings revealed linear increased metabolic activity along the PVTT with expansion in all the patients with liver tumours. The thrombi’s mean SUVmax was calculated to be 7.0 (range 4.5–11.5). Sun et al. [12] also demonstrated a high FDG uptake of malignant PVT with SUVmax ranging between 3.0 and 11.5 in HCC patients.
According to the current study, liver cirrhosis was the most common among the bland thrombosis group (81.82%), and liver cirrhosis with HCC was the most common among the tumour thrombosis group (85.19%), with a significant difference (P < 0.001). In a previous study, Lee [17] reported that venous thromboembolism is a known and a relatively common complication of cancer. According to estimates, about 25 to 30% of all cases of venous thromboembolism (VTE) involve patients who have cancer. Blom et al. [18] discovered that malignancy is responsible for about 18% of all the cases of VTE. VTE is seven times more likely in cancer patients overall; in certain cancers, the risk is even higher.
According to the current study, the ROC curve of semiqualitative analysis (SUVmax) revealed a sensitivity of 96.3%, and a specificity of 36.4%, at area under curve of 0.827 with SUVmax > 3.5 as the pathological cut-off value. Most tumoural thrombi have high SUVmax values, but differentiating tumour thrombi from bland venous thrombi by PET imaging alone may be difficult as the inflammatory lesions may also have increased 18F-FDG uptake [19, 20]. Sharma et al. [10] proposed SUVmax measurement as a valuable tool for differentiating bland from tumour thrombi with a threshold value of 3.36. Lee and Khong [11] discovered a cut-off SUVmax value of 2.25, sensitivity of 78% and a specificity of 100% in distinguishing between tumour and bland thrombi. Hu et al. [14] discovered that SUVmax has a sensitivity of 93.6%, a specificity of 80%, and an accuracy of 88.9%, with a cut-off value of 3.35. To achieve a diagnostic accuracy, the semiqualitative analysis can be used instead of the visual analysis.
There are some limitations to this study. In 7 cases (18.4%), rather than histopathological evidence, clinical and imaging findings were used to first determine the type of thrombosis. Second, the study had flaws due to the retrospective study design and the relatively small patients’ number included.
Conclusion
We concluded that using semiqualitative analysis, 18F-FDG PET/CT is a valuable new technique in differentiating neoplastic from bland PV thrombi, with optimal cut-off SUVmax value > 3.5 as a criterion.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 18F-FDG PET-CT:
-
18 Fluoro-2-deoxy-D-glucose positron emission tomography-computed tomography
- CT:
-
Computed tomography
- HCC:
-
Hepatocellular carcinoma
- MSCT:
-
Multislice computed tomography
- MRI:
-
Magnetic resonance imaging
- PVTT:
-
Portal vein tumour thrombosis
- ROI:
-
Region of interest
- SUV:
-
Standardised uptake value
References
Sotiropoulos GC, Radtke A, Schmitz KJ, Molmenti EP, Schroeder T, Saner FH, Baba HA, Fouzas I, Broelsch CE, Malagó M, Lang H (2008) Liver transplantation in the setting of hepatocellular carcinoma and portal vein thrombosis: a challenging dilemma. Dig Dis Sci 53(7):1994–1999
Samant H, Asafo-Agyei KO, Garfield K (2021) Portal Vein thrombosis. [Updated 2022 Nov 23]. In: StatPearls. StatPearls Publishing, Treasure Island. https://www.ncbi.nlm.nih.gov/books/NBK534157/
Loudin M, Ahn J (2017) Portal vein thrombosis in cirrhosis. J Clin Gastroenterol 51(7):579–585
Northup PG, Garcia-Pagan JC, Garcia-Tsao G, Intagliata NM, Superina RA, Roberts LN, Lisman T, Valla DC (2021) Vascular liver disorders, portal vein thrombosis, and procedural bleeding in patients with liver disease: 2020 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 73(1):366–413
Tarantino L, Francica G, Sordelli I, Esposito F, Giorgio A, Sorrentino P, De Stefano G, Sarno AD, Ferraioli G, Sperlongano P (2006) Diagnosis of benign and malignant portal vein thrombosis in cirrhotic patients with hepatocellular carcinoma: color Doppler US, contrast-enhanced US, and fine-needle biopsy. Abdom Imaging 31(5):537–544
Piscaglia F, Gianstefani A, Ravaioli M, Golfieri R, Cappelli A, Giampalma E, Sagrini E, Imbriaco G, Pinna AD, Bolondi L (2010) Criteria for diagnosing benign portal vein thrombosis in the assessment of patients with cirrhosis and hepatocellular carcinoma for liver transplantation. Liver Transplant 16(5):658–667
Sizemore AW, Jacobs MP, Mantil JC, Hahm GK (2007) FDG uptake in inferior vena cava tumor thrombus from renal cell carcinoma on positron emission tomography. Clin Nucl Med 32(4):309–311
Kaida H, Ishibashi M, Kurata S, Uchida M, Hayabuchi N (2007) Tumor thrombus in the inferior vena cava from colon cancer detected by 18F-FDG-PET. Ann Nucl Med 21(3):185–188
Strobel K, Steinert HC, Bhure U, Koma AY, Gassmann N, Stöckli SJ (2007) Tumour thrombus in the superior vena cava from anaplastic carcinoma of the thyroid: FDG-PET/CT imaging findings. Eur J Nucl Med Mol Imaging 34(5):813–834
Sharma P, Kumar R, Jeph S, Karunanithi S, Naswa N, Gupta A, Malhotra A (2011) 18F-FDG PET-CT in the diagnosis of tumor thrombus: can it be differentiated from benign thrombus. Nucl Med Commun 32(9):782–788
Lee EY, Khong PL (2013) The value of 18F-FDG PET/contrast-enhanced CT in detection of tumor thrombus. Clin Nucl Med 38(2):60–65
Sun L, Guan YS, Pan WM, Chen GB, Luo ZM, Wei JH, Wu H (2008) Highly metabolic thrombus of the portal vein: 18F fluorodeoxyglucose positron emission tomography/computer tomography demonstration and clinical significance in hepatocellular carcinoma. World J Gastroenterol WJG 14(8):1212
Alswat KA, Sanai FM, Altuwaijri M, Albenmousa A, Almadi M, Al-Hamoudi WK, Abdo AA (2013) Clinical characteristics of patients with hepatocellular carcinoma in a middle eastern population. Hepat Mon 13(5):e7612
Hu S, Zhang J, Cheng C, Liu Q, Sun G, Zuo C (2014) The role of 18F-FDG PET/CT in differentiating malignant from benign portal vein thrombosis. Abdom Imaging 39(6):1221–1227
Aurangabadkar HU, Palle L, Ali Z (2013) Tumour thrombosis and patterns of fluorine-18 fluorodeoxyglucose uptake: a pictorial review. Nucl Med Commun 34(7):627–637
Nguyen XC, Nguyen DS, Maurea S (2015) FDG-avid portal vein tumor thrombosis from hepatocellular carcinoma in contrast-enhanced FDG PET/CT. Asia Ocean J Nuclear Med Biol 3(1):10
Lee AY (2005) Management of thrombosis in cancer: primary prevention and secondary prophylaxis. Br J Haematol 128(3):291–302
Blom JW, Doggen CJ, Osanto S, Rosendaal FR (2005) Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 293(6):715–722
Miceli M, Atoui R, Walker R, Mahfouz T, Mirza N, Diaz J, Tricot G, Barlogie B, Anaissie E (2004) Diagnosis of deep septic thrombophlebitis in cancer patients by fluorine-18 fluorodeoxyglucose positron emission tomography scanning: a preliminary report. J Clin Oncol 22(10):1949–1956
Shimoda M, Iso Y, Tomita S, Fujimori T, Murakami K, Sawada T, Kubota K (2008) Middle bile duct cancer with portal vein tumor thrombus. World J Surg Oncol 6(1):1–5
Acknowledgements
Not applicable.
Funding
This study had no funding from any resource.
Author information
Authors and Affiliations
Contributions
HSA contributed to conceptualisation and data curation. SA was involved in formal analysis, project administration and resources, supervision, validation and visualisation, and writing review, revised, and editing. SA and HS contributed to investigation and provided software. SA, HS, and RA were involved in methodology and writing original draft. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (Institutional Review Board (IRB) of National Liver Institute Menoufia University and with the Helsinki Declaration of 1964 and later versions. Committee’s reference number is (00341/2022). No consent was obtained from the patients since it was a retrospective study.
Consent for publication
All patients included in this research gave written informed consent to publish the data contained within this study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abokoura, S., Ellaban, H.S. & Aly, R.A. The role of 18F-FDG PET/CT in distinguishing benign from malignant portal vein thrombosis. Egypt J Radiol Nucl Med 54, 111 (2023). https://doi.org/10.1186/s43055-023-01058-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-023-01058-1