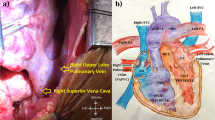
Abstract
Background
The coexisting of transposition of the great arteries (TGA) with total anomalous pulmonary venous connection (TAPVC) is one of the rare anomalies. The incidence of coexisting TAPVC and TGA is unknown with very few cases ever reported.
Case presentation
We reported a case of a 13-month-old female toddler with history of cyanosis. Echocardiography revealed atrioventricular ambiguity with pulmonary atresia, all PVs drain into the innominate vein via vertical vein (VV), ostium secundum atrial septal defect (ASD) and ventricular septal defect (VSD) were observed. The CT scan confirmed co-occurrence of TGA and TAPVC. All four confluence PVs behind the small left atrium (LA), drains into an ascending lateral large VV, coursing to innominate vein without any PV access into LA. The superior vena cava, right atrium and right ventricle (RV) were dilated. The RV is the origin of the aortic root. The aorta continues on the right side, with an arterial connection to the right pulmonary aberrant artery at the level of the aortic arch. Main pulmonary artery originates from LV and appears atretic with only connection to the left pulmonary artery. Large ASD and VSD were identified.
Conclusions
TGA and TAPVC are a rare combination and should be suspected in mild-cyanotic cases with levocardia with situs solitus. CT angiography is one of the modalities of choice to characterize the vasculature anomalies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
An anatomical description of transposition of the great arteries (TGA), specifically dextro-TGA or D-TGA with total anomalous pulmonary venous connection (TAPVC), was first published in 1933 [1]. The coexistence of D-TGA and TAPVC is uncommonly described in the scientific literature. With the unknown incidence, only a few publications describing patients who underwent anatomically anomalous diagnostic workups or surgery. The case of coexisting D-TGA and TAPVC in a toddler with cyanotic cardiac disease was described.
Case presentation
A 13-month-old female toddler was referred to the radiology department for a computed tomography (CT) angiography of the chest to evaluate the cardiac vasculature. The infant was diagnosed with cyanosis within her first week of life. Initially, two-dimensional echocardiography had been performed of which revealed atrioventricular ambiguity with pulmonary artery atresia, all pulmonary veins (PVs) drain into the innominate vein (IV) via vertical vein (VV), ostium secundum atrial septal defect (ASD) and ventricular septal defect (VSD) were observed. Left ventricle (LV) present with EF 83%. Right ventricle (RV) had good contractility with tricuspid annular plane systolic excursion of 1.3 cm.
A non-ECG-gated, free-breathing chest CT angiography was performed using a 128-multislice CT scanner (Revolution™ HD, GE HealthCare Technologies Inc., US), with 100 kVP and 150 mAs after 10.5 mL of nonionic, water-soluble, iodine contrast material (Iohexol™ 350 mg/mL) was administered. Advantage Workstation™ VolumeShare 7 (GE HealthCare Technologies Inc, US) was used for image processing.
The CT scan detected levocardia with situs solitus. All four PVs are in confluence behind the small left atrium (LA), and their confluence converges into an ascending lateral large VV, coursing to IV without any PV access into LA. TAPVC was confirmed, with no observable PV access into LA. Large atrial septal defect (ASD) and ventricular septal defect (VSD) were identified (Fig. 1). The superior vena cava (SVC), right atrium (RA) and RV were dilated. D-TGA was suggested after it was discovered that the major arteries of both ventricles were reversed. The RV is the origin of the aortic root. The aorta continues on the right side, with an apparent connection to the aberrant right pulmonary artery at the level of the aortic arch via the arterial communication favors to major aortopulmonary collateral arteries (MAPCAs). Main pulmonary artery originates from LV and appears atretic with only connection to the left pulmonary artery. The liver is located in the midline, and no intraabdominal spleen was identified (Fig. 2). Other identified intraabdominal organs were unremarkable. Schematic cardiac findings are featured in Fig. 3.
Axial and coronal section of CT angiography of the cardiac vasculature. All four pulmonary veins (PVs) are in confluence behind the small left atrium (LA), and their confluence converges to an ascending lateral large vertical vein (VV) which drains into innominate vein (IV) (A–C). The entrance of PV into LA is absent. All the finding favors to supracardiac TAPVC. Large ostium secundum atrial septal defect (ASD) and ventricular septal defect (VSD) were identified (D)
Sagittal, coronal and axial section of cardiac CT angiography of the cardiac vasculature. Aortic root is originated from the right ventricle (RV). The aorta continues on the right side, with an apparent connection to the right pulmonary aberrant artery at the level of the aortic arch via the major aortopulmonary collateral arteries (MAPCAs) (A). Main pulmonary artery originates from left ventricle and appears atretic with only connection to the left pulmonary artery (PA) (B, C). Aortic valve is located on the anterior right to the pulmonary valve confirming the spatial relationship of semilunar cusps in D-TGA (C). The liver is transversal in position and located in the midline, and no spleen was identified, suggesting a possible heterotaxy syndrome, along with the cardiac anomaly (D)
Schematic of complex cardiac abnormalities of the reported case with the main feature of co-occurrence of D-TGA and supracardiac TAPVC. The right accessory pulmonary artery (Rt-PA) is supplied by arterial communication from the aorta, favoring major aortopulmonary collateral arteries (MAPCAs). Both ASD and VSD are present. ASD Atrial septal defect, IVC Inferior vena cava, LA Left atrium, Lt-PA Left pulmonary artery, LV Left ventricle, PV Pulmonary vein, RA Right atrium, RV Right ventricle, SVC Superior vena cava, VSD Ventricular septal defect
Until this report was made, the patient underwent routine follow-up for a surgical plan at a national cardiac surgery center elsewhere. She was scheduled for an initial Blalock–Thomas–Taussig shunt surgery to increase blood flow to the pulmonary circulation before undergoing other definitive surgery.
Discussion
Epidemiology
Very few cases of D-TGA and TAPVC co-occurrence have ever been documented (Table 1). Adults with coexisting D-TGA and TAPVC are quite uncommon. The majority of these occurrences occurred in newborns, and younger children. The oldest patient documented in medical literature was 22 years old [2]. The incidence of TAPVC is increased in individuals with heterotaxy syndromes, especially asplenia [3], as is the case with our patient.
Presentation
Total anomalous pulmonary venous return (TAPVR), total anomalous pulmonary venous drainage (TAPVD), and total anomalous pulmonary venous connection (TAPVC) are terms that are arbitrarily used and generally regarded synonymous. Hines [22] defines TAPVC as the absence of confluence between the PV and the LA. In contrast, the junction of the pulmonary vein as an entrance to the LA is still maintained and the aberrant drainage is secondary to the presence of some level of left-sided structural atresia in TAPVR.
Consequently, in the isolated TAPVC, all PVs drain abnormally into the systemic venous circulation, rather than the LA. Thus, oxygenated pulmonary blood is rerouted to the right heart and pumped back to the lungs [23]. To gain survival, a portion of the RA's mixed blood is diverted to the left heart via a right-to-left shunt to supply the systemic circulation. This occurs commonly at the atrial level via an ASD, a patent foramen ovale, or less frequently a PDA [23, 24].
In isolated D-TGA, the aorta originates from the RV and is located right and anterior to the PA, which, in the contrary, originates from the LV. Thus, the deoxygenated systemic venous blood goes to the right heart and then to the systemic circulation via the aorta. Similar to TAPVC, a connectivity between the left and right sides, such as an ASD, patent foramen ovale, VSD, or PDA, is necessary for survival [25].
Cyanosis is usually present at birth in cases of isolated D-TGA or obstructed TAPVC. Both abnormalities have a high risk of inducing pulmonary arterial hypertension (PAH) at an early age, although through different mechanisms. In d-TGA, the process begins in utero with the preferential channeling of oxygen-rich inferior vena cava blood into the left heart through the VSD, causing dilatation of the pulmonary arteries and strain damage to the vascular intima, followed by secondary vasoconstriction. In obstructed TAPVC, however, the increase in pulmonary pressures is due to pulmonary venous hypertension, the severity of which is proportionate to the degree of obstruction. The coexistence of TGA and TAPVC reduces the hemodynamic impact of each individual anomaly by limiting severe desaturation and pulmonary overflow. Instead of the pulmonary circulation, the oxygen-rich blood that drains into the RA is delivered to the systemic circulation [21]. Possibly as a result of this reciprocal compensation, cyanosis is less noticeable and may go unrecognized at birth, culminating in an undeveloped and depleted LV as the individual ages [17, 18].
As oxygenated blood from the pulmonary veins is directed to the systemic circulation, the coexistence of TAPVC and D-TGA improves systemic saturations. However, it can cause a milder apparent cyanosis in a relatively asymptomatic infant, obscuring the discovery of a significant congenital heart disease [18]. Clinically, we should suspect this coexisting condition in children with a diagnosis of TGA and severe right ventricular enlargement, but who are significantly less cyanotic than the average child with uncomplicated TGA [9].
Diagnostic workup
Prompt diagnosis and surgical strategy are essential to reducing morbidity and mortality in severe cases. Some of the most intricate aspects of TAPVC may not be depicted by echocardiography, the first and safest imaging technique for cardiovascular anomalies [11, 16]. For mapping the pulmonary veins and other vascular and structural anomalous anatomy without the necessity for invasive cardiac catheterization, CT angiography provides a noninvasive and sensitive alternative. Contrast-enhanced magnetic resonance (MR) angiography is a radiation-free alternative to CT angiography, although younger children may require general anesthesia. For morphological identification in TAPVC, electrocardiography-gated CT and phase-contrast cine MR imaging (PC-MRI) are preferred above non-gated CT [26].
Treatment
The treatment strategy for a patient with coexisting D-TGA and TAPVC must be supported by clinical and radiological data indicating preservation of LV mass and the absence of irreversible pulmonary vascular disease despite the presence of PAH. [18]. A few authors reported surgery as the mainstay of treatment for coexisting TGA and TAPVC. Successful results were obtained using modified atrial switching techniques based on the principles of either Senning [2, 6, 10, 18] or Mustard [5, 7,8,9, 17]. However, in recent years, arterial switch operation (ASO) has become the preferred approach for the TGA.
Few cases have been reported of successful anatomic repair through ASO. The first successful ASO was performed by Lopes et al. on a 22-h-old infant with D-TGA and subdiaphragmatic TAPVC [11], followed by Seliem et al. on a 4-year-old toddler who had d-TGA with supracardiac TAPVC [13], and by Mykychak et al. on TGA and subdiaphragmatic TAPVC [14]. A few number procedures have been performed elsewhere [15, 16, 20]. The similar observations of the late presentation and early regression of the LV serve as limiting factors for ASO.
In D-TGA, the regression of LV mass often begins during the first 2–3 weeks. Beyond this period, the steady decline in PVR deconditions the performance of the LV with a progressive decrease in its muscle mass [18]. In the coexisting D-TGA and TAPVC, only a few have documented variability in the development of LV mass regression, which can occur early [18] or late [20, 21]. Nevertheless, ASO is encouraged within the first 2 to 3 weeks of life. Beyond that period, it may pose the risk of incapability of LV to cope with the acutely increased work of systemic circulation following surgery [9].
LV mass is preserved in TGA patients with a large VSD or PDA, obstruction of LV outflow, unregressed pulmonary vascular resistance (PVR), or a significant aortopulmonary collateral due to the presence of a greater afterload. In cases of extensive interatrial communication alone, the LV mass is often not adequately preserved following a decrease in PVR since there is no afterload and a substantial decrease in LV inflow [18].
A case report by Abah et al. [20] described a satisfactory outcome of a late-treated coexisting TAPVC and TGA, with the advantage of PAH in preserving the LV mass being highlighted. PAH and volume load from the significant right-to-left shunt at the atrial level due to the large ostium secundum ASD provide a pressure stress on the LV without resulting permanent pulmonary vascular disease. The existence of obstructed TAPVC will result in increased PA pressures, which may be beneficial for LV readiness.
Conclusions
Even in the presence of normal cardiac and abdominal situs, TGA can be associated with anomalous pulmonary venous connection. In order to aid clinical decisions and the planning of surgical and catheter-based procedures, it is essential to map the relevant anatomy. For these reasons, understanding of anomalous pulmonary venous drainage and variant anatomy is essential for optimizing the prognosis, particularly in the diagnostically challenging patient.
Availability of data and materials
Not applicable.
Abbreviations
- ASD:
-
Atrial septal defect
- ASO:
-
Arterial switch operation
- CT:
-
Computed tomography
- D-TGA:
-
Dextro-transposition of the great arteries
- LA:
-
Left atrium
- LV:
-
Left ventricle
- MAPCAs:
-
Major aortopulmonary collateral arteries
- MRI:
-
Magnetic resonance imaging
- PAH:
-
Pulmonary artery hypertension
- PV:
-
Pulmonary vein
- RA:
-
Right atrium
- RV:
-
Right ventricle
- SVC:
-
Superior vena cava
- TAPVC:
-
Total anomalous pulmonary venous connection
- TAPVD:
-
Total anomalous pulmonary venous drainage
- TAPVR:
-
Total anomalous pulmonary venous return
- VSD:
-
Ventricular septal defect
- VV:
-
Vertical vein
References
Feldman WH, Chalmes A (1933) A case of complete transposition of the great vessels of the heart with a patent foramen ovale. Br J Child Dis 30:27
Mishra A, Garg P, Agarwal V, Rana Y (2020) Transposition of great arteries with total anomalous pulmonary venous connection: a modified Senning procedure for late presentation. JTCVS Tech 4:223–226
Ghosh S, Yarmish G, Godelman A, Haramati LB, Spindola-Franco H (2009) Anomalies of visceroatrial situs. Am J Roentgenol 193(4):1107–1117. https://doi.org/10.2214/AJR.09.2411
Whitaker W, Watson D, Keates P (1964) Total anomalous pulmonary venous drainage into the left innominate vein associated with transposition of the great vessels. Circulation 30(6):918–922. https://doi.org/10.1161/01.CIR.30.6.918
Sapsford RN, Aberdeen E, Watson DA, Crew AD (1972) Transposed great arteries combined with totally anomalous pulmonary veins. J Thorac Cardiovasc Surg 63(3):360–366
Barbero-Marcial M, Verginelli G, Vila J, Zerbini EJ (1984) Transposition of the great arteries associated with total anomalous pulmonary venous connection: a surgical approach. Ann Thorac Surg 37(1):92–94
Amodeo A, Corno A, Marino B, Carta MG, Marcelletti C (1990) Combined repair of transposed great arteries and total anomalous pulmonary venous connection. Ann Thorac Surg 50(5):820–821
Thies W, Matthiers W, Minami K, Pot U, Meyer H, Korfer R (1990) Surgical repair and postoperative course of an infant with infracardiac total anomalous pulmonary venous connection, cor triatriatum sinistrum and transposition of the great arteries. Eur J Cardio-Thorac Surg 4(1):45–47. https://doi.org/10.1016/1010-7940(90)90240-Z
Gontijo B, Fantini F, Barbosa M, Gomes M, Gutierrez C, Vrandecic M (1994) Surgical repair of transposition of great arteries and total anomalous pulmonary venous return to the coronary sinus (TGA with TAPVR). Eur J Cardio-Thorac Surg 8(7):391–392. https://doi.org/10.1016/1010-7940(94)90035-3
Ueda Y, Miki S, Okita Y, Tahata T, Sakai T, Matsuyama K et al (1994) Transposition of the great arteries associated with total anomalous pulmonary venous return. Ann Thorac Surg 57(2):470–472
Lopes LM, Tavares GMP, Mailho FL, Almeida VDPCD, Mangione JA (2001) Echocardiographic diagnosis of transposition of the great arteries associated with anomalous pulmonary venous connection. Arq Bras Cardiol 77(1):66–68
Raff GW, Geiss DM, Shah JJ, Bond LM, Carroll JA (2002) Repair of transposition of the great arteries with total anomalous pulmonary venous return. Ann Thorac Surg 73(2):655–657
Seliem MA, Bouholaigah IH, Palileo MR (2004) Complete transposition of the great arteries and total anomalous pulmonary venous connection with a small atrial septal defect: a rare combination resulting in balanced pulmonary systemic circulations. Ann Saudi Med 24(2):133–135
Mykychak Y, Fedevych O, Maksymenko A, Yemets I (2017) Simultaneous arterial switch and totally anomalous pulmonary venous connection repair in a 5-hour-old child, complicated by pulmonary venous stenosis. Interact Cardiovasc Thorac Surg 24(5):809–810. https://doi.org/10.1093/icvts/ivw452
Salve GG, Jain SA, Dalvi BV, Shivaprakash K (2017) Transposition of the great arteries with total anomalous pulmonary venous connection. Ann Thorac Surg 103(4):e349–e351
Meliota G, Scalzo G, Vairo U (2019) A situs solitus transposition of great arteries with obstructed sub-diaphragmatic totally anomalous pulmonary venous connection: a rare case treated with anatomical repair. Cardiol Young 29(12):1536–1538
Samaddar A, Das M, Roychowdhury S, Roy M, Khan W, Sengupta R et al (2020) d-transposition of great arteries and total anomalous pulmonary venous connection with left ventricular regression—a rarity. World J Pediatr Congenit Hear Surg 11(1):114–116. https://doi.org/10.1177/2150135119878035
Aggarwal N, Joshi R, Paktin N, Agarwal M, Joshi R (2019) Complete transposition of great arteries associated with total anomalous pulmonary venous connection: an unusual cause for early left ventricular myocardial mass regression. Ann Pediatr Cardiol 12(3):302
Scrascia G, Grimaldi AP, Troise D, Scalzo G (2020) Unusual association of transposition of great arteries with infradiaphragmatic pulmonary venous return. Ann Pediatr Cardiol 13(1):75
Abah R, Prabhu A, Katewa A, Sahu B (2021) Transposition of the great arteries with total anomalous pulmonary venous connection in a 1½ year-old child: pulmonary arterial hypertension—an advantage. Ann Pediatr Cardiol 14(2):235
Chatterjee D, Gangopadhyay D, Narayan P (2021) Rare presentation of transposition of great arteries and total anomalous pulmonary venous connection in an 18-year-old. Echocardiography 38(10):1841–1843. https://doi.org/10.1111/echo.15197
Hines MH, Hammon JW (2001) Anatomy of total anomalous pulmonary venous connection. Oper Tech Thorac Cardiovasc Surg 6(1):2–7
Lyen S, Wijesuriya S, Ngan-Soo E, Mathias H, Yeong M, Hamilton M et al (2017) Anomalous pulmonary venous drainage: a pictorial essay with a CT focus. J Congenit Cardiol 1(1):7. https://doi.org/10.1186/s40949-017-0008-4
Dillman JR, Yarram SG, Hernandez RJ (2009) Imaging of pulmonary venous developmental anomalies. Am J Roentgenol 192(5):1272–1285. https://doi.org/10.2214/AJR.08.1526
Canan A, Ashwath R, Agarwal PP, François C, Rajiah P (2021) Multimodality imaging of transposition of the great arteries. Radiographics 41(2):338–360. https://doi.org/10.1148/rg.2021200069
Ogawa M, Nakagawa M, Hara M, Ito M, Goto T, Ohte N et al (2013) Total anomalous pulmonary venous connection in a 64-year-old man: a case report. Ann Thorac Cardiovasc Surg 19(1):46–48
Acknowledgements
Not applicable.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
YSN contributed to study design, data collection, manuscript preparation, literature search, and data interpretation; IPS contributed to data collection, manuscript preparation, and literature search; IPM contributed to study design, manuscript preparation, and data interpretation; RFR contributed to literature search and manuscript preparation; SLW contributed to data collection; data interpretation; and manuscript preparation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Non-identifiable images of the patient have been only used. Careful attention has been provided to make sure that no patient identifiable information is in the images provided.
Consent for publication
Informed consent from the patient has been obtained, and signed consent was provided by the patient.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nawawi, Y.S., Sari, I.P., Maryetty, I.P. et al. Case report of coexisting transposition of the great arteries with supracardiac total anomalous pulmonary venous connection: focus on computed tomography features. Egypt J Radiol Nucl Med 54, 66 (2023). https://doi.org/10.1186/s43055-023-01013-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-023-01013-0